Short-Term Weight Gain after Tonsillectomy Does Not Lead to Overweight: A Systematic Review
Abstract
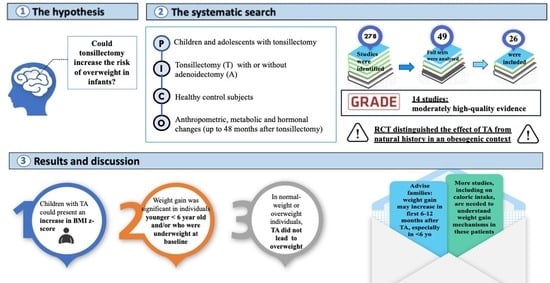
1. Introduction
- (1)
- to provide an up-to-date summary of the available evidence on the impact of TA on weight gain and eventually increased prevalence of overweight and obesity;
- (2)
- to identify subgroups of children and adolescents at risk of weight gain;
- (3)
- to elucidate mechanisms beyond increased caloric intake that underlie weight gain.
2. Materials and Methods
2.1. Search Strategy
2.2. Criteria for Study Selection
| Population | Children and Adolescents (1–18 Years) Who Had Undergone Tonsillectomy |
| Intervention | Tonsillectomy with or without adenoidectomy |
| Comparison | Healthy control subjects |
| Outcomes | Preoperative and postoperative (up to 24 months after) growth (weight, height, BMI (body mass index), and relative percentiles for age, body composition, metabolic and hormonal changes and/or comparison with a control group |
| Study design | Randomized clinical trials (RCTs), observational studies (cohort, case-control, cross-sectional studies), exploratory studies, mix of qualitative and quantitative studies |
2.3. Data Extraction and Management
2.4. Assessment of the Certainty of the Evidence
| High: | Further Research Is Very Unlikely to Change Confidence in the Estimate of the Effect |
| Moderate | Further research is likely to have an important impact on confidence in the estimate of the effect and may change the estimate |
| Low | Further research is very likely to have an important impact |
| Very low | Any estimate of effect is very uncertain |
3. Results
3.1. Changes in Growth Pattern after Tonsillectomy
3.1.1. BMI z-Score
3.1.2. Determinants of BMI z-Score after TA
3.1.3. Waist Circumference
3.1.4. Percentage of Overweight/Obesity
3.2. Changes in Body Composition after Tonsillectomy
3.3. Hormonal Changes Influencing Post-Operative Growth after Tonsillectomy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maffeis, C.; Olivieri, F.; Valerio, G.; Verduci, E.; Licenziati, M.R.; Calcaterra, V.; Pelizzo, G.; Salerno, M.; Staiano, A.; Bernasconi, S.; et al. The Treatment of Obesity in Children and Adolescents: Consensus Position Statement of the Italian Society of Pediatric Endocrinology and Diabetology, Italian Society of Pediatrics and Italian Society of Pediatric Surgery. Ital. J. Pediatr. 2023, 49, 69. [Google Scholar] [CrossRef] [PubMed]
- Frye, S.S.; Fernandez-Mendoza, J.; Calhoun, S.L.; Gaines, J.; Vgontzas, A.N.; Liao, D.; Bixler, E.O. Childhood Obesity, Weight Loss and Developmental Trajectories Predict the Persistence and Remission of Childhood Sleep-disordered Breathing. Pediatr. Obes. 2019, 14, e12461. [Google Scholar] [CrossRef]
- Paul, M.E.; Wallace, J.G.; Coakley, B.A. An Assessment of the Relationship Between BMI and Children Undergoing Surgical Procedures: A Retrospective Study. Child. Obes. 2023, 19, 249–257. [Google Scholar] [CrossRef]
- Ng, H.; Wong, E.; Curotta, J.; Trapani, S.; Cheng, A.T. Tertiary Hospital Retrospective Observational Audit of Tonsillectomy. Int. J. Pediatr. Otorhinolaryngol. 2019, 121, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.K.; Quraishi, H.A. Tonsillectomy and Adenoidectomy—Pediatric Clinics of North America. Pediatr. Clin. N. Am. 2022, 69, 247–259. [Google Scholar] [CrossRef]
- Roche, A.F. The Influence of Tonsillectomy on Growth and Caloric Intake. J. Pediatr. 1964, 65, 360–367. [Google Scholar] [CrossRef]
- Bolling, C.F. 50 Years Ago in The Journal of Pediatrics. The influence of tonsillectomy on growth and caloric intake. J. Pediatr. 2014, 165, 496. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, A.; Fettman, N.; Armbrecht, E.S.; Mitchell, R. A Systematic Review of Adenotonsillectomy as a Risk Factor for Childhood Obesity. Otolaryngol. Head Neck Surg. 2011, 144, 154–158. [Google Scholar] [CrossRef]
- Van, M.; Khan, I.; Hussain, S.S.M. Short-Term Weight Gain after Adenotonsillectomy in Children with Obstructive Sleep Apnoea: Systematic Review. J. Laryngol. Otol. 2016, 130, 214–218. [Google Scholar] [CrossRef]
- Levi, J.; Leoniak, S.; Schmidt, R. Evaluating Tonsillectomy as a Risk Factor for Childhood Obesity. Arch. Otolaryngol. Head Neck Surg. 2012, 138, 897. [Google Scholar] [CrossRef][Green Version]
