Study of the Association between Thiols and Oxidative Stress Markers in Children with Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Type
- -
- Written informed consent of the subjects legally authorized to give consent or the parent(s)/legal representative of the minors according to national law;
- -
- Consent in school-age subjects, i.e., aged ≥ 6 years;
- -
- Male and/or female subjects aged between 2 and 18 years;
- -
- Caucasian race;
- -
- Overweight (i.e., a BMI ≥ 85th (1036 standard deviation score, SDS) and < 95th percentile (1645 SDS)) according to the Italian growth curves;
- -
- Non-syndromic obesity (i.e., a BMI ≥ 95th percentile; BMI z-score for age and sex < 1064 SDS);
- -
- Absence of systemic/endocrine pathology (except overweight/obesity).
- -
- No written informed consent of the patient or parents or no consent of the minor if applicable.
- -
- Genetic obesity and other genetically determined syndromes;
- -
- Any condition associated with an increase in inflammatory parameters and, specifically, with values of erythrocyte sedimentation rate (ESR) > 15 mm or with C-reactive protein (CRP) > 0.50 mg/dL, or with a number of white blood cells > 16.00 × 103/µL.
- -
- Presence of cognitive deficits;
- -
- Presence of previous thromboembolic or hemorrhagic events;
- -
- Presence of anticoagulant therapy;
- -
- Presence of chronic corticosteroid therapy;
- -
- Positive lupus anticoagulant (LA) test;
- -
- Bariatric therapy;
- -
- Presence of allergies (total immunoglobulin E (IgE) ≥ 100.0 kU/L).
- -
- Written informed consent of the subjects legally authorized to give consent or the parent (s)/legal representative of the minors according to national law;
- -
- Consent in school-age subjects, i.e., aged ≥ 6 years;
- -
- Male and/or female subjects aged between 2 and 18 years;
- -
- Caucasian race;
- -
- Normal weight (BMI z-score for age and sex < 1.064 SDS), that is a body mass index between the 10th and 84th percentiles;
- -
- No written informed consent of the patient or parents or no consent of the minor if applicable;
- -
- Presence of inflammatory processes in the lower or upper respiratory tract, erythrocyte sedimentation rate values (ESR) ≥ 15 mm, C-Reactive Protein (CRP) ≥ 0.50 mg/dL, number of white blood cells ≥16.00 × 103/µL;
- -
- Suspected celiac disease (anti-transglutaminase antibodies ≥ 20 CU);
- -
- Presence of chronic corticosteroid therapy;
- -
- Presence of cognitive deficits;
- -
- Presence of previous thromboembolic or hemorrhagic events;
- -
- Presence of anticoagulant therapy;
- -
- Positive lupus anticoagulant (LA) test;
- -
- Presence of allergies (total IgE ≥ 100.0 kU/L).
2.2. Samples
2.3. Thiol Determination
2.4. Total Antioxidant Levels and Oxidative Stress Markers
2.5. Other Laboratory Assays
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Patients Studied
3.2. Thiol Status
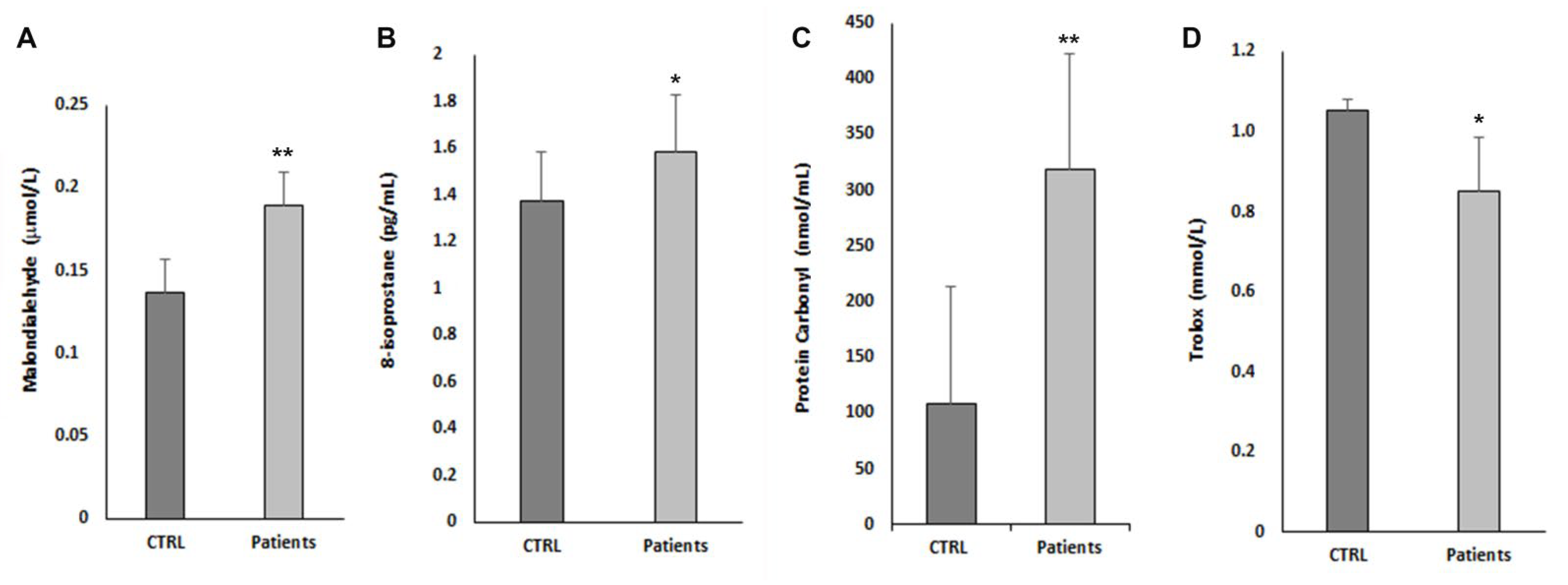
3.3. Oxidative Stress Parameter and Antioxidant Status
