Protein Restriction in Metabolic Health: Lessons from Rodent Models
Abstract
1. Introduction
2. The Effects of Protein Intake Rate on Adults and the Elderly
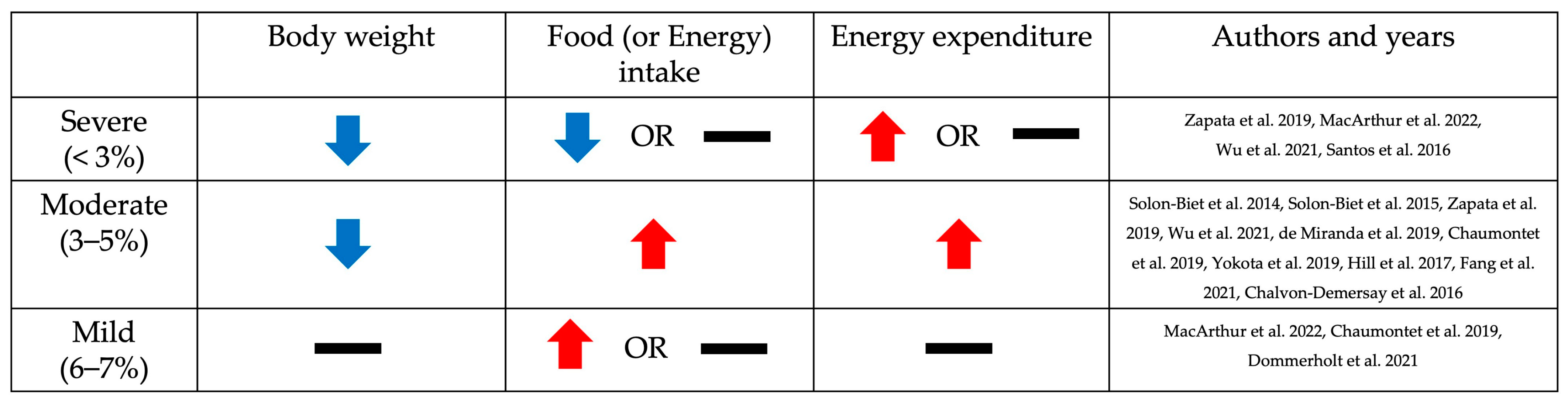
3. Phenotypic Changes Resulting from Low Dietary Protein Intake Based on the Level of Restriction: Body Weight, Food Intake, and Energy Expenditure
3.1. Body Weight
3.2. Food (or Energy) Intake
3.3. Energy Expenditure
4. Molecular Mechanisms Underlying Protein Restriction-Induced Metabolic Changes
4.1. Dietary Protein Restriction and FGF21
4.2. A Key Mechanism Underlying FGF21-Mediated Metabolic Changes
4.3. A Potential New Mechanism Responsible for FGF21 Induction
5. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mercken, E.M.; Carboneau, B.A.; Krzysik-Walker, S.M.; de Cabo, R. Of mice and men: The benefits of caloric restriction, exercise, and mimetics. Ageing Res. Rev. 2012, 11, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Solon-Biet, S.M.; McMahon, A.C.; Ballard, J.W.O.; Ruohonen, K.; Wu, L.E.; Cogger, V.C.; Warren, A.; Huang, X.; Pichaud, N.; Melvin, R.G. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014, 19, 418–430. [Google Scholar] [CrossRef]
- Solon-Biet, S.M.; Wahl, D.; Raubenheimer, D.; Cogger, V.C.; Le Couteur, D.G.; Simpson, S.J. The geometric framework: An approach for studying the impact of nutrition on healthy aging. Drug Discov. Today Dis. Models 2018, 27, 61–68. [Google Scholar] [CrossRef]
- Pezeshki, A.; Chelikani, P.K. Low protein diets and energy balance: Mechanisms of action on energy intake and expenditure. Front. Nutr. 2021, 8, 655833. [Google Scholar] [CrossRef]
- Park, Y.J.; Chung, S.; Hwang, J.-T.; Shon, J.; Kim, E. A review of recent evidence of dietary protein intake and health. Nutr. Res. Pract. 2022, 16, S37–S46. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Chung, S.; Hwang, J.-T.; Park, Y.J. 2020 Korean Dietary Reference Intakes for Protein: Estimation of protein requirements and the status of dietary protein intake in the Korean population. J. Nutr. Health 2022, 55, 10–20. [Google Scholar] [CrossRef]
- Awasthi, S.; Chauhan, M.; Pandey, M.; Singh, S.; Singh, U. Energy and protein intake during pregnancy in relation to preterm birth: A case control study. Indian Pediatr. 2015, 52, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Jyväkorpi, S.; Urtamo, A.; Kivimäki, M.; Strandberg, T. Macronutrient composition and sarcopenia in the oldest-old men: The Helsinki Businessmen Study (HBS). Clin. Nutr. 2020, 39, 3839–3841. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Locquet, M.; Touvier, M.; Reginster, J.-Y.; Bruyère, O. Association between dietary nutrient intake and sarcopenia in the SarcoPhAge study. Aging Clin. Exp. Res. 2019, 31, 815–824. [Google Scholar] [CrossRef]
- Shaw, S.; Dennison, E.; Cooper, C. Epidemiology of sarcopenia: Determinants throughout the lifecourse. Calcif. Tissue Int. 2017, 101, 229–247. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; von Kries, R.; Closa, R.; Escribano, J.; Scaglioni, S.; Giovannini, M.; Beyer, J.; Demmelmair, H.; Gruszfeld, D.; Dobrzanska, A.; et al. Lower protein in infant formula is associated with lower weight up to age 2 y: A randomized clinical trial. Am. J. Clin. Nutr. 2009, 89, 1836–1845. [Google Scholar] [CrossRef] [PubMed]