- Topal, K.; Kara, C.O.; Bozkurt, A.I.; Saatci, E. The Risk of Overweight and Obesity in Children after Tonsillectomy: A Cross-Sectional Study. Eur. Arch. Otorhinolaryngol. 2013, 270, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.L. Weight Gain after Tonsillectomy: Myth or Reality? Interpreting Research Responsibly. Otolaryngol. Head Neck Surg. 2011, 144, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Nachalon, Y.; Lowenthal, N.; Greenberg-Dotan, S.; Goldbart, A.D. Inflammation and Growth in Young Children with Obstructive Sleep Apnea Syndrome before and after Adenotonsillectomy. Mediat. Inflamm. 2014, 2014, 146893. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, P.; Löppönen, T.; Tolonen, U.; Lanning, P.; Knip, M.; Löppönen, H. Growth and biochemical markers of growth in children with snoring and obstructive sleep apnea. Pediatrics 2002, 109, e55. [Google Scholar] [CrossRef]
- Schünemann, H.J.; Cuello, C.; Akl, E.A.; Mustafa, R.A.; Meerpohl, J.J.; Thayer, K.; Morgan, R.L.; Gartlehner, G.; Kunz, R.; Katikireddi, S.V.; et al. GRADE Guidelines: 18. How ROBINS-I and Other Tools to Assess Risk of Bias in Nonrandomized Studies Should Be Used to Rate the Certainty of a Body of Evidence. J. Clin. Epidemiol. 2019, 111, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.F.; Vikani, A.R.; Benke, J.R.; Boss, E.F.; Ishman, S.L. Weight Gain after Adenotonsillectomy Is More Common in Young Children. Otolaryngol. Head Neck Surg. 2013, 148, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-S.; Guilleminault, C.; Lee, L.-A.; Lin, C.-H.; Hwang, F.-M. Treatment Outcomes of Adenotonsillectomy for Children with Obstructive Sleep Apnea: A Prospective Longitudinal Study. Sleep 2014, 37, 71–76. [Google Scholar] [CrossRef]
- Koycu, A.; Aydin, E.; Kinik, S.T. Changes in body composition and growth pattern after adenotonsillectomy in prepubertal children. Int. J. Pediatr. Otorhinolaryngol. 2016, 81, 46–50. [Google Scholar] [CrossRef]
- Inja, R.R.; Paul, R.R.; Varghese, L.; Sebastian, T.; Mathews, S.S. Impact of tonsillectomy on dysphagia symptoms and body weight in children. Am. J. Otolaryngol. 2020, 41, 102682. [Google Scholar] [CrossRef]
- Elnashar, I.; El-Anwar, M.W.; Raafat, A.; Aesa, E.; Fathy, S. Insulin-like Growth Factor Alpha Changes after Tonsillectomy for Obstructive and Nonobstructive Causes. Egypt. J. Otolaryngol. 2014, 30, 343–346. [Google Scholar] [CrossRef]
- Costa Pinto Ribeiro, A.; Capelo Candido, T.; Almeida Nascimento, P.H.; Ferraz Rodrigues, P.; Benini Guércio, W. Crescimento pôndero-estatural de crianças e adolescentes submetidos à adenoamigdalectomia. Sci. Medica 2021, 31, e39746. [Google Scholar] [CrossRef]
- Beauchamp, M.T.; Regier, B.; Nzuki, A.; Swinburne Romine, R.; Sweeney, B.; Liu, M.; Davis, A.M. Weight Change before and after Adenotonsillectomy in Children: An Analysis Based upon Pre-surgery Body Mass Category. Clin. Otolaryngol. 2020, 45, 739–745. [Google Scholar] [CrossRef]
- Ha, E.K.; Lee, S.W.; Kim, J.H.; Lee, J.E.; Jee, H.M.; Chae, K.Y.; Han, M.Y.; Rhie, S. Changes in Childhood Growth after Adenotonsillectomy: A Population-Based Cohort Study. Sleep Med. 2022, 89, 114–121. [Google Scholar] [CrossRef]
- Han, S.C.; Yang, S.K.; Han, S.Y.; Rhee, C.S.; Choi, Y.; Shin, C.H.; Lee, Y.J.; Han, D.H. Investigating factors influencing post-operative growth in pre-pubertal children after adenotonsillectomy. Eur. Arch. Otorhinolaryngol. 2023, 280, 2841–2848. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, C.H.; Kim, H.-M. Behavioral Consequences of Children with Sleep-Disordered Breathing after Adenotonsillectomy. World J. Pediatr. 2018, 14, 57–65. [Google Scholar] [CrossRef]
- Voora, R.S.; Carvalho, D.; Jiang, W. Impact of Tonsillectomy on Obesity in Pediatric Patients With Sleep-Disordered Breathing. OTO Open 2021, 5, 2473974X211059105. [Google Scholar] [CrossRef]
- Hsu, W.-C.; Kang, K.-T.; Weng, W.-C.; Lee, P.-L. Impacts of Body Weight after Surgery for Obstructive Sleep Apnea in Children. Int. J. Obes. 2013, 37, 527–531. [Google Scholar] [CrossRef]
- Koren, D.; Gozal, D.; Bhattacharjee, R.; Philby, M.F.; Kheirandish-Gozal, L. Impact of Adenotonsillectomy on Insulin Resistance and Lipoprotein Profile in Nonobese and Obese Children. Chest 2016, 149, 999–1010. [Google Scholar] [CrossRef]
- Karalok, Z.S.; Akdag, M.; Turhan, M.; Uzun, G.; Ozdem, S.; Dinc, O.; Bircan, I. Leptin and Ghrelin Levels in Children before and after Adenoidectomy or Adenotonsillectomy. Horm. Res. Paediatr. 2014, 81, 20–24. [Google Scholar] [CrossRef]
- Moghaddam, Y.J.; Golzari, S.E.J.; Saboktakin, L.; Seyedashrafi, M.H.; Sabermarouf, B.; Gavgani, H.A.E.; Haghjo, A.G.; Lotfi, A.; Ghabili, K. Does Adenotonsillectomy Alter IGF-1 and Ghrelin Serum Levels in Children with Adenotonsillar Hypertrophy and Failure to Thrive? A Prospective Study. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 1541–1544. [Google Scholar] [CrossRef]
