Nutritional Biomarkers for the Prediction of Response to Anti-TNF-α Therapy in Crohn’s Disease: New Tools for New Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
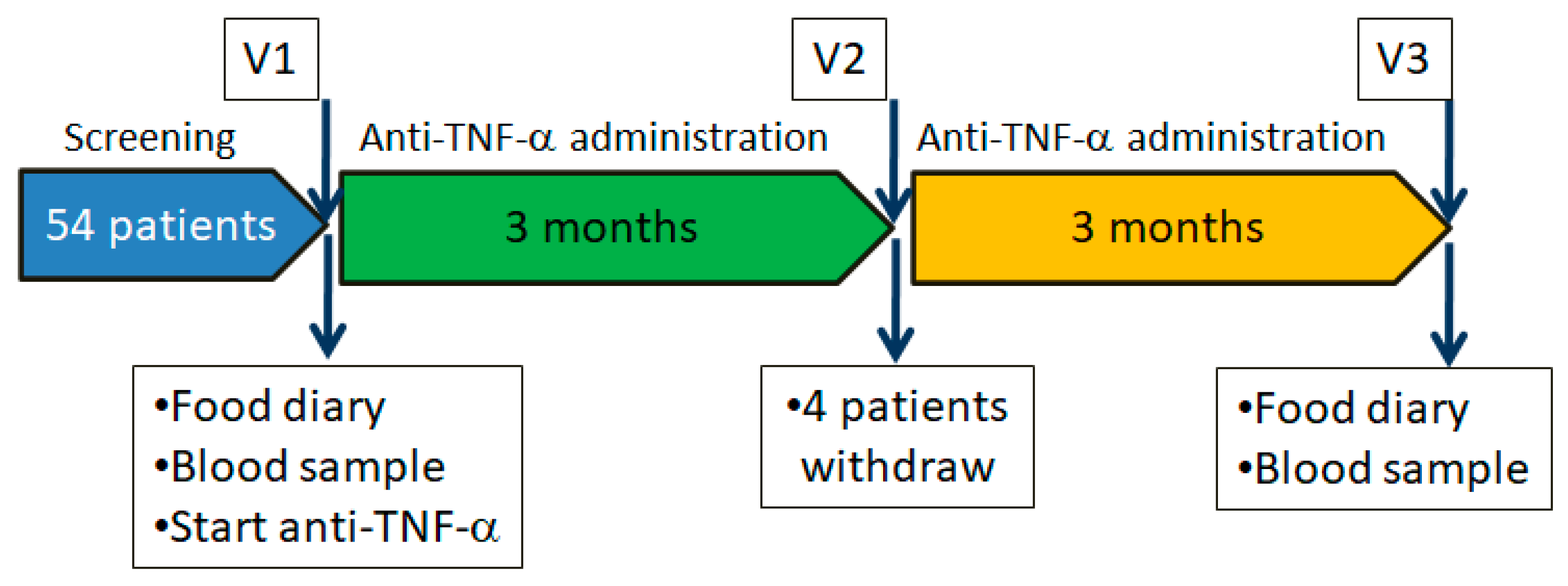
2.2. Study Design
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Study Procedures
2.6. Food Questionnaires and Nutrient Analysis
2.7. Statistical Analysis
3. Results
3.1. Study Population
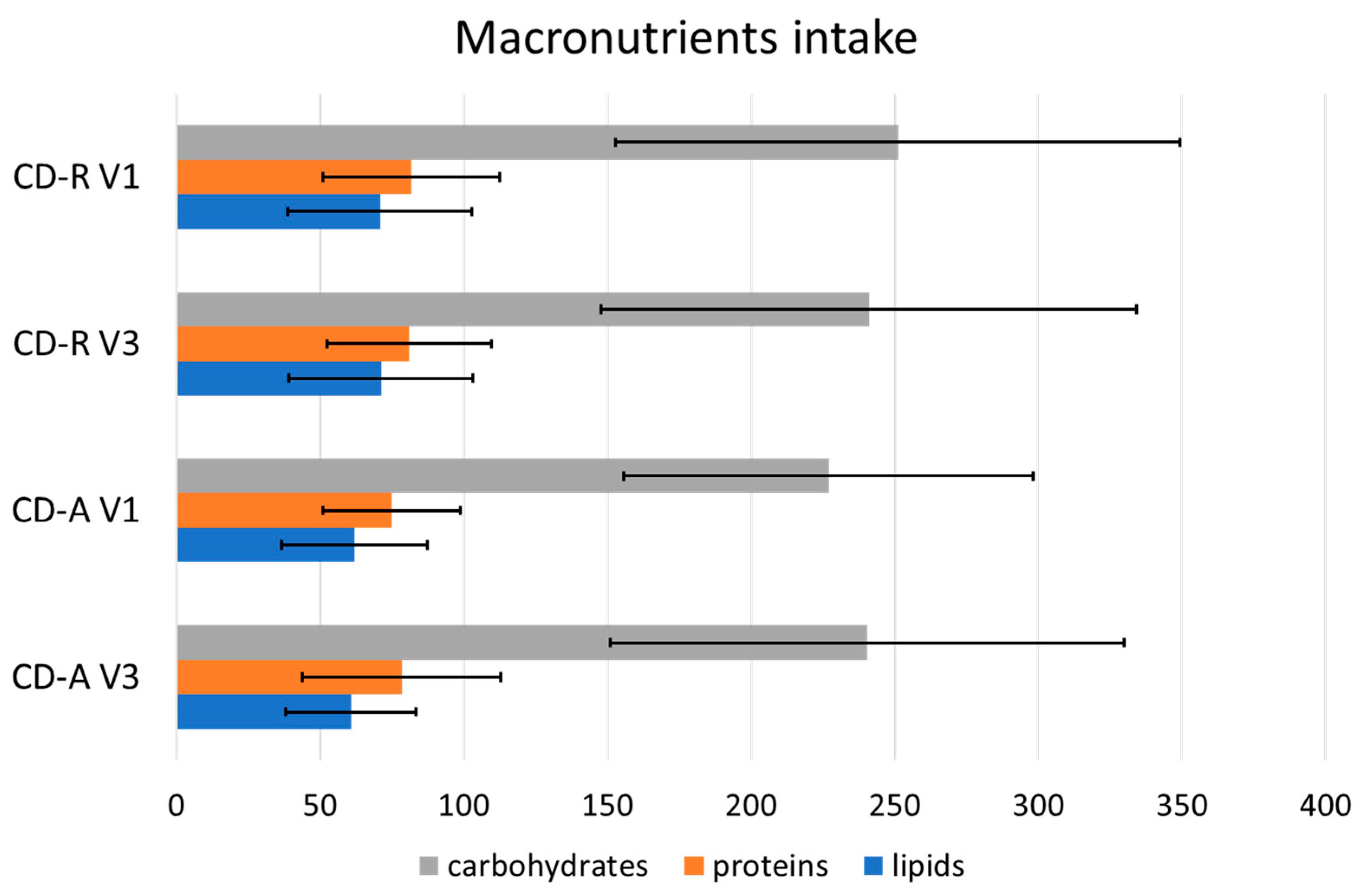
3.2. Macronutrient Intake
3.3. Vitamins and Minerals Intake
3.4. Lipids
3.5. ORAC, PRAL and Polyphenols
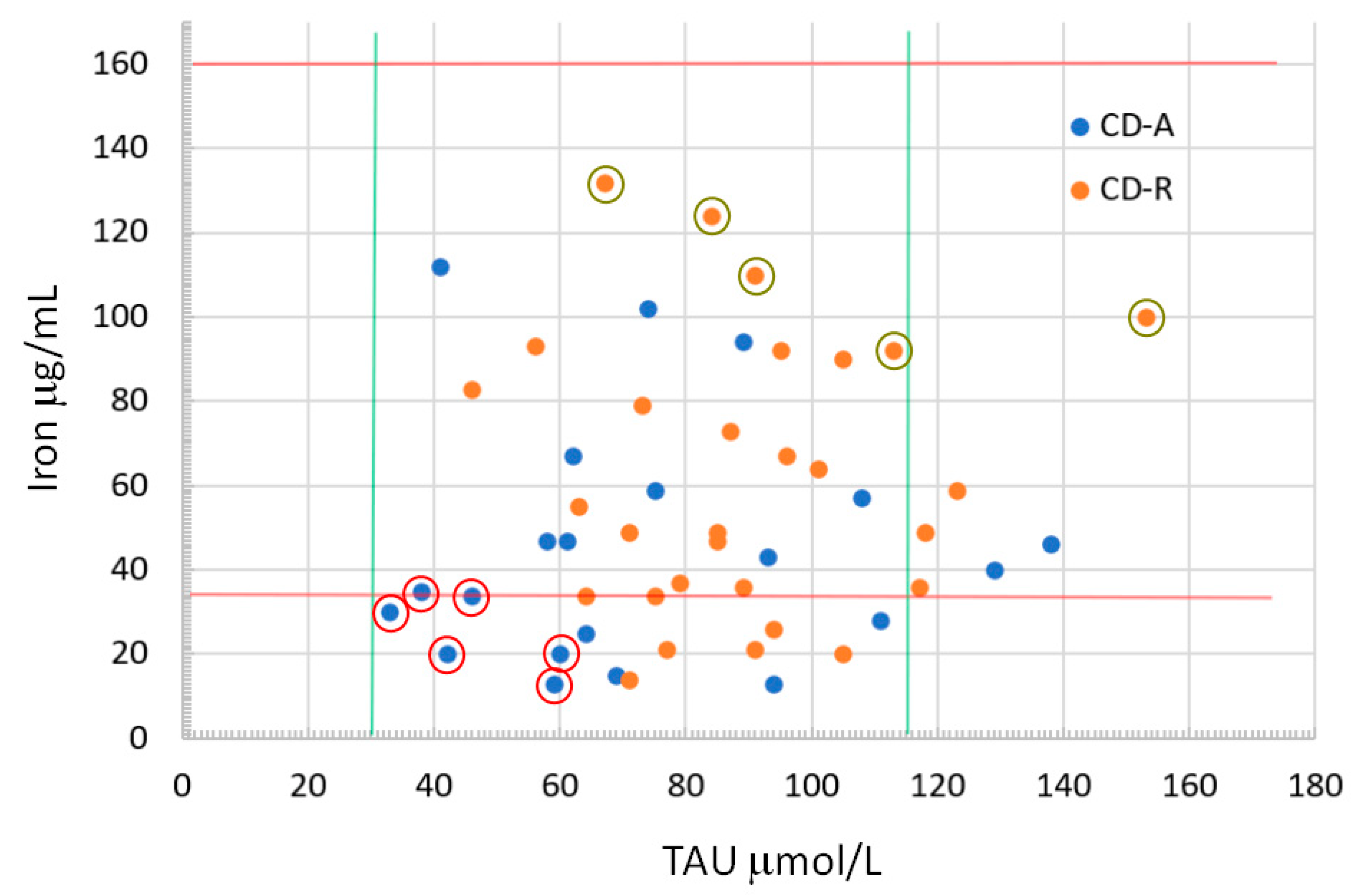
3.6. Blood Nutrient Values
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ananthakrishnan, A.N. Environmental Risk Factors for Inflammatory Bowel Diseases: A Review. Dig. Dis. Sci. 2015, 60, 290–298. [Google Scholar] [CrossRef]
- Malik, T.A. Inflammatory Bowel Disease: Historical Perspective, Epidemiology, and Risk Factors. Surg. Clin. N. Am. 2015, 95, 1105–1122. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Bernstein, C.N. Environmental risk factors for inflammatory bowel disease. United Eur. Gastroenterol. J. 2022, 10, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, F.; Spisni, E.; Giovanardi, E.; Imbesi, V.; Salice, M.; Alvisi, P.; Valerii, M.C.; Gionchetti, P. Implications of the Westernized Diet in the Onset and Progression of IBD. Nutrients 2019, 11, 1033. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, K.; Morhardt, T.L.; Kamada, N. The Role of Dietary Nutrients in Inflammatory Bowel Disease. Front. Immunol. 2019, 9, 3183. [Google Scholar] [CrossRef]
- Saracino, I.M.; Spisni, E.; Imbesi, V.; Ricci, C.; Dussias, N.K.; Alvisi, P.; Gionchetti, P.; Rizzello, F.; Valerii, M.C. The bidirectional link between nutritional factors and Inflammatory Bowel Diseases. Dietary deficits, habits, and recommended interventions. A narrative review. Foods 2023, 12, 1987. [Google Scholar] [CrossRef]
- Rizzello, F.; Gionchetti, P.; Spisni, E.; Saracino, I.M.; Bellocchio, I.; Spigarelli, R.; Collini, N.; Imbesi, V.; Dervieux, T.; Alvisi, P.; et al. Dietary Habits and Nutrient Deficiencies in a Cohort of European Crohn’s Disease Adult Patients. Int. J. Mol. Sci. 2023, 24, 1494. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Fan, Q.; Li, Z.; Goll, R.; Florholmen, J. Evaluation of anti-TNF therapeutic response in patients with inflammatory bowel disease: Current and novel biomarkers. EBioMedicine 2021, 66, 103329. [Google Scholar] [CrossRef]
- Kamata, N.; Oshitani, N.; Watanabe, K.; Watanabe, K.; Hosomi, S.; Noguchi, A.; Yukawa, T.; Yamagami, H.; Shiba, M.; Tanigawa, T.; et al. Efficacy of concomitant elemental diet therapy in scheduled infliximab therapy in patients with Crohn’s disease to prevent loss of response. Dig. Dis. Sci. 2015, 60, 1382–1388. [Google Scholar] [CrossRef]
- Watanabe, T.; Tominaga, K.; Kabata, D.; Shintani, A.; Arakawa, T.; Fujiwara, Y. Efficacy of a concomitant elemental diet to reduce the loss of response to adalimumab in patients with intractable Crohn’s disease. J. Gastroenterol. Hepatol. 2018, 33, 631–637. [Google Scholar] [CrossRef]
