Exploring the Link between Inflammatory Biomarkers and Adipometrics in Healthy Young Adults Aged 20–35 Years
Abstract
1. Introduction
2. Materials and Methods
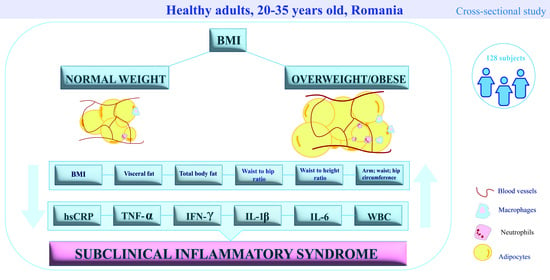
2.1. Study Design and Population
2.2. Laboratory Methods
2.2.1. Blood Sample Collection
2.2.2. Multiplex Bead-Based Immunoassay
2.2.3. The Enzyme-Linked Immunosorbent Assay
2.2.4. Turbidimetric Assay
2.2.5. White Blood Cell Count
2.3. Anthropometric Measurements
2.4. Statistical Methods
3. Results
3.1. Cohort Classification and Analyses Based on BMI
3.2. Cohort Classification and Analyses Based on hsCRP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | Arm circumference |
| AT | Adipose tissue |
| BMI | Body mass index |
| CVD | Cardiovascular disease |
| HC | Hip circumference |
| hsCRP | High-sensitivity C-reactive protein |
| IFN-γ | Interferon-γ |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| LGI | Chronic low-grade inflammation |
| NCDs | Noncommunicable diseases |
| NW | Normal weight |
| OW/OB | Overweight/obese |
| WBC | White blood cell count |
| WC | Waist circumference |
| WHR | Waist-to-hip ratio |
| WHtR | Waist-to-height ratio |
| SAT | Subcutaneous adipose tissue |
| TBFM | Total body fat mass |
| TNF-α | Tumor necrosis factor-α |
| VAT | Visceral adipose tissue |
| VFL | Visceral fat level |
References
- WHO. Acceleration Plan to Stop Obesity. Available online: https://iris.who.int/bitstream/handle/10665/370281/9789240075634-eng.pdf?sequence=1 (accessed on 1 December 2023).
- Bacârea, A.; Bacârea, V.C.; Cînpeanu, C.; Teodorescu, C.; Seni, A.G.; Guiné, R.P.F.; Tarcea, M. Demographic, Anthropometric and Food Behavior Data towards Healthy Eating in Romania. Foods 2021, 10, 487. [Google Scholar] [PubMed]
- Steenblock, C.; Hassanein, M.; Khan, E.G.; Yaman, M.; Kamel, M.; Barbir, M.; Bornstein, S.R. Obesity and COVID-19: What are the Consequences? Horm. Metab. Res. 2022, 54, 496–502. [Google Scholar] [PubMed]
- Restrepo, B.J. Obesity Prevalence Among U.S. Adults during the COVID-19 Pandemic. Am. J. Prev. Med. 2022, 63, 102–106. [Google Scholar] [PubMed]
- World Health Organization. WHO European Regional Obesity Report 2022. World Health Organization. Regional Office for Europe. 2022. Available online: https://iris.who.int/handle/10665/353747 (accessed on 1 December 2023).