- Yogeesha, B.S.; Arumugam, S.; Maradi, N. Impact of Adenotonsillectomy on Weight Gain in Children. Bengal J. Otolaryngol. Head Neck Surg. 2020, 28, 157–160. [Google Scholar]
- Czechowicz, J.A.; Chang, K.W. Analysis of Growth Curves in Children After Adenotonsillectomy. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 491. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.L.; Johnson, R.F.; Choi, J.; Mitchell, R.B. Weight Gain after Adenotonsillectomy: A Case Control Study. Otolaryngol. Head Neck Surg. 2015, 152, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.S.; Moore, R.H.; Rosen, C.L.; Mitchell, R.B.; Amin, R.; Arens, R.; Muzumdar, H.; Chervin, R.D.; Marcus, C.L.; Paruthi, S.; et al. Growth After Adenotonsillectomy for Obstructive Sleep Apnea: An RCT. Pediatrics 2014, 134, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.M.; Herrmann, B.W.; Mitchell, R.B.; Friedman, N.R. Growth After Adenotonsillectomy for Obstructive Sleep Apnea: Revisited. Laryngoscope 2022, 132, 1289–1294. [Google Scholar] [CrossRef]
- Kirkham, E.M.; Leis, A.M.; Chervin, R.D. Weight Gain in Children after Adenotonsillectomy: Undesirable Weight Gain or Catch-up Growth? Sleep Med. 2021, 85, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Al Abdulla, A.; Prabhu, S.; Behzad, K. Prospective Controlled Study on Post-Tonsillectomy Weight Gain-by Objective and Subjective Methods. Egypt. J. Ear Nose Throat Allied Sci. 2018, 1, 24–26. [Google Scholar] [CrossRef]
- AlAbdullah, Z.A.; Alali, K.; Al Jabr, I. Clinical Assessment of Weight Gain in Pediatric Patients Post-Tonsillectomy: A Retrospective Study. Cureus 2020, 12, e12005. [Google Scholar] [CrossRef]
- Kevat, A.; Bernard, A.; Harris, M.-A.; Heussler, H.; Black, R.; Cheng, A.; Waters, K.; Chawla, J. Impact of Adenotonsillectomy on Growth Trajectories in Preschool Children with Mild–Moderate Obstructive Sleep Apnea. J. Clin. Sleep Med. 2023, 19, 55–62. [Google Scholar] [CrossRef]
- Au, C.T.; Chan, K.C.C.; Lee, D.L.Y.; Leung, N.; Chow, S.M.W.; Chow, J.S.; Wing, Y.K.; Li, A.M. Effect of Surgical Intervention for Mild Childhood Obstructive Sleep Apnoea on Attention and Behavioural Outcomes: A Randomized Controlled Study. Respirology 2021, 26, 690–699. [Google Scholar] [CrossRef]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity among Adults: United States, 2017–2018; NCHS Data Brief; 2020; pp. 1–8. [Google Scholar]
- Stierman, B.; Afful, J.; Carroll, M.D.; Chen, T.C.; Fink, S.; Fryar, C.D.; Gu, Q.; Hales, C.M.; Hughes, J.P.; Ostchega, Y.; et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files; National Health Statistics Reports Number 158; National Center for Health Statistics: Hyattsville, MD, USA, 2021. [CrossRef]
- Bonuck, K.; Parikh, S.; Bassila, M. Growth failure and sleep disordered breathing: A review of the literature. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Blaser, M.J. Antibiotics in early life and obesity. Nat. Rev. Endocrinol. 2015, 11, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Saari, A.; Virta, L.J.; Sankilampi, U.; Dunkel, L.; Saxen, H. Antibiotic exposure in infancy and risk of being overweight in the first 24 months of life. Pediatrics 2015, 135, 617–626. [Google Scholar] [CrossRef]
- Trasande, L.; Blustein, J.; Liu, M.; Corwin, E.; Cox, L.M.; Blaser, M.J. Infant antibiotic exposures and early-life body mass. Int. J. Obes. 2013, 37, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Georgalas, C.C.; Tolley, N.S.; Narula, P.A. Tonsillitis. BMJ Clin. Evid. 2014, 22, 0503. [Google Scholar]
- Samara, P.; Athanasopoulos, M.; Athanasopoulos, I. Unveiling the Enigmatic Adenoids and Tonsils: Exploring Immunology, Physiology, Microbiome Dynamics, and the Transformative Power of Surgery. Microorganisms 2023, 11, 1624. [Google Scholar] [CrossRef]
- Paramaesvaran, S.; Ahmadzada, S.; Eslick, G.D. Incidence and potential risk factors for adenoid regrowth and revision adenoidectomy: A meta-analysis. Int. J. Pediatr. Otorhinolaryngol. 2020, 137, 110220. [Google Scholar] [CrossRef]
- Lesinskas, E.; Drigotas, M. The incidence of adenoidal regrowth after adenoidectomy and its effect on persistent nasal symptoms. Eur. Arch. Otorhinolaryngol. 2009, 266, 469–473. [Google Scholar] [CrossRef]
- Marcus, C.L.; Carroll, J.L.; Koerner, C.B.; Hamer, A.; Lutz, J.; Loughlin, G.M. Determinants of Growth in Children with the Obstructive Sleep Apnea Syndrome. J. Pediatr. 1994, 125, 556–562. [Google Scholar] [CrossRef]