- Gnagnarella, P.; Parpinel, M.; Salvini, S.; Franceschi, S.; Palli, D.; Boyle, P. The update of the Italian Food Composition Database. J. Food Compos. Anal. 2004, 17, 509–522. [Google Scholar] [CrossRef]
- Carvalho, J.R.B.; Meireles, A.N.; Marques, S.S.; Gregório, B.J.R.; Ramos, I.I.; Silva, E.M.P.; Barreiros, L.; Segundo, M.A. Exploiting Kinetic Features of ORAC Assay for Evaluation of Radical Scavenging Capacity. Antioxidants 2023, 12, 505. [Google Scholar] [CrossRef]
- Remer, T.; Manz, F. Potential renal acid load of foods and its influence on urine pH. J. Am. Diet Assoc. 1995, 95, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Mertens, E.; Colizzi, C.; Peñalvo, J.L. Ultra-processed food consumption in adults across Europe. Eur. J. Nutr. 2022, 61, 1521–1539. [Google Scholar] [CrossRef]
- Zhou, X.; Qiao, K.; Wu, H.; Zhang, Y. The Impact of Food Additives on the Abundance and Composition of Gut Microbiota. Molecules 2023, 28, 631. [Google Scholar] [CrossRef]
- Di Giorgio, F.M.; Melatti, P.; Ciminnisi, S.; Cappello, M. A Narrative Review on Eating Disorders and Disordered Eating in Inflammatory Bowel Diseases: Need for Increased Awareness. Dietetics 2023, 2, 150–160. [Google Scholar] [CrossRef]
- Sakurai, K.; Furukawa, S.; Katsurada, T.; Otagiri, S.; Yamanashi, K.; Nagashima, K.; Onishi, R.; Yagisawa, K.; Nishimura, H.; Ito, T.; et al. Effectiveness of administering zinc acetate hydrate to patients with inflammatory bowel disease and zinc deficiency: A retrospective observational two-center study. Intest. Res. 2020, 20, 78–89. [Google Scholar] [CrossRef]
- Zupo, R.; Sila, A.; Castellana, F.; Bringiotti, R.; Curlo, M.; De Pergola, G.; De Nucci, S.; Giannelli, G.; Mastronardi, M.; Sardone, R. Prevalence of Zinc Deficiency in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4052. [Google Scholar] [CrossRef]
- Siva, S.; Rubin, D.T.; Gulotta, G.; Wroblewski, K.; Pekow, J. Zinc Deficiency is Associated with Poor Clinical Outcomes in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 152–157. [Google Scholar] [CrossRef]
- Brownson, E.; Saunders, J.; Jatkowska, A.; White, B.; Gerasimidis, K.; Seenan, J.P.; Macdonald, J. Micronutrient Status and Prediction of Disease Outcome in Adults with Inflammatory Bowel Disease Receiving Biologic Therapy. Inflamm. Bowel Dis. 2023, 23, izad174. [Google Scholar] [CrossRef]
- Balestrieri, P.; Ribolsi, M.; Guarino, M.P.L.; Emerenziani, S.; Altomare, A.; Cicala, M. Nutritional Aspects in Inflammatory Bowel Diseases. Nutrients 2020, 12, 372. [Google Scholar] [CrossRef]
- Hwang, C.; Ross, V.; and Mahadevan, U. Micronutrient deficiencies in inflammatory bowel disease: From A to zinc. Inflamm. Bowel Dis. 2012, 18, 1961–1981. [Google Scholar] [CrossRef]
- Kilby, K.; Mathias, H.; Boisvenue, L.; Heisler, C.; Jones, J.L. Micronutrient Absorption and Related Outcomes in People with Inflammatory Bowel Disease: A Review. Nutrients 2019, 11, 1388. [Google Scholar] [CrossRef] [PubMed]