- Collell, R.; Closa-Monasterolo, R.; Ferré, N.; Luque, V.; Koletzko, B.; Grote, V.; Janas, R.; Verduci, E.; Escribano, J. Higher protein intake increases cardiac function parameters in healthy children: Metabolic programming by infant nutrition—Secondary analysis from a clinical trial. Pediatr. Res. 2016, 79, 880–888. [Google Scholar] [CrossRef]
- Oropeza-Ceja, L.G.; Rosado, J.L.; Ronquillo, D.; García, O.P.; Caamaño, M.d.C.; García-Ugalde, C.; Viveros-Contreras, R.; Duarte-Vázquez, M.Á. Lower protein intake supports normal growth of full-term infants fed formula: A randomized controlled trial. Nutrients 2018, 10, 886. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Choi, J.-E.; Hwang, H.-S. Protein supplementation improves muscle mass and physical performance in undernourished prefrail and frail elderly subjects: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2018, 108, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Kimura, Y.; Ishiyama, D.; Nishio, N.; Otobe, Y.; Tanaka, T.; Ohji, S.; Koyama, S.; Sato, A.; Suzuki, M. Synergistic effect of bodyweight resistance exercise and protein supplementation on skeletal muscle in sarcopenic or dynapenic older adults. Geriatr. Gerontol. Int. 2019, 19, 429–437. [Google Scholar] [CrossRef]
- Westerterp-Plantenga, M.S.; Lemmens, S.G.; Westerterp, K.R. Dietary protein-its role in satiety, energetics, weight loss and health. Br. J. Nutr. 2012, 108, S105–S112. [Google Scholar] [CrossRef]
- Sutton, E.F.; Bray, G.A.; Burton, J.H.; Smith, S.R.; Redman, L.M. No evidence for metabolic adaptation in thermic effect of food by dietary protein. Obesity 2016, 24, 1639–1642. [Google Scholar] [CrossRef]
- Mittendorfer, B.; Klein, S.; Fontana, L. A word of caution against excessive protein intake. Nat. Rev. Endocrinol. 2020, 16, 59–66. [Google Scholar] [CrossRef]
- Fappi, A.; Mittendorfer, B. Dietary protein intake and obesity-associated cardiometabolic function. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 380. [Google Scholar] [CrossRef]
- Malik, V.S.; Li, Y.; Tobias, D.K.; Pan, A.; Hu, F.B. Dietary protein intake and risk of type 2 diabetes in US men and women. Am. J. Epidemiol. 2016, 183, 715–728. [Google Scholar] [CrossRef]
- Lang, S.; Martin, A.; Farowski, F.; Wisplinghoff, H.; Vehreschild, M.J.; Liu, J.; Krawczyk, M.; Nowag, A.; Kretzschmar, A.; Herweg, J. High protein intake is associated with histological disease activity in patients with NAFLD. Hepatol. Commun. 2020, 4, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Solon-Biet, S.M.; Mitchell, S.J.; Coogan, S.C.; Cogger, V.C.; Gokarn, R.; McMahon, A.C.; Raubenheimer, D.; de Cabo, R.; Simpson, S.J.; Le Couteur, D.G. Dietary protein to carbohydrate ratio and caloric restriction: Comparing metabolic outcomes in mice. Cell Rep. 2015, 11, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.E.; Suarez, J.A.; Brandhorst, S.; Balasubramanian, P.; Cheng, C.-W.; Madia, F.; Fontana, L.; Mirisola, M.G.; Guevara-Aguirre, J.; Wan, J. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014, 19, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Cummings, N.E.; Apelo, S.I.A.; Neuman, J.C.; Kasza, I.; Schmidt, B.A.; Cava, E.; Spelta, F.; Tosti, V.; Syed, F.A. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep. 2016, 16, 520–530. [Google Scholar] [CrossRef]