- Gkouskou, K.K.; Vlastos, I.M.; Hajiioannou, I.; Hatzaki, I.; Houlakis, M.; Fragkiadakis, G.A. Dietary habits of preschool aged children with tonsillar hypertrophy, pre- and post-operatively. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 1025–1030. [Google Scholar]
- Selimoğlu, E.; Selimoğlu, M.; Orbak, Z. Does Adenotonsillectomy Improve Growth in Children with Obstructive Adenotonsillar Hypertrophy? J. Int. Med. Res. 2003, 31, 84–87. [Google Scholar] [CrossRef] [PubMed]

| References | Main Objective | Study Design | Population and Comparator | Methods | Outcomes | Results | Study Limitations and Level of Evidence |
|---|---|---|---|---|---|---|---|
| Smith DF et al. [16] | Post-op WT and demographic risk factors | Retrospective | 115 children with TA (85 had surgery for OSA; 30 for RT) Pre-op BMI z-score: 0.98 ± 1.5 Age: 1–18 y (7.2 ± 4.3 y) Follow-up: 6 m Period: 2008–2011 Region: USA | Chart review | HT, WT, BMIz changes: pre-op vs. post-op Risk factors: age, gender, race | BMIz increased from 0.98 ± 1.5 to 1.21 ± 1.25 (p = 0.0009) No difference by surgical indication, gender, race Younger age (<6 years) was a significant predictor of postoperative weight gain (p = 0.015) | Retrospective Moderate |
| Huang YS et al. [17] | Efficacy of TA in children with OSA | Prospective | 88 children with OSA underwent TA Pre-op BMI z-score: −0.7 ± 1.0 Age: 6–12 y (8.9 ± 2.7 y) Follow-up: 36 m Period: 2007–2010 Region: Taiwan | Medical history, physical examination | BMIz pre-op vs. post-op | BMIz increased not significantly from −0.7 ± 1.0 to −0.33 ± 0.98 at 24 m (p > 0.05) | No control group Selection bias (no Obese by choice) Moderate |
| Koycu A et al. [18] | Effect of TA on muscle and fat composition | Prospective | 30 prepubertal children with TA or A only vs. 28 controls Pre-op BMIz 0.09 ± 1.09 Age: 3–9 y (5.0 ± 1.2) Follow-up: 6 m Period: 2013–2014 Region: Turkey | Questionnaire at 0 and 6 m post-op Growth and body composition data at 0 and 6 m with bioelectrical impedance analysis (BIA) | Dietary habits and physical activity HT and WT z-scores, body fat and muscle mass, BMI z-scores, basal metabolic scores | At 6 m, in both groups: increased body muscle mass vs. baseline (p < 0.05) Relative BMIz (relBMI), pre-post intervention, increased significantly (p = 0.018) only in the patient group No change (pre–post) in body fat mass, body fat %, HT z-scores, WT z-scores, and BMIz Children with TA (n = 14): BMIz improved significantly (p < 0.05), whereas children who underwent A alone (n = 16) had no significant change in BMI after surgery The number of OW and Ob children did not change significantly in either group (p < 0.05) BMIz were similar at the end of 6 m in the three groups | Small sample size Low |
| Inja RR et al. [19] | Effect of T on swallowing | Prospective | 29 with TA and 2 T only Pre-op WT: 25.4 ± 12.4 Kg Age: 4–14 y (8.3 ± 2.9) Follow-up: 3 m Period: not reported Region: South India | WT at 0, 1 and 3 m post-op | WT | The increase in WT post-op (+3 m) was 26.9 ± 12.7 (vs. pre-op visit, p-value < 0.001) | Small sample size Short f/up No control group HT and BMI and z-scores not evaluated. Low |
| Elnashar I et al. [20] | Effect of T on the serum IGF-1 in patients undergoing T | Prospective | 50 children with T (24 because of obstructive symptoms and 25 because of RT) Pre-Op BMI: n.a. Age: 4.5–10 y Follow-up: 3 m Period: 2013–2014 Region: Egypt | IGF-1 pre-op and 3 m post-op | IGF-1 levels | In the obstructive group, there was an increase in IGF-1 (p < 0.0001) but not in the infection group (p = 0.0883) The pre-op IGF-1 levels in the obstructive group was less than that in the infective group (p = 0.026), whereas the post-op IGF-1 level was higher in the obstructive group than in the infective group (0.01) | Short f/up No control group HT, WT, BMI not evaluated. Low |
| Costa Pinto R et al. [21] | Effect of TA on WT and HT | Retrospective | 29 children with TA Pre-op WT 29.1 ± 17.4 kg, HT 122 ± 27.1 cm Age: 3–13 y Follow-up: 4 months Period: not reported Region: Brazil | WT and HT pre-op and 4 m post-op | WT and HT | WT: from 29.1 ± 17.4 kg to 32.8 ± 18.7 kg (p < 0.0001). HT: from 122 ± 27.1 cm to 125 ± 25.4 (p < 0.0001) Pre-scholar, Scholar, Adolescent age, WT and HT increases are not significant. | Small sample size Short f/up Retrospective No z-score Low |