3.4. Correlations between Thiols, Oxidative Stress, and the Other Parameters Studied
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Noncommunicable Diseases: Childhood Overweight and Obesity. Available online: http://www.who.int/dietphysicalactivity/childhood/en/ (accessed on 16 June 2022).
- Lobstein, T.; Jackson-Leach, R.; Moodie, M.L.; Hall, K.D.; Gortmaker, S.L.; Swinburn, B.A.; James, W.P.T.; Wang, Y.; McPherson, K. Child and adolescent obesity: Part of a bigger picture. Lancet 2015, 385, 2510–2520. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. Available online: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 16 July 2022).
- Karacabeyli, D.; Allender, S.; Pinkney, S.; Amed, S. Evaluation of complex community-based childhood obesity prevention interventions. Obes. Rev. 2018, 19, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.J.; Fierz, Y.; Ferguson, R.D.; LeRoith, D. The Pathway from Diabetes and Obesity to Cancer, on the Route to Targeted Therapy. Endocr. Pract. 2010, 16, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Woloshynowych, M.; Britto, J.C.; Bilkevic, I.; Glassar, B.; Chapman, S.; Ford-Adams, M.E.; Desai, A.; Bain, M.; Tewfik, I.; et al. Obesity, oxidative DNA damage and vitamin D as predictors of genomic instability in children and adolescents. Int. J. Obes. 2021, 45, 2095–2107. [Google Scholar] [CrossRef] [PubMed]
- Al-Aubaidy, A.H.; Jelinek, H.F. Oxidative DNA damage and obesity in type 2 diabetes mellitus. Eur. J. Endocrinol. 2011, 164, 899–904. [Google Scholar] [CrossRef]
- Keane, K.N.; Cruzat, V.F.; Carlessi, R.; de Bittencourt, P.I.H.; Newsholme, P. Molecular Events Linking Oxidative Stress and Inflammation to Insulin Resistance and β-Cell Dysfunction. Oxidative Med. Cell. Longev. 2015, 2015, 181643. [Google Scholar] [CrossRef]
- Elmas, B. Dynamic thiol/disulphide homeostasis as a novel indicator of oxidative stress in obese children and its relationship with inflammatory-cardiovascular markers. Anatol. J. Cardiol. 2017, 18, 361–369. [Google Scholar] [CrossRef]
- Ates, E.; Set, T.; Karahan, S.C.; Biçer, C.; Erel, Ö. Thiol/Disulphide homeostasis, ischemia modified albumin, and ferroxidase as oxidative stress markers in women with obesity with insulin resistance. J. Med. Biochem. 2019, 38, 445–451. [Google Scholar] [CrossRef]
- Sreckovic, B.; Sreckovic, V.D.; Soldatovic, I.; Colak, E.; Sumarac-Dumanovic, M.; Janeski, H.; Janeski, N.; Gacic, J.; Mrdovic, I. Homocysteine is a marker for metabolic syndrome and atherosclerosis. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, 179–182. [Google Scholar] [CrossRef]