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [PubMed]
- Surmi, B.; Hasty, A. Macrophage infiltration into adipose tissue: Initiation, propagation and remodeling. Future Lipidol. 2008, 3, 545–556. [Google Scholar]
- Chen, Y.; Liu, S.; Leng, S.X. Chronic Low-grade Inflammatory Phenotype (CLIP) and Senescent Immune Dysregulation. Clin. Ther. 2019, 41, 400–409. [Google Scholar]
- Ahmed, A.U. An overview of inflammation: Mechanism and consequences. Front. Biol. 2011, 6, 274. [Google Scholar]
- Antonelli, M.; Kushner, I. It’s time to redefine inflammation. FASEB J. 2017, 31, 1787–1791. [Google Scholar]
- Figueroa, A.L.; Takx, R.A.P.; MacNabb, M.H.; Abdelbaky, A.; Lavender, Z.R.; Kaplan, R.S.; Tawakol, A. Relationship Between Measures of Adiposity, Arterial Inflammation, and Subsequent Cardiovascular Events. Circ. Cardiovasc. Imaging 2016, 9, e004043. [Google Scholar]
- Alvehus, M.; Burén, J.; Sjöström, M.; Goedecke, J.; Olsson, T. The Human Visceral Fat Depot Has a Unique Inflammatory Profile. Obesity 2010, 18, 879–883. [Google Scholar]
- Picarelli, A.; Borghini, R.; Marino, M.; Casale, R.; Di Tola, M.; Lubrano, C.; Masselli, G. Piermattei, A.; Gualdi, G.; Bella, A.; Donato, G.; et al.Visceral and subcutaneous adipose tissue as markers of local and systemic inflammation: A comparison between celiac and obese patients using MRI. Tech. Coloproctol. 2020, 24, 553–562. [Google Scholar]
- Kushner, I.; Antonelli, M.J. What Should We Regard as an “Elevated” C-Reactive Protein Level? Ann. Intern. Med. 2015, 163, 326. [Google Scholar] [PubMed]
- Krabbe, K.S.; Pedersen, M.; Bruunsgaard, H. Inflammatory mediators in the elderly. Exp. Gerontol. 2004, 39, 687–699. [Google Scholar]
- Minciullo, P.L.; Catalano, A.; Mandraffino, G.; Casciaro, M.; Crucitti, A.; Maltese, G.; Morabito, N.; Lasco, A.; Gangemi, S.; Basile, G. Inflammaging and Anti-Inflammaging: The Role of Cytokines in Extreme Longevity. Arch. Immunol. Ther. Exp. 2016, 64, 111–126. [Google Scholar]
- World Health Organization. Obesity and Overweight—Fact Sheets. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 December 2023).
- Proinflammatory Cytokines in CRP Baseline Regulation. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0065242309480053 (accessed on 1 December 2023).
- Ridker, P.M.; Hennekens, C.H.; Buring, J.E.; Rifai, N. C-Reactive Protein and Other Markers of Inflammation in the Prediction of Cardiovascular Disease in Women. N. Engl. J. Med. 2000, 342, 836–843. [Google Scholar]
- Lee, J.; Yoon, K.; Ryu, S.; Chang, Y.; Kim, H.R. High-normal levels of hs-CRP predict the development of non-alcoholic fatty liver in healthy men. PLoS ONE 2017, 12, e0172666. [Google Scholar]
- Ebrahimi, M.; Heidari-Bakavoli, A.R.; Shoeibi, S.; Mirhafez, S.R.; Moohebati, M.; Esmaily, H.; Ghayour-Mobarhan, M. Association of Serum hs-CRP Levels with the Presence of Obesity, Diabetes Mellitus, and Other Cardiovascular Risk Factors. J. Clin. Lab. Anal. 2016, 30, 672–676. [Google Scholar]
- Berenson, G.S.; Srinivasan, S.R.; Bao, W.; Newman, W.P.; Tracy, R.E.; Wattigney, W.A. Association between Multiple Cardiovascular Risk Factors and Atherosclerosis in Children and Young Adults. N. Engl. J. Med. 1998, 338, 1650–1656. [Google Scholar]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar]