| Beauchamp MT et al. [22] | Effect of T or TA on BMIz | Retrospective | 1751 children with T or TA Age 3–11 y Groups for WT: (383 Ob, 266 Ow, 1041 Nw and 61 Uw) Groups for age: “young” (ages 3–5 y) and “older” (ages 6–11 y) Follow-up: 24 m Period: 2004–2017 Region: USA | Data from medical records | Change in BMIz trajectories pre-op vs. post-op Predictors: age at time of surgery and gender | Age at time of surgery and gender were not significant predictors of BMIz change Children with Ow/Ob decreased in BMIz, whereas children with Uw or Nw experienced an increase in BMIz. | Retrospective Wide range period Moderate |
| Ha EK et al. [23] | Effect of TA on BMIz and HTz | Prospective | 3172 children with TA and 31,663 in the control group Age: 34–69 m Follow-up: 66–71 m Period: 2008–2009 Region: Korea | Physical measurement by a primary clinician | BMI, WT and HT | TA was related to increased WT and HT in the operation group vs. the control group. Shortly after TA, WT and BMIz increased significantly in the operation group (0.41 ± 0.02) vs. control group (0.18 ± 0.01; p < 0.001); a significant increase in the HT z-score was observed more than 1 y after TA. | Moderate |
| Han SC et al. [24] | Effect of TA on change in growth-for-age | Retrospective | 206 prepubertal children with TA; 167 Nw; 19 Uw; and 20 Ob Age: 3–10 y Follow-up: 12 m Period: 2011–2014 Region: Seoul | OSA-18 questionnaire, symptom questionnaire. Data from medical records | Change in HT, WT, BMIz pre-op vs. post-op Factors affecting the change in growth after TA | After TA, HTz, WTz, and BMIz all increased both in 167 Nw patients and 19 Uw patients (p-value < 0.05). In 20 Ob patients, only HTz increased (p value = 0.028). The multiple regression test showed that the sleep disturbance domain of OSA-18 was positively correlated with HT z-score change (p-value = 0.041), and age was negatively correlated with WT z-score change (p value = 0.016). Pre-op BMIz was negatively correlated (p-value = 0.019) and adenoid grade was positively correlated (p-value = 0.023) with BMIz change. | Retrospective Small number of patients in the Uw and Ob group Moderate |
| Kim JY et al. [25] | Effect of TA on behavior in children with sleep-disordered breathing (SDB) | Prospective | 170 children who underwent TA and 150 controls BMI pc: 57.1 ± 30.7 Age 5–11 y Follow-up: 15.4 ± 2.7 m Period: 2013–2014 Region: South Korea | Standardized questionnaires for parental sleep-related breathing disorder (SRBD); clinical assessments | Change in HT, WT, BMI pc | Significant increase in BMI pc (p < 0.001) and percentage of Ow (p < 0.05) after surgery compared to controls | Moderate |
| Voora RS et al. [26] | Impact of T on obesity in Pediatric Patients With SDB | Retrospective | 560 (of the cohort of 1153) had available follow-up BMI data. They underwent T or TA due to SDB (87.8%) or (12.2%) for recurrent tonsillitis (RT). Age: 2.0–19.5 y (7.6 ± 4.0 y) Follow-up: 6 m (50–605 d) Period: 2016–2017 Region: USA | HT, WT, BMI pre-op and post-op | Change in BMI pc pre-op vs. post-op | Higher post-op BMI pc Post-op BMI pc strongly correlated to higher pre-op BMI pc (p < 0.001), as well as younger age (p < 0.001), male sex (p = 0.0005), and SDB as a surgical indication (p = 0.003). | Retrospective Limited % of patients with available follow-up BMI data Wide f/up period Low |
| Hsu WC et al. [27] | Impacts of WT status on surgical outcomes and shifts of WT status after TA in children with OSA | Prospective | 161 children with OSA who underwent TA. Children were divided into four WT status groups (24 Uw, 79 Nw, 22 Ow and 36 Ob), based on age- and gender-corrected BMI Age: 7.0 ± 3.4 y Follow-up: 6 m Period: 2009–2011 Region: Taiwan | Demographic data, clinical symptoms, and physical examinations | Change in WT pre-op vs. post-op | 54% (13/24) of the Uw children gained WT and shifted to Nw. Nw group had a 9% (7/79) shift to Ow status and 4% (3/79) shift to Uw. The Ow group had a 27% (6/22) shift to Ob and a 14% (3/22) shift to Nw status. Most Ob children (92%, 33/36) remained Ob after TA, with only 8% (3/36) shifting to Ow status | No control group Moderate |
| Koren D et al. [28] | Impact of TA upon Ob and metabolic outcomes in children with OSA | Retrospective | 69 children with moderate-severe baseline OSA who underwent TA Age: 6.33 ± 2.04 y (3.8–12.2) Follow-up: 7.9 ± 3.1 m (range, 2.2–12.2 m) Period: n.a. Region: USA | Baseline and at follow-up, overnight PSG anthropometric and metabolic measurements | BMIz, insulin, glucose, HOMA-IR, McAuley index, total-HDL-LDL cholesterol, triglycerides | BMIz increased significantly (from 1.43 ± 0.78 to 1.52 ± 0.62, p < 0.001) whereas fasting plasma insulin and HOMA-IR were significantly lower and McAuley index trended toward being higher, indicating improved insulin resistance. HDL was significantly higher and total cholesterol/HDL and LDL/HDL ratios were significantly lower, indicating more favorable lipoprotein profiles. | Retrospective Short follow up per some patients Wide age distribution and range of f/up Low |