- Lind, M.; Lauritzen, L.; Vestergaard, H.; Hansen, T.; Pedersen, O.; Kristensen, M.; Ross, A. One-carbon metabolism markers are associated with cardiometabolic risk factors. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Pastore, A.; Ciampalini, P.; Tozzi, G.; Pecorelli, L.; Passarelli, C.; Bertini, E.; Piemonte, F. All glutathione forms are depleted in blood of obese and type 1 diabetic children. Pediatr. Diabetes 2012, 13, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Pastore, A.; Panera, N.; Mosca, A.; Caccamo, R.; Camanni, D.; Crudele, A.; De Stefanis, C.; Alterio, A.; Di Giovamberardino, G.; De Vito, R.; et al. Changes in Total Homocysteine and Glutathione Levels After Laparoscopic Sleeve Gastrectomy in Children with Metabolic-Associated Fatty Liver Disease. Obes. Surg. 2022, 32, 82–89. [Google Scholar] [CrossRef]
- Pastore, A.; Massoud, R.; Motti, C.; Russo, A.L.; Fucci, G.; Cortese, C.; Federici, G. Fully automated assay for total homocysteine, cysteine, cysteinylglycine, glutathione, cysteamine, and 2-mercaptopropionylglycine in plasma and urine. Clin. Chem. 1998, 44, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Klop, B.; Elte, J.W.F.; Cabezas, M.C. Dyslipidemia in Obesity: Mechanisms and Potential Targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef]
- Shashaj, B.; Luciano, R.; Contoli, B.; Morino, G.S.; Spreghini, M.R.; Rustico, C.; Sforza, R.W.; Dallapiccola, B.; Manco, M. Reference ranges of HOMA-IR in normal-weight and obese young Caucasians. Acta Diabetol. 2016, 53, 251–260. [Google Scholar] [CrossRef]
- Tobisch, B.; Blatniczky, L.; Barkai, L. Cardiometabolic risk factors and insulin resistance in children with obesity and adolescents: Relation to puberty. Pediatric Obes. 2015, 10, 37–44. [Google Scholar] [CrossRef]
- Blachier, F.; Andriamihaja, M.; Blais, A. Sulfur-Containing Amino Acids and Lipid Metabolism. J. Nutr. 2020, 150, 2524S–2531S. [Google Scholar] [CrossRef]
- Lonn, E.; Yusuf, S.; Arnold, M.J.; Sheridan, P.; Pogue, J.; Micks, M.; McQueen, M.J.; Probstfield, J.; Fodor, G.; Held, C.; et al. Homocysteine Lowering with Folic Acid and B Vitamins in Vascular Disease. New Engl. J. Med. 2006, 354, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Perła-Kaján, J.; Jakubowski, H. Dysregulation of Epigenetic Mechanisms of Gene Expression in the Pathologies of Hyperhomocysteinemia. Int. J. Mol. Sci. 2019, 20, 3140. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.C.; Stanisic, D.; Singh, M. Epigenetic memory: Gene writer, eraser and homocysteine. Mol. Cell. Biochem. 2021, 476, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Bajic, V.P.; Van Neste, C.; Obradovic, M.; Zafirovic, S.; Radak, D.; Bajic, V.B.; Essack, M.; Isenovic, E.R. Glutathione “Redox Homeostasis” and Its Relation to Cardiovascular Disease. Oxidative Med. Cell. Longev. 2019, 2019, 50281812019. [Google Scholar] [CrossRef]
- Pastore, A.; Federici, G.; Bertini, E.; Piemonte, F. Analysis of glutathione: Implication in redox and detoxification. Clin. Chim. Acta 2003, 333, 19–39. [Google Scholar] [CrossRef]