- Kuilman, T.; Michaloglou, C.; Vredeveld, L.C.W.; Douma, S.; Van Doorn, R.; Desmet, C.J.; Aarden, L.A.; Mooi, W.J.; Peeper, D.S. Oncogene-Induced Senescence Relayed by an Interleukin-Dependent Inflammatory Network. Cell 2008, 133, 1019–1031. [Google Scholar]
- El-Mikkawy, D.M.E.; EL-Sadek, M.A.; EL-Badawy, M.A.; Samaha, D. Circulating level of interleukin-6 in relation to body mass indices and lipid profile in Egyptian adults with overweight and obesity. Egypt Rheumatol. Rehabil. 2020, 47, 7. [Google Scholar]
- Park, H.S.; Park, J.Y.; Yu, R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-α and IL-6. Diabetes Res. Clin. Pract. 2005, 69, 29–35. [Google Scholar] [PubMed]
- Van De Veerdonk, F.L.; Netea, M.G.; Dinarello, C.A.; Joosten, L.A.B. Inflammasome activation and IL-1β and IL-18 processing during infection. Trends Immunol. 2011, 32, 110–116. [Google Scholar] [PubMed]
- Migliorini, P.; Italiani, P.; Pratesi, F.; Puxeddu, I.; Boraschi, D. The IL-1 family cytokines and receptors in autoimmune diseases. Autoimmun. Rev. 2020, 19, 102617. [Google Scholar] [PubMed]
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [PubMed]
- Jager, J.; Grémeaux, T.; Cormont, M.; Le Marchand-Brustel, Y.; Tanti, J.F. Interleukin-1β-Induced Insulin Resistance in Adipocytes through Down-Regulation of Insulin Receptor Substrate-1 Expression. Endocrinology 2007, 148, 241–251. [Google Scholar]
- Negrin, K.A.; Roth Flach, R.J.; DiStefano, M.T.; Matevossian, A.; Friedline, R.H.; Jung, D.; Kim, J.K.; Czech, M.P. IL-1 Signaling in Obesity-Induced Hepatic Lipogenesis and Steatosis. PLoS ONE 2014, 9, e107265. [Google Scholar]
- Lucas, R.; Parikh, S.J.; Sridhar, S.; Guo, D.H.; Bhagatwala, J.; Dong, Y.; Caldwell, R.; Mellor, A.; Caldwell, W.; Zhu, H.; et al. Cytokine profiling of young overweight and obese female African American adults with prediabetes. Cytokine 2013, 64, 310–315. [Google Scholar]
- Ridker, P.M.; MacFadyen, J.G.; Glynn, R.J.; Koenig, W.; Libby, P.; Everett, B.M.; Cornel, J.H. Inhibition of Interleukin-1β by Canakinumab and Cardiovascular Outcomes in Patients With Chronic Kidney Disease. J. Am. Coll. Cardiol. 2018, 71, 2405–2414. [Google Scholar]
- Tack, C.J.; Stienstra, R.; Joosten, L.A.B.; Netea, M.G. Inflammation links excess fat to insulin resistance: The role of the interleukin-1 family. Immunol. Rev. 2012, 249, 239–252. [Google Scholar]
- Borges, M.D.; Franca, E.L.; Fujimori, M.; Silva, S.M.C.; de Marchi, P.G.F.; Deluque, A.L.; de Abreu, L.C. Relationship between Proinflammatory Cytokines/Chemokines and Adipokines in Serum of Young Adults with Obesity. Metab. Immune Disord. Drug Targets 2018, 18, 260–267. [Google Scholar]
- El-Wakkad, A.; Hassan, N.E.M.; Sabai, H.; El-Zayat, S.R. Proinflammatory, anti-inflammatory cytokines and adiponkines in students with central obesity. Cytokine 2013, 61, 682–687. [Google Scholar]