| Karalok ZS et al. [29] | Impact of T or TA on serum leptin and plasma ghrelin levels in children | Prospective | 31 patients with T or TA 29 age and sex-matched healthy control children Age: 5.70 ± 2.37 y Follow-up: 12 m Period: 2010 Region: Turkey | Auxologic evaluation and biochemical investigations | WT, HT, BMI, IGF-1, IGFBP-3, HOMA-IR, leptin, and ghrelin | 1 y post-op, HTz (p = 0.001) and WTz (p = 0.004) were significantly increased in both groups. No changes in BMIz (p = 0.105) were observed Pre-op leptin levels were significantly higher in patients than controls (p < 0.001). IGF-1, IGFBP-3, HOMA-IR, and ghrelin values were not significantly different between the groups. 1-year post-op, IGF-1 (p = 0.001) and IGFBP-3 (p = 0.001) were significantly increased, while ghrelin (p < 0.001) was significantly decreased; leptin levels were also significantly higher than pre-op values (p = 0.036). | Small sample size Low |
| Moghaddam YJ et al. [30] | Impact of TA on IGF-1 and ghrelin in children | Prospective | 40 pre-pubertal children with AT hypertrophy, SDB, snoring, open mouth breathing and growth retardation Age: 6.57 ± 1.28 y Follow-up: 12 m Period: 2010–2011 Region: Iran | Auxologic evaluation and biochemical investigations | WT, HT, BMI percentiles, IGF-1, ghrelin | WT, HT, and BMI percentiles were increased significantly post-op (p < 0.001). Serum IGF-1 and ghrelin levels increased significantly post-op vs. pre-op (p < 0.001). | Small sample size No control group Low |
| S YB et al. [31] | Influence of TA on WT | Prospective | 45 children divided in 2 groups: 28 (5–10 y) 17 (11–15 y) Mean age 9.32 y ± n.a Follow-up: 6 m Period: n.a. Region: India | Growth data | Change in HT, WT pre and post op | No significant change in WT between two groups and between pre-op and post-op phasis | Small sample size No control group Low |
| Czechowicz et al. [32] | Changes in WT, HT, BMIz, before and after TA | Retrospective | 815 children with TA Pre-op WT pc 1st–60th: 342 pt 61st–80th 142 pt 81st–99th 259 pt <1st 11 pt, >99th 61 pt Pre-op BMI z-score: n.a. Age: <18 y <4 y: 206 4–8 y: 338 >8 y: 199 Follow-up: 18 m Period: 2007–2012 Region: Stanford, California | Pre- and post-op WT and HT | HT, WT, BMIz changes: pre-op vs. post-op | Mean WT pc increased by 6.3 pc points (p < 0.001). No significant changes in HT pc. Weight percentile plateaued 1 year after surgery, with 90% of gain occurring by 41⁄2 to 6 months. Mean BMI pc increased from 64 to 72 (p < 0.001) Lower BMI at the time of surgery (1st–80th pc) was a significant predictor of post-op WT gain (p < 0.001); no significant increase in BMI pc in those with higher BMI (81st–99th). African-American children had a higher WT pc increase (p < 0.001). Tendency to greatest increases in WT were in the younger population (<4 y: mean increase of 10.7 points; children > 8 years had a smaller increase: 3.8 points). | Retrospective Moderate |
| Lewis TL et al. [33] | Association between WT gain and TA | Retrospective | 154 children with TA; 182 as the control group Study populations divided into 3 groups: Nw (BMI < 85 °C), Ow (85–95 °C), Ob (>95 °C) Pre-op BMI pc: Nw 45.7, OW 90, Ob 96.7 Age: 2–16 y (mean: Nw 4.9, Ow 6.6, Ob 8.3) Follow-up: 6–24 months Period: 2010–2011 Region: USA | Pre- and post-op WT, HT, BMIz | WT, HT, BMIz Risk factors: Baseline WT, age, gender, ethnicity, indication for surgery | WT gain in the TA group significantly increased at 6, 12, 18, 24 months (p 0.01, <0.001, <0.001, 0.002, respectively) HT changes were significantly different at 24 months (TA + 1.8 cm, p 0.04) Ob: significant WT gain at 12, 18, 24 m (p < 0.001); significant increases in HT at 6–12 m Nw: significant WT gain at 12 months (p 0.02) Ow: no differences BMIz significantly higher in Ob at 24 m Differences not affected by age, gender, ethnicity, indication for surgery | Retrospective Incomplete follow-up data Nw in TA group significantly younger than controls Moderate |