- Hsu, K.-J.; Chien, K.-Y.; Tsai, S.-C.; Tsai, Y.-S.; Liao, Y.-H.; Chen, J.-J.; Chen, Y.-R.; Chen, C.-N. Effects of exercise alone or in combination with high-protein diet on muscle function, aerobic capacity, and physical function in middle-aged obese adults: A randomized controlled trial. J. Nutr. Health Aging 2021, 25, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, N.; Ehsani, B.; Mirmiran, P.; Hojjat, P.; Azizi, F. Association of dietary proportions of macronutrients with visceral adiposity index: Non-substitution and iso-energetic substitution models in a prospective study. Nutrients 2015, 7, 8859–8870. [Google Scholar] [CrossRef]
- Zapata, R.C.; Singh, A.; Pezeshki, A.; Avirineni, B.S.; Patra, S.; Chelikani, P.K. Low-Protein Diets with Fixed Carbohydrate Content Promote Hyperphagia and Sympathetically Mediated Increase in Energy Expenditure. Mol. Nutr. Food Res. 2019, 63, 1900088. [Google Scholar] [CrossRef]
- MacArthur, M.R.; Mitchell, S.J.; Chadaideh, K.S.; Treviño-Villarreal, J.H.; Jung, J.; Kalafut, K.C.; Reynolds, J.S.; Mann, C.G.; Trocha, K.M.; Tao, M. Multiomics assessment of dietary protein titration reveals altered hepatic glucose utilization. Cell Rep. 2022, 40, 111187. [Google Scholar] [CrossRef]
- Wu, Y.; Li, B.; Li, L.; Mitchell, S.E.; Green, C.L.; D’Agostino, G.; Wang, G.; Wang, L.; Li, M.; Li, J. Very-low-protein diets lead to reduced food intake and weight loss, linked to inhibition of hypothalamic mTOR signaling, in mice. Cell Metab. 2021, 33, 888–904.e886. [Google Scholar] [CrossRef]
- Santos, E.W.; de Oliveira, D.C.; Hastreiter, A.; Beltran, J.S.d.O.; Rogero, M.M.; Fock, R.A.; Borelli, P. High-fat diet or low-protein diet changes peritoneal macrophages function in mice. Nutrire 2016, 41, 6. [Google Scholar] [CrossRef]
- de Miranda, M.B.; Lanna, M.F.; Nascimento, A.L.B.; de Paula, C.A.; de Souza, M.E.; Felipetto, M.; da Silva Barcelos, L.; de Moura, S.A.L. Hydroalcoholic extract of Brazilian green propolis modulates inflammatory process in mice submitted to a low protein diet. Biomed. Pharmacother. 2019, 109, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Chaumontet, C.; Azzout-Marniche, D.; Blais, A.; Piedcoq, J.; Tomé, D.; Gaudichon, C.; Even, P.C. Low-protein and methionine, high-starch diets increase energy intake and expenditure, increase FGF21, decrease IGF-1, and have little effect on adiposity in mice. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2019, 316, R486–R501. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.-I.; Ando, M.; Aoyama, S.; Nakamura, K.; Shibata, S. Leucine restores murine hepatic triglyceride accumulation induced by a low-protein diet by suppressing autophagy and excessive endoplasmic reticulum stress. Amino Acids 2016, 48, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.