- Massironi, S.; Viganò, C.; Palermo, A.; Pirola, L.; Mulinacci, G.; Allocca, M.; Peyrin-Biroulet, L.; Danese, S. Inflammation and malnutrition in inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2023, 8, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Stacy, A.; Andrade-Oliveira, V.; McCulloch, J.A.; Hild, B.; Oh, J.H.; Perez-Chaparro, P.J.; Sim, C.K.; Lim, A.I.; Link, V.M.; Enamorado, M.; et al. Infection trains the host for microbiota-enhanced resistance to pathogens. Cell 2021, 184, 615–627.e17. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.M.; Denger, K. Metabolism of taurine in microorganisms: A primer in molecular biodiversity? Adv. Exp. Med. Biol. 2006, 583, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Lambert, I.H.; Kristensen, D.M.; Holm, J.B.; Mortensen, O.H. Physiological role of taurine—From organism to organelle. Acta Physiol. 2015, 213, 191–212. [Google Scholar] [CrossRef]
- Fu, Q.; Song, T.; Ma, X.; Cui, J. Research progress on the relationship between intestinal microecology and intestinal bowel disease. Animal Model Exp. Med. 2022, 5, 297–310. [Google Scholar] [CrossRef]
- Stipanuk, M.H.; Jurkowska, H.; Roman, H.B.; Niewiadomski, J.; Hirschberger, L.L. Insights into Taurine Synthesis and Function Based on Studies with Cysteine Dioxygenase (CDO1) Knockout Mice. Adv. Exp. Med. Biol. 2015, 803, 29–39. [Google Scholar] [CrossRef]
- Zhao, Z.; Satsu, H.; Fujisawa, M.; Hori, M.; Ishimoto, Y.; Totsuka, M.; Nambu, A.; Kakuta, S.; Ozaki, H.; Shimizu, M. Attenuation by dietary taurine of dextran sulfate sodium-induced colitis in mice and of THP-1-induced damage to intestinal Caco-2 cell monolayers. Amino Acids 2008, 35, 217–224. [Google Scholar] [CrossRef]
- Shimizu, M.; Zhao, Z.; Ishimoto, Y.; Satsu, H. Dietary taurine attenuates dextran sulfate sodium (DSS)-induced experimental colitis in mice. Adv. Exp. Med. Biol. 2009, 643, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.; Schmitt-Kopplin, P. The role of fecal sulfur metabolome in inflammatory bowel diseases. Int. J. Med. Microbiol. 2021, 311, 151513. [Google Scholar] [CrossRef] [PubMed]
- Kaitha, S.; Bashir, M.; Ali, T. Iron deficiency anemia in inflammatory bowel disease. World J. Gastrointest. Pathophysiol. 2015, 6, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Nairz, M.; Weiss, G. Iron in infection and immunity. Mol. Aspects Med. 2020, 75, 100864. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; An, S.; Park, D.K.; Kwon, K.A.; Kim, K.O.; Chung, J.W.; Kim, J.H.; Kim, Y.J. Efficacy of iron supplementation in patients with inflammatory bowel disease treated with anti-tumor necrosis factor-alpha agents. Ther. Adv. Gastroenterol. 2020, 13, 1756284820961302. [Google Scholar] [CrossRef]
- Dudkowiak, R.; Neubauer, K.; Poniewierka, E. Hepcidin and its role in inflammatory bowel disease. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2013, 22, 585–591. [Google Scholar]
- Loveikyte, R.; Bourgonje, A.R.; van der Reijden, J.J.; Bulthuis, M.L.C.; Hawinkels, L.J.A.C.; Visschedijk, M.C.; Festen, E.A.M.; van Dullemen, H.M.; Weersma, R.K.; van Goor, H.; et al. Hepcidin and Iron Status in Patients with Inflammatory Bowel Disease Undergoing Induction Therapy with Vedolizumab or Infliximab. Inflamm. Bowel Dis. 2023, 29, 1272–1284. [Google Scholar] [CrossRef] [PubMed]