| Katz ES et al. [34] | WT gain post-TA | RCT (CHAT) | 396: 204 children with OSA underwent TA; 192 watchful waiting control group Pre-op BMI pc: Nw: 48.3%, Ow: 14.9% Ob: 32.7% Uw: 4.5% Age: 5–9.9 y (6.57 ± 1.43 in the TA group) Follow-up: 7 m Period: 2008–2011 Region: USA | Pre- and post-op WT, HT, BMIz | WT, HT, BMIz Risk factors: Baseline WT, age, gender, ethnicity, indication for surgery | WTz and BMIz increases (0.13 vs. 0.31) in both groups, greater with TA (p < 0.0001) A greater proportion of Ow randomized to TA developed Ob compared with controls (52% vs. 21%; p = 0.05) Race and gender were not significantly associated with BMIz change | Statistical model did not consider variability of baseline WT status (Kirkham et al.) Low |
| Jensen AM et al. [35] | Determine if TA increases the risk of Ob | Re-analysis of RCT (CHAT) | 396: 204 children with OSA underwent TA; 192 watchful waiting control group Pre-op BMI pc: Nw: 48.3%, Ow: 14.9% Ob: 32.7% Uw: 4.5% Age: 5–9.9 y (6.57 ± 1.43 in the TA group) Follow-up: 7 m Period: 2008–2011 Region: USA | Pre- and post-op %BMI pc 95 (as a percentage of the 95th percentile) | %BMI pc 95 Baseline WT, Age, sex, race, Sleep apnea | Utilizing a linear mixed-effects model accounting for the variability of baseline weight status, OSA resolution status, and time, no significant association between AT and %BMIp95 was found WT significantly increased in the TA group, especially in Uw at baseline (p = 0.01). No gender difference. Resolution of OSA was associated with a decreased weight trajectory (p < 0.001) | High |
| Kirkham et al. [36] | Effect of TA on BMIz Factors influencing post-op growth | Reanalysis of RCT (CHAT) CHAT: children 5–9.9 y with OSA (proven by PSG) | 396: 204 children with OSA underwent TA 192 watchful waiting control group Pre-op BMI pc: Nw: 48.3%, Ow: 14.9% Ob: 32.7% Uw: 4.5% Age: 5–9.9 y (6.57 ± 1.43 y in the TA group) Follow-up: 7 m Period: 2008–2011 Region: USA | Pre- and post-op BMIz | WT, HT, BMIz Baseline WT, Age, Sleep apnea, maternal BMI | A similar percentage of children in both arms experienced undesirable WT gain (45% TA vs. 41% watchful waiting). Regression models excluded children who were Uw at baseline; there was no longer a significant effect of TA on BMIz (different from Katz 2014). | High |
| Al Abdulla A et al. [37] | WT gain post-TA | Retrospective | 240 children Age: 1–15 (7.45 ± 2.89 y) Pre-operative BMI 22.35 ± 3.98 kg/m2 Follow-up: 6 m Period: 2018–2019 Region: Saudi Arabia | Pre- and post-op BMI | WT, HT, BMI, and classification in Uw, Nw, Ow, Ob according to percentile cut-off | Significant differences in mean WT and BMI at 1–6 months post-op (p < 0.0001) Positive linear correlation between WT and BMI at 6 m | Retrospective No z-scores calculation Low |
| AlAbdullah ZA et al. [38] | Impact of TA on growth | Prospective | 53 children underwent T. 52 controls Age: 2–14 y Pre-operative BMIz −0.30 ± 2.06 Follow-up: 12 m Period: 2012–2015 Region: Saudi Arabia | Pre- and post-op BMIz | WT, HT, BMI, BMIz | BMIz in the study group increased compared to controls, but this gain was not statistically significant (p = 0.053). | Moderate |
| Kevat A et al. [39] | Impact of TA on growth trajectories in children with OSA | RCT | 126 children with OSA randomly assigned to early TA (within 2 m) routine (12 m) Age: 49.46 ± 8.44 m 48.97 ± 8.23 m pre-operative BMIz 0.22 (−0.43–0.97) BMIz 0.56 (−0.24–1.21) Follow-up: 2 y Period: 2018–2019 Region: Australia | BMIz pre-op and at 12–24 m | WTz, HTz, BMIz | Significant increase in BMIz in the 12 months after TA. Early surgery group (0–12 m): median 0.4, IC95 0.1–0.8. Routine surgery group (12–24 m) median 0.45, IC 95 0.1–0.8, but not from 0–12 months (preoperative time). Findings for WTz were like the findings for BMIz. HTz: no significant change between different time points or intervention groups. | High |
| Nachalon Y et al. [13] | Impact of TA on growth in children with OSA | Prospective | 16 children with OSA Age: 23 ± 6 m Follow-up: 5 ± 2 m Period: 2013 Region: Israel | Pre- and post-op WT, HT, BMIz Caloric intake questionnaire | HT, WT, C-reactive protein, IGF-1 | Increase in HT, WT (p < 0.001 for both) WTz, BMI and BMIz (p = 0.002). Increased caloric intake after TA (p < 0.001) with increased protein and decreased fat intake. The decrease in C-reactive protein levels correlated with the increase in WT in boys (p < 0.05, adjusted for caloric intake). | Small sample size No control group Low |