-I.; Nakamura, K.; Ando, M.; Haraguchi, A.; Omori, K.; Shibata, S. A low-protein diet eliminates the circadian rhythm of serum insulin and hepatic lipid metabolism in mice. J. Nutr. Biochem. 2019, 63, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.M.; Laeger, T.; Albarado, D.C.; McDougal, D.H.; Berthoud, H.-R.; Münzberg, H.; Morrison, C.D. Low protein-induced increases in FGF21 drive UCP1-dependent metabolic but not thermoregulatory endpoints. Sci. Rep. 2017, 7, 8209. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Ghosh, S.; Sims, L.C.; Stone, K.P.; Hill, C.M.; Spires, D.; Ilatovskaya, D.V.; Morrison, C.D.; Gettys, T.W.; Stadler, K. FGF21 prevents low-protein diet-induced renal inflammation in aged mice. Am. J. Physiol.-Ren. Physiol. 2021, 321, F356–F368. [Google Scholar] [CrossRef] [PubMed]
- Dommerholt, M.B.; Blankestijn, M.; Vieira-Lara, M.A.; van Dijk, T.H.; Wolters, H.; Koster, M.H.; Gerding, A.; van Os, R.P.; Bloks, V.W.; Bakker, B.M. Short-term protein restriction at advanced age stimulates FGF21 signalling, energy expenditure and browning of white adipose tissue. FEBS J. 2021, 288, 2257–2277. [Google Scholar] [CrossRef] [PubMed]
- Chalvon-Demersay, T.; Even, P.C.; Tomé, D.; Chaumontet, C.; Piedcoq, J.; Gaudichon, C.; Azzout-Marniche, D. Low-protein diet induces, whereas high-protein diet reduces hepatic FGF21 production in mice, but glucose and not amino acids up-regulate FGF21 in cultured hepatocytes. J. Nutr. Biochem. 2016, 36, 60–67. [Google Scholar] [CrossRef]
- Lin, X.; Liu, Y.B.; Hu, H. Metabolic role of fibroblast growth factor 21 in liver, adipose and nervous system tissues. Biomed. Rep. 2017, 6, 495–502. [Google Scholar] [CrossRef]
- Laeger, T.; Henagan, T.M.; Albarado, D.C.; Redman, L.M.; Bray, G.A.; Noland, R.C.; Münzberg, H.; Hutson, S.M.; Gettys, T.W.; Schwartz, M.W. FGF21 is an endocrine signal of protein restriction. J. Clin. Investig. 2014, 124, 3913–3922. [Google Scholar] [CrossRef]
- Hill, C.M.; Albarado, D.C.; Coco, L.G.; Spann, R.A.; Khan, M.S.; Qualls-Creekmore, E.; Burk, D.H.; Burke, S.J.; Collier, J.J.; Yu, S. FGF21 is required for protein restriction to extend lifespan and improve metabolic health in male mice. Nat. Commun. 2022, 13, 1897. [Google Scholar] [CrossRef]
- Solon-Biet, S.M.; Cogger, V.C.; Pulpitel, T.; Heblinski, M.; Wahl, D.; McMahon, A.C.; Warren, A.; Durrant-Whyte, J.; Walters, K.A.; Krycer, J.R. Defining the nutritional and metabolic context of FGF21 using the geometric framework. Cell Metab. 2016, 24, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Garza, Ú.; Torres-Oteros, D.; Yarritu-Gallego, A.; Marrero, P.F.; Haro, D.; Relat, J. Fibroblast growth factor 21 and the adaptive response to nutritional challenges. Int. J. Mol. Sci. 2019, 20, 4692. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.M.; Laeger, T.; Dehner, M.; Albarado, D.C.; Clarke, B.; Wanders, D.; Burke, S.J.; Collier, J.J.; Qualls-Creekmore, E.; Solon-Biet, S.M. FGF21 signals protein status to the brain and adaptively regulates food choice and metabolism. Cell Rep. 2019, 27, 2934–2947.e2933. [Google Scholar] [CrossRef] [PubMed]