- Nguyen, D.; Samson, S.L.; Reddy, V.T.; Gonzalez, E.V.; Sekhar, R.V. Impaired mitochondrial fatty acid oxidation and insulin resistance in aging: Novel protective role of glutathione. Aging Cell 2013, 12, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, Y.; Miller, M.L.; Shen, D.; Shertzer, H.G.; Stringer, K.F.; Wang, B.; Schneider, S.N.; Nebert, D.W.; Dalton, T.P. Hepatocyte-specific Gclc deletion leads to rapid onset of steatosis with mitochondrial injury and liver failure. Hepatology 2007, 45, 1118–1128. [Google Scholar] [CrossRef]
- Alves, A.; Bassot, A.; Bulteau, A.-L.; Pirola, L.; Morio, B. Glycine Metabolism and Its Alterations in Obesity and Metabolic Diseases. Nutrients 2019, 11, 1356. [Google Scholar] [CrossRef]
- Okekunle, A.P.; Li, Y.; Liu, L.; Du, S.; Wu, X.; Chen, Y.; Li, Y.; Qi, J.; Sun, C.; Feng, R. Abnormal circulating amino acid profiles in multiple metabolic disorders. Diabetes Res. Clin. Pract. 2017, 132, 45–58. [Google Scholar] [CrossRef]
- McCarty, M.F.; O’Keefe, J.H.; Di Nicolantonio, J.J. Dietary Glycine Is Rate-Limiting for Glutathione Synthesis and May Have Broad Potential for Health Protection. Ochsner J. 2018, 18, 81–87. [Google Scholar]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Constantin, A.; Constantinescu, E.; Dumitrescu, M.; Calin, A.; Popov, D. Effects of ageing on carbonyl stress and antioxidant defense in RBCs of obese Type 2 diabetic patients. J. Cell. Mol. Med. 2005, 9, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Krzystek-Korpacka, M.; Patryn, E.; Boehm, D.; Berdowska, I.; Zielinski, B.; Noczynska, A. Advanced oxidation protein products (AOPPs) in juvenile overweight and obesity prior to and following weight reduction. Clin. Biochem. 2008, 41, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, R.V.; McKay, S.V.; Patel, S.G.; Guthikonda, A.P.; Reddy, V.T.; Balasubramanyam, A.; Jahoor, F. Glutathione Synthesis Is Diminished in Patients with Uncontrolled Diabetes and Restored by Dietary Supplementation with Cysteine and Glycine. Diabetes Care 2011, 34, 162–167. [Google Scholar] [CrossRef]
- Sekhar, R.V.; Patel, S.G.; Guthikonda, A.P.; Reid, M.; Balasubramanyam, A.; Taffet, E.G.; Jahoor, F. Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation. Am. J. Clin. Nutr. 2011, 94, 847–853. [Google Scholar] [CrossRef]
- Jobgen, W.; Fu, W.J.; Gao, H.; Li, P.; Meininger, C.J.; Smith, S.B.; Spencer, T.E.; Wu, G. High fat feeding and dietary l-arginine supplementation differentially regulate gene expression in rat white adipose tissue. Amino Acids 2009, 37, 187–198. [Google Scholar] [CrossRef]
- Codoñer-Franch, P.; López-Jaén, A.B.; De La Mano-Hernández, A.; Sentandreu, E.; Simó-Jordá, R.; Valls-Bellés, V. Oxidative markers in children with severe obesity following low-calorie diets supplemented with mandarin juice. Acta Paediatr. 2010, 99, 1841–1846. [Google Scholar] [CrossRef]
- Zhang, C.; Ren, W.; Li, M.; Wang, W.; Sun, C.; Liu, L.; Fang, Y.; Yang, X.; Zhang, X.; Li, S. Association Between the Children’s Dietary Inflammatory Index (C-DII) and Markers of Inflammation and Oxidative Stress Among Children and Adolescents: NHANES 2015–2018. Front. Nutr. 2022, 9, 894966. [Google Scholar] [CrossRef]


| Variable | All Patients | Male | Female | p |