| CD (n:50) N (%) or Mdn (IQR) | CD-R (n:29) N (%) or Mdn (IQR) | CD-A (n:21) N (%) or Mdn (IQR) | P (CD-A vs. CD-R) | |
|---|---|---|---|---|
| Gender | ||||
| Female | 18 (36.0) | 9 (31.1) | 9 (42.8) | 0.39 |
| Male | 32 (64.0) | 20 (68.9) | 12 (57.2) | |
| Age | ||||
| Female | 39.1 (20–55) | 43.0 (42.0–48.0) | 33.0 (21.0–45.0) | 0.19 |
| Male | 35.7 (20.0–64.0) | 32.5 (24.0–40.5) | 34.5 (27.0–45.0) | 0.47 |
| BMI | ||||
| Female | 22.3 (18.0–25.0) | 23.62 (22.48–24.91) | 20.76 (19.59–23.53) | 0.22 |
| Male | 22.8 (18.0–25.0) | 23.84 (21.2–25.9) | 23.71 (22.17–26.4) | 0.58 |
| Smokers | 11 | 8 | 3 | 0.26 |
| CDAI | 150.0 (99.0–209.0) | 134.0 (86.5–201.0) | 172.0 (145.5–220.0) | 0.12 |
| SES CD | 10.0 (7.0–14.0) | 11.0 (7.0–14.0) | 8.0 (7.0–13.0) | 0.56 |
| Symptoms duration * | 8 (0.57–14.0) | 8 (0.75–13.0) | 6 (0.36–14.5) | 0.92 |
| CD-A V3 | CD-R V3 | CD-A V1 | CD-R V1 | |
|---|---|---|---|---|
| Soluble fibers | 2.26 (1.50–3.00) | 2.40 (1.70–1.350) | 2.45 (1.31–2.87) | 2.25 (1.64–2.85) |
| Insoluble fibers | 3.02 (2.27–5.63) | 4.54 (2.42–6.90) | 4.24 (2.71–5.22) | 3.84 (2.99–6.03) |
| Milk | 0.00 (0.00–85.75) | 20.00 (0.00–108.95) | 0.00 (0.00–50.71) | 5.71 (0.00–46.43) |
| Dairy products | 25.60 (5.70–55.35) | 52.86 (27.50–78.57) | 47.86 (19.29–77.14) | 55.71 (34.29–96.07) |
| Fish | 17.1 (0.00–42.10) | 17.14 (11.43–52.86) | 7.14 (0–12.86) | 15.71 (0.00–42.86) |
| White meat | 25.60 (5.7–55.35) | 21.43 (7.86–38.57) | 42.86 (16.43–62.14) | 14.29 (0.00–32.14) |
| Red meat | 51.40 (28.60–62.85) | 68.57 (23.57–97.14) | 52.86 (20.71–76.43) | 68.57 (27.86–114.64) |
| Processed meat | 38.57 (17.14–60.00) | 25.00 (14.29–42.14) | 50.00 (29.29–82.14) | 37.14 (17.14–50.00) |
| Eggs | 0.00 (0.00–4.30) | 0.00 (0.00–15.71) | 0.00 (0.00–14.29) | 0.00 (0.00–17.14) |
| Starchy foods # | 232.90 (202.85–298.55) | 245.29 (141.64–319.79) | 262.86 (182.5–317.86) | 246.14 (142.86–299.64) |
| Fruit | 47.86 (4.29–195.00) | 85.71 (28.57–155.71) | 69.28 (28.57–121.43) | 81.43 (21.43–112.86) |
| Vegetables | 75.36 (40.00–132.86) | 85.71 (44.29–135.71) | 60.35 (25.71–102.86) | 85.71 (40.00–165.71) |
| V3 CD-A vs. CD-R | V1 CD-A vs. CD-R | |||
|---|---|---|---|---|
| Soluble fibers | 0.54 | 0.99 | ||
| Insoluble fibers | 0.44 | 0.65 | ||
| Milk | 0.58 | 0.47 | ||
| Dairy products | 0.04 * | CD-A < CD-R | 0.39 | |
| Fish | 0.16 | 0.05 ** | CD-A < CD-R | |
| White meat | 0.93 | 0.03 * | CD-A > CD-R | |
| Red Meat | 0.65 | 0.21 | ||
| Processed meat | 0.22 | 0.16 | ||
| Eggs | 0.10 | 0.85 | ||
| Starchy foods # | 0.80 | 0.96 | ||
| Fruit | 0.44 | 0.94 | ||
| Vegetables | 0.72 | 0.24 |
| CD-A V3 | CD-R V3 | CD-A V1 | CD-R V1 | |
|---|---|---|---|---|
| Vitamin A ** | 404.82 (230.47–693.89) | 559.6 (319.62–837.73) | 436.39 (339.21–585.39) | 497.32 (284.14–856.07) |
| Vitamin B1 * | 0.96 (0.78–1.06) | 0.91 (0.68–1.15) | 0.95 (0.66–1.15) | 0.95 (0.75–1.26) |