- Owen, B.M.; Ding, X.; Morgan, D.A.; Coate, K.C.; Bookout, A.L.; Rahmouni, K.; Kliewer, S.A.; Mangelsdorf, D.J. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 2014, 20, 670–677. [Google Scholar] [CrossRef]
- Brown, M.R.; Fisher, L.A.; Spiess, J.; RIVIER, C.; RIVIER, J.; VALE, W. Corticotropin-releasing factor: Actions on the sympathetic nervous system and metabolism. Endocrinology 1982, 111, 928–931. [Google Scholar] [CrossRef] [PubMed]
- Arase, K.; York, D.; Shimizu, H.; Shargill, N.; Bray, G. Effects of corticotropin-releasing factor on food intake and brown adipose tissue thermogenesis in rats. Am. J. Physiol.-Endocrinol. Metab. 1988, 255, E255–E259. [Google Scholar] [CrossRef]
- Liu, M.; Cao, H.; Hou, Y.; Sun, G.; Li, D.; Wang, W. Liver plays a major role in FGF-21 mediated glucose homeostasis. Cell. Physiol. Biochem. 2018, 45, 1423–1433. [Google Scholar] [CrossRef]
- Kleiner, S.; Douris, N.; Fox, E.C.; Mepani, R.J.; Verdeguer, F.; Wu, J.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E.; Spiegelman, B.M. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012, 26, 271–281. [Google Scholar]
- Xu, J.; Stanislaus, S.; Chinookoswong, N.; Lau, Y.Y.; Hager, T.; Patel, J.; Ge, H.; Weiszmann, J.; Lu, S.-C.; Graham, M. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models—Association with liver and adipose tissue effects. Am. J. Physiol.-Endocrinol. Metab. 2009, 297, E1105–E1114. [Google Scholar] [CrossRef]
- Geng, L.; Lam, K.S.; Xu, A. The therapeutic potential of FGF21 in metabolic diseases: From bench to clinic. Nat. Rev. Endocrinol. 2020, 16, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Coskun, T.; Bina, H.A.; Schneider, M.A.; Dunbar, J.D.; Hu, C.C.; Chen, Y.; Moller, D.E.; Kharitonenkov, A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 2008, 149, 6018–6027. [Google Scholar] [CrossRef]
- Serin, Y.; Tek, N.A. Effect of circadian rhythm on metabolic processes and the regulation of energy balance. Ann. Nutr. Metab. 2019, 74, 322–330. [Google Scholar] [CrossRef]
- Oishi, K.; Uchida, D.; Itoh, N. Low-carbohydrate, high-protein diet affects rhythmic expression of gluconeogenic regulatory and circadian clock genes in mouse peripheral tissues. Chronobiol. Int. 2012, 29, 799–809. [Google Scholar] [CrossRef]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Pendergast, J.S.; Branecky, K.L.; Yang, W.; Ellacott, K.L.; Niswender, K.D.; Yamazaki, S. High-fat diet acutely affects circadian organisation and eating behavior. Eur. J. Neurosci. 2013, 37, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Chaix, A.; Lin, T.; Le, H.D.; Chang, M.W.; Panda, S. Time-restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metab. 2019, 29, 303–319.e304. [Google Scholar] [CrossRef] [PubMed]
- Mezhnina, V.; Ebeigbe, O.P.; Velingkaar, N.; Poe, A.; Sandlers, Y.; Kondratov, R.V. Circadian clock controls rhythms in ketogenesis by interfering with PPARα transcriptional network. Proc. Natl. Acad. Sci. USA 2022, 119, e2205755119. [Google Scholar] [CrossRef]