- Koenen, T.B.; Stienstra, R.; Van Tits, L.J.; Joosten, L.A.B.; Van Velzen, J.F.; Hijmans, A.; de Graaf, J. The Inflammasome and Caspase-1 Activation: A New Mechanism Underlying Increased Inflammatory Activity in Human Visceral Adipose Tissue. Endocrinology 2011, 152, 3769–3778. [Google Scholar] [PubMed]
- Moschen, A.R.; Molnar, C.; Enrich, B.; Geiger, S.; Ebenbichler, C.F.; Tilg, H. Adipose and Liver Expression of Interleukin (IL)-1 Family Members in Morbid Obesity and Effects of Weight Loss. Mol. Med. 2011, 17, 840–845. [Google Scholar]
- Unamuno, X.; Gómez-Ambrosi, J.; Ramírez, B.; Rodríguez, A.; Becerril, S.; Valentí, V.; Moncada, R.; Silva, C.; Salvador, J.; Frühbeck, G.; et al. NLRP3 inflammasome blockade reduces adipose tissue inflammation and extracellular matrix remodeling. Cell Mol. Immunol. 2021, 18, 1045–1057. [Google Scholar] [PubMed]
- Esser, N.; L’homme, L.; De Roover, A.; Kohnen, L.; Scheen, A.J.; Moutschen, M.; Paquot, N. Obesity phenotype is related to NLRP3 inflammasome activity and immunological profile of visceral adipose tissue. Diabetologia 2013, 56, 2487–2497. [Google Scholar]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar]
- Kursunel, M.A.; Esendagli, G. The untold story of IFN-γ in cancer biology. Cytokine Growth Factor Rev. 2016, 31, 73–81. [Google Scholar]
- Langer, V.; Vivi, E.; Regensburger, D.; Winkler, T.H.; Waldner, M.J.; Rath, T.; Schmid, B.; Skottke, L.; Lee, S.; Jeon, N.L.; et al. IFN-γ drives inflammatory bowel disease pathogenesis through VE-cadherin–directed vascular barrier disruption. J. Clin. Investig. 2019, 129, 4691–4707. [Google Scholar]
- Akkoc, T.; De Koning, P.J.A.; Rückert, B.; Barlan, I.; Akdis, M.; Akdis, C.A. Increased activation-induced cell death of high IFN-γ–producing TH1 cells as a mechanism of TH2 predominance in atopic diseases. J. Allergy Clin. Immunol. 2008, 121, 652–658.e1. [Google Scholar]
- Zheng, C.; Zhou, X.W.; Wang, J.Z. The dual roles of cytokines in Alzheimer’s disease: Update on interleukins, TNF-α, TGF-β and IFN-γ. Transl. Neurodegener. 2016, 5, 7. [Google Scholar] [PubMed]
- O’Rourke, R.W.; Metcalf, M.D.; White, A.E.; Madala, A.; Winters, B.R.; Maizlin, I.I.; Marks, D.L. Depot-specific differences in inflammatory mediators and a role for NK cells and IFN-γ in inflammation in human adipose tissue. Int. J. Obes. 2009, 33, 978–990. [Google Scholar]
- O’Rourke, R.W.; White, A.E.; Metcalf, M.D.; Winters, B.R.; Diggs, B.S.; Zhu, X.; Marks, D.L. Systemic inflammation and insulin sensitivity in obese IFN-γ knockout mice. Metabolism 2012, 61, 1152–1161. [Google Scholar]
- García-Hernández, M.; Rodríguez-Varela, E.; García-Jacobo, R.; la Torre, M.H.-D.; Uresti-Rivera, E.; González-Amaro, R.; Portales-Pérez, D. Frequency of regulatory B cells in adipose tissue and peripheral blood from individuals with overweight, obesity and normal-weight. Obes. Res. Clin. Pract. 2018, 12, 513–519. [Google Scholar] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar]
- Singhai, M.; Faizy, A.; Goyal, R.; Siddiqui, S. Evaluation of TNF-α and IL-6 levels in obese and non-obese diabetics: Pre- and postinsulin effects. N. Am. J. Med. Sci. 2012, 4, 180. [Google Scholar]
- TNF-Α and Obesity. Available online: https://www.karger.com/Article/FullText/289203 (accessed on 15 December 2023).