| Vitamin B2 * | 1.16 (0.82–1.41) | 1.12 (1.00–1.55) | 1.1 (0.9–1.32) | 1.11 (0.92–1.60) |
| Vitamin B3 * | 15.57 (9.8–18.43) | 15.06 (11.04–17.34) | 13.94 (9.57–19.77) | 14.47 (10.39–19.44) |
| Vitamin B5 * | 1.31 (1.16–1.76) | 1.45 (0.98–2.21) | 1.65 (1.09–1.95) | 1.44 (1.11–1.83) |
| Vitamin B6 * | 1.34 (0.91–1.63) | 1.38 (1.03–1.68) | 1.15 (0.92–1.47) | 1.38 (0.90–1.91) |
| Vitamin B8 * | 5.98 (4.18–7.69) | 6.9 (4.91–9.82) | 7.48 (5.19–8.97) | 5.74 (4.3–11.06) |
| Vitamin B9 ** | 151.64 (119.28–185) | 155.79 (117.36–207.67) | 142.68 (98.12–206.77) | 167.7 (118.51–216.56) |
| Vitamin B12 * | 1.6 (0.90–2.16) | 1.51 (0.8–2.35) | 1.51 (0.58–2.02) | 1.41 (0.91–2.33) |
| Vitamin C * | 48.93 (20.5–88.43) | 50.47 (41.51–79.52) | 45.36 (34.2–76.05) | 57.38 (31.78–85.54) |
| Vitamin D * | 2.49 (0.99–3.63) | 2.37 (1.36–4.33) | 1.76 (1.09–2.79) | 1.90 (0.73–2.99) |
| Vitamin E *# | 0.51 (0.26–0.66) | 0.61 (0.26–0.76) | 0.46 (0.32–0.75) | 0.45 (0.30–0.79) |
| Vitamin k * | 13.19 (8.95–17.6) | 18.42 (9.76–51.13) | 10.88 (6.43–25.88) | 17.3 (8.54–36.09) |
| b-carotene ** | 726.36 (201.2–1493.8) | 719.16 (338.79–1452.84) | 716.04 (320.40–1338.57) | 823.90 (314.54–1232.04) |
| V3 CD-A vs. CD-R | V1 CD-A vs. CD-R | |
|---|---|---|
| Vitamin A | 0.19 | 0.53 |
| Vitamin B1 | 0.90 | 0.93 |
| Vitamin B2 | 0.60 | 0.72 |
| Vitamin B3 | 0.96 | 0.89 |
| Vitamin B5 | 0.92 | 0.42 |
| Vitamin B6 | 0.63 | 0.39 |
| Vitamin B8 | 0.45 | 0.49 |
| Vitamin B9 | 0.56 | 0.45 |
| Vitamin B12 | 0.92 | 0.43 |
| Vitamin C | 0.56 | 0.55 |
| Vitamin D | 0.59 | 0.62 |
| Vitamin E | 0.53 | 0.96 |
| Vitamin k | 0.23 | 0.35 |
| b-carotene | 0.71 | 0.82 |
| CD-A V3 | CD-R V3 | CD-A V1 | CD-R V1 | |
|---|---|---|---|---|
| Calcium * | 345.47 (290.36–510.18) | 555.04 (434.38–690.43) | 391.76 (290.67–526.19) | 455.92 (302.24–669.57) |
| Sodium * | 2500.25 (1950.9–2763.57) | 1889.36 (1453.21–2882.46) | 2240.23 (1385.68–3154.97) | 2037.94 (1454.79–3250.07) |
| Potassium * | 2171.81 (1643.54–2385.75) | 2022.13 (1614.41–2799.31) | 1964.24 (1514.08–2345.31) | 2044.52 (1469.3–2676.46) |
| Phosphorus * | 971.19 (811.83–1049.06) | 1121.89 (839.01–1397.77) | 1028.58 (764.02–1104.56) | 1035.85 (837.84–1250.53) |
| Iron * | 8.05 (5.84–10.56) | 9.39 (7.55–12.06) | 7.94 (6.11–10.49) | 9.03 (7.53–10.82) |
| Zinc * | 8.11 (6.26–9.72) | 8.89 (6.82–10.13) | 7.76 (5.25–9.49) | 9.15 (5.8–11.58) |
| Magnesium * | 112.14 (87.22–157.5) | 137.00 (110.66–164.56) | 127.37 (99.22–151.12) | 129.14 (93.18–161.37) |
| Copper * | 0.75 (0.44–0.9) | 0.77 (0.59–1.01) | 0.73 (0.44–0.86) | 0.78 (0.57–0.95) |
| Selenium ** | 19.15 (13.27–24.85) | 21.47 (14.89–32.28) | 17.23 (14.23–23.47) | 18.93 (11.88–25.33) |
| Chromium ** | 0 (0.00–0.0028) | 0.0014 (0.00–0.0028) | 0.00 (0.00–0.00143) | 0.00 (0.00–0.00143) |
| Fluorine * | 87.07 (60.27–153.55) | 88.71 (44.43–171.69) | 110.27 (50.78–169.97) | 93.02 (30.85–203.84) |
| Iodine ** | 48.61 (37.94–81.21) | 51.46 (35.03–66.94) | 45.43 (33.94–59.38) | 53.46 (29.34–73.85) |
| Manganese * | 3.89 (1.52–8.40) | 2.80 (1.38–8.93) | 1.70 (1.13–3.67) | 1.62 (1.06–4.94) |