- Oosterman, J.E.; Kalsbeek, A.; la Fleur, S.E.; Belsham, D.D. Impact of nutrients on circadian rhythmicity. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2015, 308, R337–R350. [Google Scholar] [CrossRef]
- Hardin, P.E.; Panda, S. Circadian timekeeping and output mechanisms in animals. Curr. Opin. Neurobiol. 2013, 23, 724–731. [Google Scholar] [CrossRef]
- Pickel, L.; Sung, H.-K. Feeding rhythms and the circadian regulation of metabolism. Front. Nutr. 2020, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Charoensuksai, P.; Xu, W. PPARs in rhythmic metabolic regulation and implications in health and disease. PPAR Res. 2010, 2010, 243643. [Google Scholar] [CrossRef]
- Tong, X.; Muchnik, M.; Chen, Z.; Patel, M.; Wu, N.; Joshi, S.; Rui, L.; Lazar, M.A.; Yin, L. Transcriptional repressor E4-binding protein 4 (E4BP4) regulates metabolic hormone fibroblast growth factor 21 (FGF21) during circadian cycles and feeding. J. Biol. Chem. 2010, 285, 36401–36409. [Google Scholar] [CrossRef] [PubMed]
- Erickson, A.; Moreau, R. The regulation of FGF21 gene expression by metabolic factors and nutrients. Horm. Mol. Biol. Clin. Investig. 2017, 30, 20160016. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Solt, L.A.; Burris, T.P. Regulation of FGF21 expression and secretion by retinoic acid receptor-related orphan receptor α. J. Biol. Chem. 2010, 285, 15668–15673. [Google Scholar] [CrossRef] [PubMed]
- Estall, J.L.; Ruas, J.L.; Choi, C.S.; Laznik, D.; Badman, M.; Maratos-Flier, E.; Shulman, G.I.; Spiegelman, B.M. PGC-1α negatively regulates hepatic FGF21 expression by modulating the heme/Rev-Erbα axis. Proc. Natl. Acad. Sci. USA 2009, 106, 22510–22515. [Google Scholar] [CrossRef]
- Lundåsen, T.; Hunt, M.C.; Nilsson, L.-M.; Sanyal, S.; Angelin, B.; Alexson, S.E.; Rudling, M. PPARα is a key regulator of hepatic FGF21. Biochem. Biophys. Res. Commun. 2007, 360, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Capelo-Diz, A.; Lachiondo-Ortega, S.; Fernández-Ramos, D.; Cañas-Martín, J.; Goikoetxea-Usandizaga, N.; Serrano-Maciá, M.; González-Rellan, M.J.; Mosca, L.; Blazquez-Vicens, J.; Tinahones-Ruano, A. Hepatic levels of S-adenosylmethionine regulate the adaptive response to fasting. Cell Metab. 2023, 35, 1373–1389.e1378. [Google Scholar] [CrossRef]
- Kosakamoto, H.; Obata, F.; Kuraishi, J.; Aikawa, H.; Okada, R.; Johnstone, J.N.; Onuma, T.; Piper, M.D.; Miura, M. Early-adult methionine restriction reduces methionine sulfoxide and extends lifespan in Drosophila. bioRxiv 2023. [Google Scholar] [CrossRef]
- Chung, S.; Chung, M.-Y.; Choi, H.-K.; Park, J.H.; Hwang, J.-T.; Joung, H. Animal protein intake is positively associated with metabolic syndrome risk factors in middle-aged Korean men. Nutrients 2020, 12, 3415. [Google Scholar] [CrossRef]