- Farhangi, M.A.; Keshavarz, S.A.; Eshraghian, M.; Ostadrahimi, A.; Saboor-Yaraghi, A.A. White Blood Cell Count in Women: Relation to Inflammatory Biomarkers, Haematological Profiles, Visceral Adiposity, and Other Cardiovascular Risk Factors. J. Health Popul. Nutr. 2013, 31, 58–64. [Google Scholar]
- Ghannadiasl, F. Associations between white blood cells count and obesity in apparently healthy young adults. Nutr. Food Sci. 2020, 50, 1013–1019. [Google Scholar]
- Pérez-de-Heredia, F.; Gómez-Martínez, S.; Díaz, L.E.; Veses, A.M.; Nova, E.; Wärnberg, J.; HELENA Study Group. Influence of sex, age, pubertal maturation and body mass index on circulating white blood cell counts in healthy European adolescents—The HELENA study. Eur. J. Pediatr. 2015, 174, 999–1014. [Google Scholar]
- Gu, Y.; Hu, K.; Huang, Y.; Zhang, Q.; Liu, L.; Meng, G.; Wu, H.; Xia, Y.; Bao, X.; Shi, H.; et al. White blood cells count as an indicator to identify whether obesity leads to increased risk of type 2 diabetes. Diabetes Res. Clin. Pract. 2018, 141, 140–147. [Google Scholar]
- Twig, G.; Afek, A.; Shamiss, A.; Derazne, E.; Tzur, D.; Gordon, B.; Tirosh, A. White Blood Cell Count and the Risk for Coronary Artery Disease in Young Adults. PLoS ONE 2012, 7, e47183. [Google Scholar]
- Sung, K.; Ryu, S.; Sung, J.; Kim, Y.B.; Won, Y.S.; Cho, D.S.; Liu, A. Inflammation in the Prediction of Type 2 Diabetes and Hypertension in Healthy Adults. Arch. Med. Res. 2017, 48, 535–545. [Google Scholar] [PubMed]
- Jung, C.H.; Lee, W.Y.; Kim, B.Y.; Park, S.E.; Rhee, E.J.; Park, C.Y.; Oh, K.-W.; Mok, J.-O.; Kim, C.-H.; Park, S.-W.; et al. The Risk of Metabolic Syndrome According to the White Blood Cell Count in Apparently Healthy Korean Adults. Yonsei Med. J. 2013, 54, 615. [Google Scholar]
- Brown, D.W.; Giles, W.H.; Croft, J.B. White blood cell count. J. Clin. Epidemiol. 2001, 54, 316–322. [Google Scholar]
- Dixon, J.; O’ Brien, P. Obesity and the White Blood Cell Count: Changes with Sustained Weight Loss. Obes. Surg. 2006, 16, 251–257. [Google Scholar]
- Nishida, C.; Ko, G.T.; Kumanyika, S. Body fat distribution and noncommunicable diseases in populations: Overview of the 2008 WHO Expert Consultation on Waist Circumference and Waist–Hip Ratio. Eur. J. Clin. Nutr. 2010, 64, 2–5. [Google Scholar] [PubMed]
- Cheng, C.H.; Ho, C.C.; Yang, C.F.; Huang, Y.C.; Lai, C.H.; Liaw, Y.P. Waist-to-hip ratio is a better anthropometric index than body mass index for predicting the risk of type 2 diabetes in Taiwanese population. Nutr. Res. 2010, 30, 585–593. [Google Scholar] [PubMed]
- Pou, K.M.; Massaro, J.M.; Hoffmann, U.; Vasan, R.S.; Maurovich-Horvat, P.; Larson, M.G.; Fox, C.S. Visceral and Subcutaneous Adipose Tissue Volumes Are Cross-Sectionally Related to Markers of Inflammation and Oxidative Stress: The Framingham Heart Study. Circulation 2007, 116, 1234–1241. [Google Scholar] [PubMed]
- Rana, M.N.; Neeland, I.J. Adipose Tissue Inflammation and Cardiovascular Disease: An Update. Curr. Diab. Rep. 2022, 22, 27–37. [Google Scholar]