| Molybdenum ** | 4.00 (2.37–9.22) | 5.00 (2.37–9.03) | 3.59 (1.93–7.91) | 3.72 (2.01–6.89) |
| V3 CD-A vs. CD-R | V1 CD-A vs. CD-R | |||
|---|---|---|---|---|
| Calcium | <0.01 * | CD-A < CD-R | 0.27 | |
| Sodium | 0.32 | 0.73 | ||
| Potassium | 0.66 | 0.38 | ||
| Phosphorus | 0.14 | 0.38 | ||
| Iron | 0.39 | 0.37 | ||
| Zinc | 0.33 | 0.09 ** | CD-A < CD-R | |
| Magnesium | 0.33 | 0.85 | ||
| Copper | 0.34 | 0.19 | ||
| Selenium | 0.43 | 0.85 | ||
| Chromium | 0.3 | 0.88 | ||
| Fluorine | 0.76 | 0.55 | ||
| Iodine | 0.73 | 0.43 | ||
| Manganese | 0.54 | 0.99 | ||
| Molybdenum | 0.56 | 0.97 |
| CD-A V3 | CD-R V3 | CD-A V1 | CD-R V1 | |
|---|---|---|---|---|
| Cholesterol | 194.63 (165.84–285.71) | 238.24 (166.39–321.36) | 232.28 (143.78–301.25) | 263.38 (169.3–320.02) |
| Saturated | 20.52 (15.15–22.89) | 24.65 (14.56–29.11) | 20.42 (15.01–26.07) | 22.41 (15.51–31.64) |
| Unsaturated | 29.16 (24.91–37.87) | 36.87 (26.42–44.45) | 31.10 (24.21–37.03) | 33.58 (23.29–48.73) |
| Polyunsaturated | 7.26 (5.35–11.18) | 9.32 (5.51–11.02) | 7.50 (6.02–8.53) | 7.69 (5.75–11.45) |
| Monounsaturated | 22.37 (19.56–28.77) | 26.36 (19.81–33.00) | 24.05 (17.89–27.70) | 25.58 (18.05–37.77) |
| C10:0 capric acid | 0.11 (0.07–0.35) | 0.34 (0.16–0.50) | 0.19 (0.04–0.46) | 0.25 (0.07–0.39) |
| C12:0 lauric acid | 0.09 (0.03–0.15) | 0.17 (0.09–0.23) | 0.07 (0.02–0.22) | 0.12 (0.04–0.19) |
| C14:0 myristic acid | 0.33 (0.25–0.65) | 0.67 (0.36–0.80) | 0.38 (0.22–0.85) | 0.50 (0.27–0.70) |
| C16:0 palmitic acid | 4.05 (3.12–4.83) | 4.17 (3.25–5.73) | 4.09 (2.66–5.93) | 3.56 (2.37–4.82) |
| C18:0 stearic acid | 1.72 (1.28–2.09) | 1.65 (1.37–2.89) | 1.85 (1.09–2.79) | 1.58 (0.96–2.21) |
| C20:0 arachidic acid | 0.07 (0.03–0.13) | 0.07 (0.04–0.11) | 0.0 (0.03–0.10) | 0.08 (0.04–0.14) |
| C14:1 myristoleic acid | 0.01 (0–0.05) | 0.01 (0–0.03) | 0.02 (0.01–0.07) | 0.01 (0.00–0.04) |
| C16:1 palmitoleic acid | 0.42 (0.31–0.52) | 0.37 (0.3–0.59) | 0.46 (0.28–0.60) | 0.36 (0.22–0.55) |
| C18:1 oleic acid | 12.46 (10.05–16.00) | 14.5 (9.5–17.78) | 13.26 (8.15–15.67) | 12.76 (9.02–18.86) |
| C20:1 eicosanoic acid | 0.14 (0.08–0.18) | 0.12 (0.07–0.22) | 0.12 (0.09–0.18) | 0.13 (0.07–0.22) |
| C22:1 erucic acid | 0.01 (0.00–0.06) | 0.01 (0.00–0.03) | 0.00 (0.00- 0.02) | 0.01 (0.00–0.03) |
| C18:2 linoleic acid | 3.36 (2.49–4.47) | 3.75 (2.68–4.47) | 3.59 (2.60–4.87) | 3.52 (2.28–5.36) |
| C18:3 α-linolenic acid | 0.37 (0.24–0.49) | 0.37 (0.24–0.51) | 0.34 (0.27–0.52) | 0.40 (0.23–0.6) |
| C20:4 arachidonic acid | 0.12 (0.08–0.2) | 0.12 (0.07–0.19) | 0.14 (0.08–0.24) | 0.12 (0.07–0.2) |
| C20:5 EPA | 0.03 (0.00–0.04) | 0.02 (0.01–0.04) | 0.01 (0.00–0.03) | 0.01 (0.00–0.03) |
| C22:6 DHA | 0.01 (0.00–0.06) | 0.02 (0.00–0.05) | 0.00 (0.00–0.02) | 0.01 (0.00–0.04) |
| Total ω3 | 0.42 (0.3–0.6) | 0.45 (0.26–0.65) | 0.42 (0.28–0.66) | 0.42 (0.26–0.62) |
| Total ω6 | 3.59 (2.62–4.59) | 3.87 (2.72–4.62) | 3.79 (2.69–5.11) | 3.67 (2.43–5.63) |
| ω3/ω6 | 0.12 | 0.12 | 0.11 | 0.11 |
| V3 CD-A vs. CD-R | V1 CD-A vs. CD-R | |||
|---|---|---|---|---|