| Au CT et al. [40] | Impact of T or TA on growth | RCT | 71 pre-pubertal children with OSA 35 with early surgical intervention (18TA, 17T) 36 watchful waiting Age: 6–11 y (8.4 ± 1.6) Follow-up: 10.5 ± 2.2 m Period: 2010–2018 Region: Hong Kong | Pre- and post-op WT, BMIz, waist circumference z-score | WT, BMIz, waist circumference z-score | The intervention group had a higher baseline waist circumference z-score (p = 0.063). The intervention group had a significantly greater increase in WT +3.3 ± 2.1 vs. +2.2 ± 1.5 kg, p = 0.014. The BMI z-score remained similar at baseline and follow-up for both groups. The waist circumference z-score increased significantly in the control group (p < 0.05). | Wide f/up range High |
| Reference | Initial WT Status | Number/Design | Intervention (f/up) | WT | HT | BMIz | Risk Factors for WT/BMI Change | Level of Evidence (GRADE) |
|---|---|---|---|---|---|---|---|---|
| Smith DF et al. [16] | Nw-Ow | 115/R | TA (6 m) | ↑ | <age (<6 y) No OSA indication | M | ||
| Huang YS et al. [17] | Nw | 88/P | TA (36 m) | = | M | |||
| Beauchamp MT et al. [22] | Uw, Nw, Ow, Ob | 1751/R | TA or T (24 m) | ↑ in Uw/Nw ↓ in Ob-Ow | Uw No age, no surgery indication | M | ||
| Ha EK et al. [23] | Uw, Nw, Ow, Ob | 3172/P | TA (66–71) | ↑ | ↑ HTz | ↑ | M | |
| Han SC et al. [24] | Nw, Ow, Ob | 206/R | TA (12 m) | ↑ WTz | ↑ HTz | ↑ | <age <BMI pre >adenoid grade | M |
| Kim JY et al. [25] | Nw, Ow, Ob | 170/P | TA (15 m) | ↑ (and >Ow) | TA | M | ||
| Hsu WC et al. [27] | Uw, Nw, Ow, Ob | 161/P | TA (6 m) | ↑ | Uw | M | ||
| Czechowicz et al. [32] | Uw, Nw, Ow, Ob | 815/R | TA (18 m) | ↑ WTz | =HTz | ↑ | <age (<4 y) <BMI pre African-American | M |
| Lewis TL et al. [33] | Nw, Ow, Ob | 154/R | TA (24 m) | ↑ | ↑ | ↑ | No age Ob and Nw pre No surgery indication | M |
| Jensen AM et al. [35] | Uw, Nw, Ow, Ob | 396/RCT | TA (7 m) | ↑ | = | Uw at baseline | H | |
| Kirkham et al. [36] | Uw, Nw, Ow, Ob | 396/RCT | TA (7 m) | = | = | Uw at baseline | H | |
| AlAbdullah ZA et al. [38] | Nw | 53/P | T (12 m) | = | M | |||
| Kevat A et al. [39] | Uw, Nw, Ow, Ob | 126/RCT | TA (24 m) | ↑ | =HTz | ↑ | H | |
| Au CT et al. [40] | Nw | 71/RCT | T-TA (10 m) | ↑ | = | H |
| Reference | Intervention (f/up) | WT | HT | BMIz | relBMI% | Body Composition | Hormonal Change | Risk Factors for WT/BMI Change | Level of Evidence (GRADE) |
|---|---|---|---|---|---|---|---|---|---|
| Koycu A et al. [18] | TA or A (6 m) Nw | = | = | ↑ relBMI% ↑ BMIz in TA | ↑ body muscle mass | TA vs. T | L | ||
| Inja RR et al. [19] | TA or A (3 m) | ↑ | L | ||||||
| Elnashar I et al. [20] | T (3 m) | ↑ IGF-1 in obstructive group, not in infective | L | ||||||
| Costa Pinto R et al. [21] | TA (4 m) | ↑ | ↑ | L | |||||
| Voora RS et al. [26] | T or TA (6 m) | ↑ | ↑ BMI pre-op Younger Male Obstruction as indication | ||||||
| Koren D et al. [28] | TA (8 m)-OSA | ↑ | L | ||||||
| Karalok ZS et al. [29] | T or TA (12 m) | ↑ WTz | ↑ WTz | = | Pre-OP. ↑ leptin = GHrelin, IGF.1 +1 y post-op: ↑ IGF-1, IGFBP3, leptin and ↓ GHrelin | L | |||
| Moghaddam YJ et al. [30] | TA (12 m)-OSA | ↑ | ↑ | ↑ | Post-op: ↑ IGF-1, leptin, and Ghrelin | L | |||
| S YB et al. [31] | TA (6 m) | = | L | ||||||
| Katz ES et al. [34] | TA (7 m)-OSA Uw, Nw, Ow, Ob | ↑ WTz | = | ↑ | No race and gender | L | |||
| Nachalon Y et al. [13] | TA (5 m)-OSA | ↑/WTz | ↑ | ↑ | Increased caloric intake | L | |||
| Al Abdulla A et al. [37] | TA (6 m) | ↑ | L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buono, P.; Maines, E.; Azzolini, N.; Franceschi, R.; Ludovica, F.; Leonardi, L.; Occhiati, L.; Mozzillo, E.; Maffeis, C.; Marigliano, M. Short-Term Weight Gain after Tonsillectomy Does Not Lead to Overweight: A Systematic Review. Nutrients 2024, 16, 324. https://doi.org/10.3390/nu16020324
Buono P, Maines E, Azzolini N, Franceschi R, Ludovica F, Leonardi L, Occhiati L, Mozzillo E, Maffeis C, Marigliano M. Short-Term Weight Gain after Tonsillectomy Does Not Lead to Overweight: A Systematic Review. Nutrients. 2024; 16(2):324. https://doi.org/10.3390/nu16020324
Chicago/Turabian StyleBuono, Pietro, Evelina Maines, Nicolò Azzolini, Roberto Franceschi, Fedi Ludovica, Letizia Leonardi, Luisa Occhiati, Enza Mozzillo, Claudio Maffeis, and Marco Marigliano. 2024. "Short-Term Weight Gain after Tonsillectomy Does Not Lead to Overweight: A Systematic Review" Nutrients 16, no. 2: 324. https://doi.org/10.3390/nu16020324
APA StyleBuono, P., Maines, E., Azzolini, N., Franceschi, R., Ludovica, F., Leonardi, L., Occhiati, L., Mozzillo, E., Maffeis, C., & Marigliano, M. (2024). Short-Term Weight Gain after Tonsillectomy Does Not Lead to Overweight: A Systematic Review. Nutrients, 16(2), 324. https://doi.org/10.3390/nu16020324