- Chung, S.; Park, J.H.; Joung, H.; Ha, K.; Shin, S. Amino acid intake with protein food source and incident dyslipidemia in Korean adults from the Ansan and Ansung Study and the Health Examinee Study. Front. Nutr. 2023, 10, 1195349. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Ogura, Y.; Monno, I.; Koya, D. The impact of dietary protein intake on longevity and metabolic health. EBioMedicine 2019, 43, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Latimer, M.N.; Freij, K.W.; Cleveland, B.M.; Biga, P.R. Physiological and molecular mechanisms of methionine restriction. Front. Endocrinol. 2018, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ren, B.; Zhang, Q.; Chu, C.; Zhao, Z.; Wu, J.; Zhao, W.; Liu, Z.; Liu, X. Methionine restriction alleviates high-fat diet-induced obesity: Involvement of diurnal metabolism of lipids and bile acids. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2020, 1866, 165908. [Google Scholar] [CrossRef]
- Richardson, N.E.; Konon, E.N.; Schuster, H.S.; Mitchell, A.T.; Boyle, C.; Rodgers, A.C.; Finke, M.; Haider, L.R.; Yu, D.; Flores, V. Lifelong restriction of dietary branched-chain amino acids has sex-specific benefits for frailty and life span in mice. Nat. Aging 2021, 1, 73–86. [Google Scholar] [CrossRef]
- Mu, W.-C.; VanHoosier, E.; Elks, C.M.; Grant, R.W. Long-term effects of dietary protein and branched-chain amino acids on metabolism and inflammation in mice. Nutrients 2018, 10, 918. [Google Scholar] [CrossRef]
- Lee, H.J.; Shon, J.; Park, Y.J. Association of NAFLD with FGF21 Polygenic Hazard Score, and Its Interaction with Protein Intake Level in Korean Adults. Nutrients 2023, 15, 2385. [Google Scholar] [CrossRef]
- Shon, J.; Han, Y.; Park, Y.J. Effects of Dietary Fat to Carbohydrate Ratio on Obesity Risk Depending on Genotypes of Circadian Genes. Nutrients 2022, 14, 478. [Google Scholar] [CrossRef]


| Life Stages | Initial Ages | Periods | Comparison Groups | Study Designs | Subject No. | Results | References |
|---|---|---|---|---|---|---|---|
| Adults (<65 years) | ≥18 years | - | Patients with biopsy-proven NAFLD with an NAS of 5–8 vs. patients with an NAS of 0–4 1 | Cross- sectional | 61 |
| [21] |
| 3 years | 5% increase in intake of protein (14.3 ± 2.6% and 14.2 ± 1.9% energy intake from protein for women and men, respectively) | Prospective cohort | 1254 |
| [26] | ||
| ≥24 years | - | Quintile of protein intake (14–22% energy of total protein) | Cross- sectional | 205,802 2 |
| [20] | |
| 50–65 years | - | LP diet (less than 10% of calories from proteins) vs. MP diet (10–19% of calories from proteins) vs. HP diet (20% or more of calories from proteins) | Cross- sectional | 6381 |
| [23] | |
| 12 weeks | Ex group 3 vs. Ex + HP group 3 vs. Control group 3 | RCT | 69 |
| [25] | ||
| 52–53 years | 43 days (mean) | LP diet (7–9% of protein diet) vs. MP diet (~50% more protein diet) | RCT | 38 |
| [24] | |
| Elderly (≥65 years) | ≥65 years | - | LP diet (less than 10% of calories from proteins) vs. MP diet (10–19% of calories from proteins) vs. HP diet (20% or more of calories from proteins) | Cross- sectional | 6381 |
| [23] |
| 70–85 years | 12 weeks | 0.8, 1.2, or 1.5 g/kg/day group | RCT | 120 |
| [14] | |
| 74.8 ± 5.9 years | - | Sarcopenia (70.2 ± 20.2 g protein/day) vs. non-sarcopenia (85 ± 28.3 g protein/day) | Cross- sectional | 331 |
| [9] | |
| 87 years (mean) | - | Robust (78 g protein/day) vs. Probable sarcopenia (72 g protein/day) vs. Sarcopenia (66 g protein/day) | Cross- sectional | 126 |
| [8] |