- Spoto, B.; Di Betta, E.; Mattace-Raso, F.; Sijbrands, E.; Vilardi, A.; Parlongo, R.M.; Pizzini, P.; Pisano, A.; Vermi, W.; Testa, A.; et al. Pro- and anti-inflammatory cytokine gene expression in subcutaneous and visceral fat in severe obesity. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1137–1143. [Google Scholar]
| Overweight and Obese (n = 91) | Normal Weight (n = 37) | ||
|---|---|---|---|
| Age—Mean ± SD (Median) | 28.08 ± 4.66 (28.00) | 28.03 ± 3.63 (27.00) | |
| Sex | male—n (%) | 53 (58.24%) | 7 (18.92%) |
| female—n (%) | 38 (41.76%) | 30 (81.08%) | |
| Provenience | urban—n (%) | 78 (86.67%) | 31 (83.78%) |
| rural—n (%) | 12 (13.33%) | 6 (16.22%) | |
| Overweight and Obese (n = 91) Mean ± SD (Median) | Normal Weight (n = 37) Mean ± SD (Median) | p * | |
|---|---|---|---|
| The adipometrics | |||
| BMI | 30.89 ± 5.36 (28.80) | 21.35 ± 1.82 (21.20) | <0.0001 |
| Total body fat mass (kg) | 31.03 ± 13.38 (28.20) | 13.67 ± 3.89 (13.40) | <0.0001 |
| Visceral fat level | 8.65 ± 5.07 (7.00) | 2.00 ± 1.08 (2.00) | <0.0001 |
| Arm circumference (cm) | 32.80 ± 4.00 (32.00) | 26.59 ± 3.14 (26.00) | <0.0001 |
| Waist circumference (cm) | 98.48 ± 14.19 (97.00) | 73.70 ± 9.18 (72.00) | <0.0001 |
| Hip circumference (cm) | 113.20 ± 9.90 (111.00) | 97.65 ± 4.83 (97.00) | <0.0001 |
| Waist-to-hip ratio | 0.86 ± 0.08 (0.87) | 0.75 ± 0.07 (0.72) | <0.0001 |
| Waist-to-height ratio | 0.56 ± 0.07 (0.55) | 0.43 ± 0.04 (0.42) | <0.0001 |
| The inflammatory cytokines | |||
| hsCRP (mg/L) | 2.46 ± 3.55 (1.15) | 0.53 ± 0.51 (0.38) | <0.0001 |
| IL-1β (pg/mL) | 0.89 ± 1.70 (0.42) | 0.78 ± 0.93 (0.46) | 0.8746 |
| IL-6 (pg/mL) | 19.49 ± 42.89 (3.36) | 13.42 ± 37.68 (0.71) | 0.0201 |
| TNF-α (pg/mL) | 5.63 ± 4.30 (4.92) | 3.44 ± 1.43 (3.44) | <0.0001 |
| IFN-γ (pg/mL) | 21.35 ± 3.40 (21.84) | 19.63 ± 2.51 (18.77) | 0.0216 |
| WBC (103/µL) | 6.91 ± 1.58 (6.57) | 6.07 ± 1.32 (5.92) | 0.0016 |
| AC | WC | HC | WHR | WHtR | BMI | TBFM | VFL | |
|---|---|---|---|---|---|---|---|---|
| r (CI 95%) | r (CI 95%) | r (CI 95%) | r (CI 95%) | r (CI 95%) | r (CI 95%) | r (CI 95%) | r (CI 95%) | |
| hsCRP (mg/L) | 0.5238 (0.3808 to 0.6423) *** | 0.5389 (0.3986 to 0.6545) *** | 0.5332 (0.3920 to 0.6499) *** | 0.4361 (0.2793 to 0.5703) *** | 0.6005 (0.4726 to 0.7037) *** | 0.6419 (0.5233 to 0.7361) *** | 0.6369 (0.5171 to 0.7322) *** | 0.5970 (0.4683 to 0.7008) *** |
| IL-1β (pg/mL) | −0.0507 (−0.2273 to 0.1290) | −0.03595 (−0.2132 to 0.1436) | −0.0645 (−0.2404 to 0.1153) | 0.0135 (−0.1654 to 0.1917) | −0.0091 (−0.1874 to 0.1697) | −0.0058 (−0.1843 to 0.1729) | −0.0379 (−0.2151 to 0.1416) | 0.0387 (−0.1408 to 0.2158) |
| IL-6 (pg/mL) | 0.0924 (−0.0875 to 0.2666) | 0.1734 (−0.0053 to 0.3414) | 0.1212 (−0.0586 to 0.2934) | 0.2026 (0.0248 to 0.3679) * | 0.2540 (0.0789 to 0.4138) ** | 0.2265 (0.0499 to 0.3894) * | 0.1979 (0.0200 to 0.3636) * | 0.2354 (0.0593 to 0.3973) * |