| Cholesterol | 0.33 | 0.49 | ||
| Saturated | 0.38 | 0.44 | ||
| Unsaturated | 0.55 | 0.48 | ||
| Polyunsaturated | 0.37 | 0.57 | ||
| Monounsaturated | 0.12 | 0.55 | ||
| C10:0 capric acid | 0.04 * | CD-A < CD-R | 0.85 | |
| C12:0 lauric acid | 0.05 ** | CD-A < CD-R | 0.62 | |
| C14:0 myristic acid | 0.28 | 0.59 | ||
| C16:0 palmitic acid | 0.22 | 0.59 | ||
| C18:0 stearic acid | 0.69 | 0.74 | ||
| C20:0 arachidic acid | 0.4 | 0.33 | ||
| C14:1 myristoleic acid | 0.77 | 0.06 ** | CD-A > CD-R | |
| C16:1 palmitoleic acid | 0.78 | 0.43 | ||
| C18:1 oleic acid | 0.63 | 0.87 | ||
| C20:1 eicosanoic acid | 0.86 | 0.9 | ||
| C22:1 erucic acid | 0.68 | 0.77 | ||
| C18:2 linoleic acid | 0.86 | 0.94 | ||
| C18:3 α-linolenic acid | 0.84 | 0.76 | ||
| C20:4 arachidonic acid | 0.83 | 0.55 | ||
| C20:5 EPA | 0.93 | 0.87 | ||
| C22:6 DHA | 0.58 | 0.92 | ||
| Total ω3 | 0.83 | 0.90 | ||
| Total ω6 | 0.82 | 1.00 |
| CD-A V3 | CD-R V3 | CD-A V1 | CD-R V1 | |
|---|---|---|---|---|
| Cholesterol # | 169.0 (153.0–188.0) | 181.0 (160.0–224.0) | 155.0 (141.0–173.0) | 162.0 (138.0–198.0) |
| Vitamin B9 § | 5.2 (4.7–7.5) | 7.3 (5.8–9.6) | 5.5 (3.0–6.9) | 5.3 (3.8–6.9) |
| Vitamin B12 ** | 153.0 (120.0–279.0) | 268.0 (202.0–333.0) | 170.0 (154.0- 309.0) | 243.0 (186.0–332.0) |
| Potassium * | 4.0 (3.8–4.2) | 4.1 (3.9–4.2) | 4.1 (3.7–4.5) | 4.0 (3.9–4.3) |
| Magnesium * | 2.1 (2.0–2.2) | 2.2 (2.1–2.4) | 2.0 (1.8–2.1) | 2.0 (1.9–2.1) |
| Iron ● | 65.0 (31.0–115.0) | 122.0 (97.0–167.0) | 34.0 (20.0–47.0) | 55.0 (36.0–90.0) |
| Sodium * | 139.0 (138.0–141.0) | 140.0 (137.0–141.0) | 138.0 (138.0–140.0) | 139.0 (138.0–141.0) |
| Phosphorus * | 3.3 (2.9–3.7) | 3.3 (3.1–3.6) | 3.2 (2.7–3.6) | 3.2 (3.0–3.5) |
| TAU * | 83.5 (64.0–100.5) | 97.0 (76.0–107.0) | 64.0 (58.0–93.0) | 87.0 (73.0–101.0) |
| ASP * | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | 3.0 (3.0-4.0) | 3.0 (3.0–5.0) |
| THR * | 98.0 (91.5–124.5) | 122.0 (111.0–136.0) | 96.0 (87.0–126.0) | 109.0 (97.0–142.0) |
| SER * | 110.0 (102.5–122.5) | 111.0 (98.0–127.0) | 102.0 (90.0–111.0) | 109.0 (84.0–117.0) |
| GLU * | 671.5 (615.5–779.0) | 806.0 (708.0–885.0) | 639.0 (556.0–731.0) | 651.0 (587.0–773.0) |
| PRO * | 192.0 (135–224.0) | 168.0 (150.0–198.0) | 182.0 (145.0–246.0) | 191.0 (164.0–247.0) |
| GLY * | 219.0 (199.0–243.0) | 235.0 (207.0–262.0) | 226.0 (193.0–253.0) | 234.0 (191.0–285.0) |
| ALA * | 318.5 (287–381.5) | 350.0 (293.0–396.0) | 297.0 (238.0–367.0) | 306.0 (263.0–376.0) |
| CIT * | 31.5 (23.5–41.5) | 39.0 (35.0–44.0) | 30.0 (23.0–32.0) | 33.0 (28.0–40.0) |
| VAL * | 202.0 (156.0–241.0) | 230.0 (198.0–256.0) | 198.0 (171.0–217.0) | 201.0 (171.0–222.0) |
| CYS * | 40.5 (34.0–48.0) | 47.0 (41.0–58.0) | 38.0 (31.0–44.0) | 40.0 (35.0–48.0) |
| MET * | 21.5 (19.0–26.0) | 27.0 (21.0–29.0) | 21.0 (19.0–26.0) | 21.0 (16.0–24.0) |
| ILE * | 64.5 (54.5–74.0) | 70.0 (58.0–76.0) | 63.0 (52.0–73.0) | 60.0 (50.0–68.0) |
| LEU * | 135.5 (105.5–175.5) | 159.0 (140.0–172.0) | 134.0 (98.0–163.0) | 131.0 (114.0–145.0) |
| TYR * | 55.0 (52.5–64.5) | 62.0 (55.0–75.0) | 61.0 (48.0–74.0) | 59.0 (50.0–67.0) |