|---|---|---|---|---|
| n | 283 | 162 | 121 | |
| Age, y, mean (standard deviation) | 12.11 (5.69) | 12.04 (2.89) | 12.85 (3.47) | 0.037 |
| Height, cm, mean (standard deviation) | 154.28 (14.62) | 77.37 (25.44) | 74.97 (23.67) | 0.16 |
| Weight, Kg, mean (standard deviation) | 76.30 (24.66) | 155.30 (15.28) | 153.02 (13.72) | 0.39 |
| BMI, mean (standard deviation) | 32.41 (7.19) | 31.01 (6.20) | 31.41 (3.47) | 0.59 |
| CRP, mg/dL, median (25th and 75th percentile) | 0.20 (0.07–0.42) | 0.21 (0.07–0.41) | 0.19 (0.07–0.42) | 0.46 |
| HOMA-IR, median (25th and 75th percentile) | 4.00 (2.22–5.97) | 4.95 (2.27–5.72) | 4.70 (2.80–5.70) | 0.90 |
| Triglycerides, mg/dL, mean (standard deviation) | 89.83 (42.92) | 96.30 (56.65) | 88.19 (36.30) | 0.14 |
| Cholesterol, mg/dL, mean (standard deviation) | 155.80 (31.91) | 152.10 (24.73) | 155.10 (28.11) | 0.35 |
| HDL, mg/dL, mean (standard deviation) | 46.00 (40.00–53.00) | 47.50 (10.54) | 48.36 (10.98) | 0.51 |
| LDL, mg/dL, mean (standard deviation) | 92.00 (75.00–108.50) | 92.35 (22.94) | 94.62 (26.78) | 0.45 |
| AST, U/L, median (25th and 75th percentile) | 20.00 (16.00–27.25) | 22.00 (19.00–28.00) | 19.00 (16.00–24.00) | 0.0002 |
| ALT, U/L, median (25th and 75th percentile) | 19.00 (15.00–29.00) | 22.00 (16.00–34.00) | 16.00 (13.00–23.00) | 0.0006 |
| Cys, μmol/L, mean (standard deviation) | 223.30 (41.83) | 221.00 (40.28) | 227.00 (42.44) | 0.23 |
| Cys-Gly, μmol/L, mean (standard deviation) | 125.80 (25.60) | 119.20 (25.15) | 116.50 (25.95) | 0.37 |
| GSH, μmol/L, mean (standard deviation) | 6.06 (2.45) | 6.26 (1.65) | 6.65 (2.08) | 0.09 |
| Hcy, μmol/L, mean (standard deviation) | 10.00 (4.43) | 10.80 (5.12) | 10.95 (4.34) | 0.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Alessandro, A.; Di Felice, G.; Manco, M.; Pastore, A.; Pezzi, S.; Mariani, M.; Fintini, D.; Onetti Muda, A.; Porzio, O. Study of the Association between Thiols and Oxidative Stress Markers in Children with Obesity. Nutrients 2022, 14, 3637. https://doi.org/10.3390/nu14173637
D’Alessandro A, Di Felice G, Manco M, Pastore A, Pezzi S, Mariani M, Fintini D, Onetti Muda A, Porzio O. Study of the Association between Thiols and Oxidative Stress Markers in Children with Obesity. Nutrients. 2022; 14(17):3637. https://doi.org/10.3390/nu14173637
Chicago/Turabian StyleD’Alessandro, Annamaria, Giovina Di Felice, Melania Manco, Anna Pastore, Simona Pezzi, Michela Mariani, Danilo Fintini, Andrea Onetti Muda, and Ottavia Porzio. 2022. "Study of the Association between Thiols and Oxidative Stress Markers in Children with Obesity" Nutrients 14, no. 17: 3637. https://doi.org/10.3390/nu14173637
APA StyleD’Alessandro, A., Di Felice, G., Manco, M., Pastore, A., Pezzi, S., Mariani, M., Fintini, D., Onetti Muda, A., & Porzio, O. (2022). Study of the Association between Thiols and Oxidative Stress Markers in Children with Obesity. Nutrients, 14(17), 3637. https://doi.org/10.3390/nu14173637