| TNF-α (pg/mL) | 0.2771 (0.1036 to 0.4342) ** | 0.3356 (0.1670 to 0.4851) *** | 0.2619 (0.0873 to 0.4208) ** | 0.3089 (0.1379 to 0.4620) ** | 0.2973 (0.1253 to 0.4519) ** | 0.2499 (0.0746 to 0.4102) ** | 0.1839 (0.0054 to 0.3510) * | 0.3002 (0.1284 to 0.4544) ** |
| IFN-γ (pg/mL) | 0.1695 (−0.0093 to 0.3379) | 0.1518 (−0.0275 to 0.3217) | 0.2149 (0.0377 to 0.3790) * | 0.0774 (−0.1026 to 0.2525) | 0.1401 (−0.0395 to 0.3109) | 0.1977 (0.0197 to 0.3634) * | 0.1814 (0.0028 to 0.3487) * | 0.1661 (−0.0128 to 0.3348) |
| WBC (×103/µL) | 0.2405 (0.0646 to 0.4018) * | 0.3097 (0.1388 to 0.4627) ** | 0.4417 (0.2856 to 0.5749) *** | 0.1863 (0.0079 to 0.3531) * | 0.3327 (0.1639 to 0.4826) *** | 0.3799 (0.2159 to 0.5230) *** | 0.4058 (0.2450 to 0.5449) *** | 0.3187 (0.1485 to 0.4705) ** |
| Group 1 (n = 71) Mean ± SD (Median) | Group 2 (n = 37) Mean ± SD (Median) | Group 3 (n = 20) Mean ± SD (Median) | p-Value | Post Hoc Test p < 0.05 | |
|---|---|---|---|---|---|
| AC | 29.08 ± 3.88 (29.00) | 32.22 ± 4.17 (32.00) | 35.60 ± 4.52 (37.00) | <0.0001 * | Group 2 vs. Group 3 NS ‡ |
| WC | 84.92 ± 14.36 (86.00) | 93.86 ± 14.84 (93.00) | 109.40 ± 16.71 (107.50) | <0.0001 * | Yes ‡ |
| HC | 104.40 ± 8.45 (104.00) | 110.60 ± 11.18 (109.00) | 120.40 ± 11.02 (119.50) | <0.0001 ⸷ | Yes § |
| WHR | 0.80 ± 0.09 (0.81) | 0.84 ± 0.10 (0.84) | 0.90 ± 0.08 (0.92) | 0.0005 * | Yes ‡ |
| WHtR | 0.49 ± 0.06 (0.49) | 0.54 ± 0.07 (0.54) | 0.62 ± 0.08 (0.63) | <0.0001 * | Yes ‡ |
| BMI | 25.12 ± 4.26 (25.30) | 29.51 ± 5.16 (28.70) | 36.30 ± 6.59 (37.95) | <0.0001 * | Yes ‡ |
| TBFM | 18.20 ± 7.52 (18.20) | 26.30 ± 10.95 (26.30) | 45.20 ± 17.53 (45.20) | <0.0001 * | Yes ‡ |
| VFL | 4.52 ± 3.08 (4.00) | 7.62 ± 5.11 (7.00) | 12.95 ± 6.35 (13.00) | <0.0001 * | Yes ‡ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosovski, I.B.; Bacârea, V.; Ghiga, D.; Ciurea, C.N.; Cucoranu, D.C.; Hutanu, A.; Bacârea, A. Exploring the Link between Inflammatory Biomarkers and Adipometrics in Healthy Young Adults Aged 20–35 Years. Nutrients 2024, 16, 257. https://doi.org/10.3390/nu16020257
Kosovski IB, Bacârea V, Ghiga D, Ciurea CN, Cucoranu DC, Hutanu A, Bacârea A. Exploring the Link between Inflammatory Biomarkers and Adipometrics in Healthy Young Adults Aged 20–35 Years. Nutrients. 2024; 16(2):257. https://doi.org/10.3390/nu16020257
Chicago/Turabian StyleKosovski, Irina Bianca, Vladimir Bacârea, Dana Ghiga, Cristina Nicoleta Ciurea, Dragos Constantin Cucoranu, Adina Hutanu, and Anca Bacârea. 2024. "Exploring the Link between Inflammatory Biomarkers and Adipometrics in Healthy Young Adults Aged 20–35 Years" Nutrients 16, no. 2: 257. https://doi.org/10.3390/nu16020257
APA StyleKosovski, I. B., Bacârea, V., Ghiga, D., Ciurea, C. N., Cucoranu, D. C., Hutanu, A., & Bacârea, A. (2024). Exploring the Link between Inflammatory Biomarkers and Adipometrics in Healthy Young Adults Aged 20–35 Years. Nutrients, 16(2), 257. https://doi.org/10.3390/nu16020257