| PHE * | 55.5 (50–58) | 65.0 (58.0–69.0) | 59.0 (51.0–64.0) | 59.0 (48.0–69.0) |
| ORN * | 84.5 (75–102.5) | 102.0 (70.0–122.0) | 82.0 (62.0–105.0) | 93.0 (74.0–118.0) |
| LYS * | 183.5 (137.5–213.5) | 211.0 (173.0–243.0) | 168.0 (140.0–187.0) | 174.0 (140.0–200.0) |
| HIS * | 75.0 (63.5–88.0) | 85.0 (78.0–96.0) | 69.0 (52.0–77.0) | 73.0 (63.0–82.0) |
| ARG * | 42.0 (31.0–57.0) | 56.0 (45.0–72.0) | 37.0 (32.0–51.0) | 47.0 (33.0–65.0) |
| V3 CD-A vs. CD-R | V1 CD-A vs. CD-R | |||
|---|---|---|---|---|
| Cholesterol | 0.15 | 0.65 | ||
| Vitamin B9 | 0.03 * | CD-A < CD-R | 0.54 | |
| Vitamin B12 | 0.05 ** | CD-A < CD-R | 0.05 ** | CD-A < CD-R |
| Potassium | 0.63 | 0.79 | ||
| Magnesium | 0.04 * | CD-A < CD-R | 0.68 | |
| Iron | <0.01 * | CD-A < CD-R | <0.01 * | CD-A < CD-R |
| Sodium | 0.46 | 0.63 | ||
| Phosphorus | 0.95 | 0.97 | ||
| TAU | 0.23 | 0.02 * | CD-A < CD-R | |
| ASP | 0.67 | 0.78 | ||
| THR | 0.01 * | CD-A < CD-R | 0.35 | |
| SER | 0.64 | 0.76 | ||
| GLU | 0.02 * | CD-A < CD-R | 0.67 | |
| PRO | 0.32 | 0.58 | ||
| GLY | 0.21 | 0.53 | ||
| ALA | 0.34 | 0.60 | ||
| CIT | 0.03 * | CD-A < CD-R | 0.93 | |
| VAL | 0.04 * | CD-A < CD-R | 0.46 | |
| CYS | 0.02 * | CD-A < CD-R | 0.52 | |
| MET | <0.01 * | CD-A < CD-R | 0.92 | |
| ILE | 0.42 | 0.68 | ||
| LEU | 0.09 ** | CD-A < CD-R | 0.99 | |
| TYR | 0.07 ** | CD-A < CD-R | 0.77 | |
| PHE | <0.01* | CD-A < CD-R | 0.95 | |
| ORN | 0.32 | 0.47 | ||
| LYS | 0.06 ** | CD-A < CD-R | 0.71 | |
| HIS | <0.01 * | CD-A < CD-R | 0.28 | |
| ARG | 0.04 * | CD-A < CD-R | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzello, F.; Saracino, I.M.; Gionchetti, P.; Valerii, M.C.; Ricci, C.; Imbesi, V.; Filippone, E.; Bellocchio, I.; Dussias, N.K.; Dervieux, T.; et al. Nutritional Biomarkers for the Prediction of Response to Anti-TNF-α Therapy in Crohn’s Disease: New Tools for New Approaches. Nutrients 2024, 16, 280. https://doi.org/10.3390/nu16020280
Rizzello F, Saracino IM, Gionchetti P, Valerii MC, Ricci C, Imbesi V, Filippone E, Bellocchio I, Dussias NK, Dervieux T, et al. Nutritional Biomarkers for the Prediction of Response to Anti-TNF-α Therapy in Crohn’s Disease: New Tools for New Approaches. Nutrients. 2024; 16(2):280. https://doi.org/10.3390/nu16020280
Chicago/Turabian StyleRizzello, Fernando, Ilaria Maria Saracino, Paolo Gionchetti, Maria Chiara Valerii, Chiara Ricci, Veronica Imbesi, Eleonora Filippone, Irene Bellocchio, Nikolas Konstantine Dussias, Thierry Dervieux, and et al. 2024. "Nutritional Biomarkers for the Prediction of Response to Anti-TNF-α Therapy in Crohn’s Disease: New Tools for New Approaches" Nutrients 16, no. 2: 280. https://doi.org/10.3390/nu16020280
APA StyleRizzello, F., Saracino, I. M., Gionchetti, P., Valerii, M. C., Ricci, C., Imbesi, V., Filippone, E., Bellocchio, I., Dussias, N. K., Dervieux, T., & Spisni, E. (2024). Nutritional Biomarkers for the Prediction of Response to Anti-TNF-α Therapy in Crohn’s Disease: New Tools for New Approaches. Nutrients, 16(2), 280. https://doi.org/10.3390/nu16020280