| Level of Protein Restriction | Low- Protein Diets | Control Diets | Rodent Models (Strain, Sex, Initial Age) | Periods | Body Weight | Food Intake (FI) or Energy Intake (EI) | Indirect Energy Expenditure | References |
|---|---|---|---|---|---|---|---|---|
| Severe (<3%) | 1% | 15% |
| 3 weeks | Decreased | EI: No significant difference initially and decreased from day 8 of dietary intervention | Initially increased, but its level significantly decreased from day 10 of dietary intervention | [27] |
| 20% |
| 12 weeks | Decreased | EI: Decreased | No significant difference | [29] | ||
| 2% | 12% |
| 5 weeks | Decreased | FI: No significant difference | - | [30] | |
| 18% |
| 1 week | Decreased | FI: Decreased | - | [28] | ||
| 2.5% | 20% |
| 12 weeks | Decreased | EI: No significant difference | Increased | [29] | |
| Moderate (3–5%) | 3% | 12% |
| 5–6 weeks | Decreased | - | - | [31] |
| 20% |
| 1 week | Decreased | FI: No significant difference | - | [34] | ||
| 8 weeks | Decreased | EI: Increased | Increased | [32] | |||
| 5% | 14–60% |
| 24 weeks | - | FI: Increased | - | [2] | |
| 14.5% |
| 3 weeks | - | FI: Increased | - | [38] | ||
| 15% |
| 3 weeks | Decreased | EI: Increased | Increased | [27] | ||
| 20% |
| 6 weeks | Decreased | FI: Increased | Increased | [35] | ||
| 12 weeks | Decreased | EI: Increased | Increased | [29] | |||
| 76 weeks | Decreased | - | - | [36] | |||
| 33% |
| 8 weeks | - | EI and FI: Increased | Increased | [22] | ||
| Mild (6–7%) | 6% | 18% |
| 1 week | No significant difference | FI: No significant difference | - | [28] |
| 20% |
| 8 weeks | No significant difference | EI: No significant difference | No significant difference | [32] | ||
| 7% | 20% |
| 12 weeks | No significant difference | FI: Increased | - | [37] |
| Level of Protein Restriction | Low Protein Diets | Control Diets | Rodent Models (Strain, Sex, Initial Age) | Periods | Fgf21 | References |
|---|---|---|---|---|---|---|
| Severe (<3%) | 1% | 20% |
| 12 weeks | Increased | [29] |
| 2% | 18% |
| 1 week | Increased | [28] | |
| 2.5% | 20% |
| 12 weeks | Increased | [29] | |
| Moderate (3–5%) | 3% | 20% |
| 8 weeks | Increased | [32] |
| 5% | 14.5% |
| 3 weeks | Increased | [38] | |
| 5% | 20% |
| 6 weeks | Increased | [35] | |
| 5% | 20% |
| 12 weeks | Increased | [29] | |
| 6% | 18% |
| 1 week | Increased | [28] | |
| Mild (6–7%) | 6% | 20% |
| 8 weeks | No significant difference | [32] |
| 7% | 20% |
| 12 weeks |
| [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, K.; Park, Y.J. Protein Restriction in Metabolic Health: Lessons from Rodent Models. Nutrients 2024, 16, 229. https://doi.org/10.3390/nu16020229
Na K, Park YJ. Protein Restriction in Metabolic Health: Lessons from Rodent Models. Nutrients. 2024; 16(2):229. https://doi.org/10.3390/nu16020229
Chicago/Turabian StyleNa, Khuhee, and Yoon Jung Park. 2024. "Protein Restriction in Metabolic Health: Lessons from Rodent Models" Nutrients 16, no. 2: 229. https://doi.org/10.3390/nu16020229
APA StyleNa, K., & Park, Y. J. (2024). Protein Restriction in Metabolic Health: Lessons from Rodent Models. Nutrients, 16(2), 229. https://doi.org/10.3390/nu16020229






