Diagnostic Ultrasound-Based Investigation of Central vs. Peripheral Arterial Changes Consequent to Low-Dose Caffeine Ingestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Protocols and Data Acquisition
2.3. Statistical Analysis
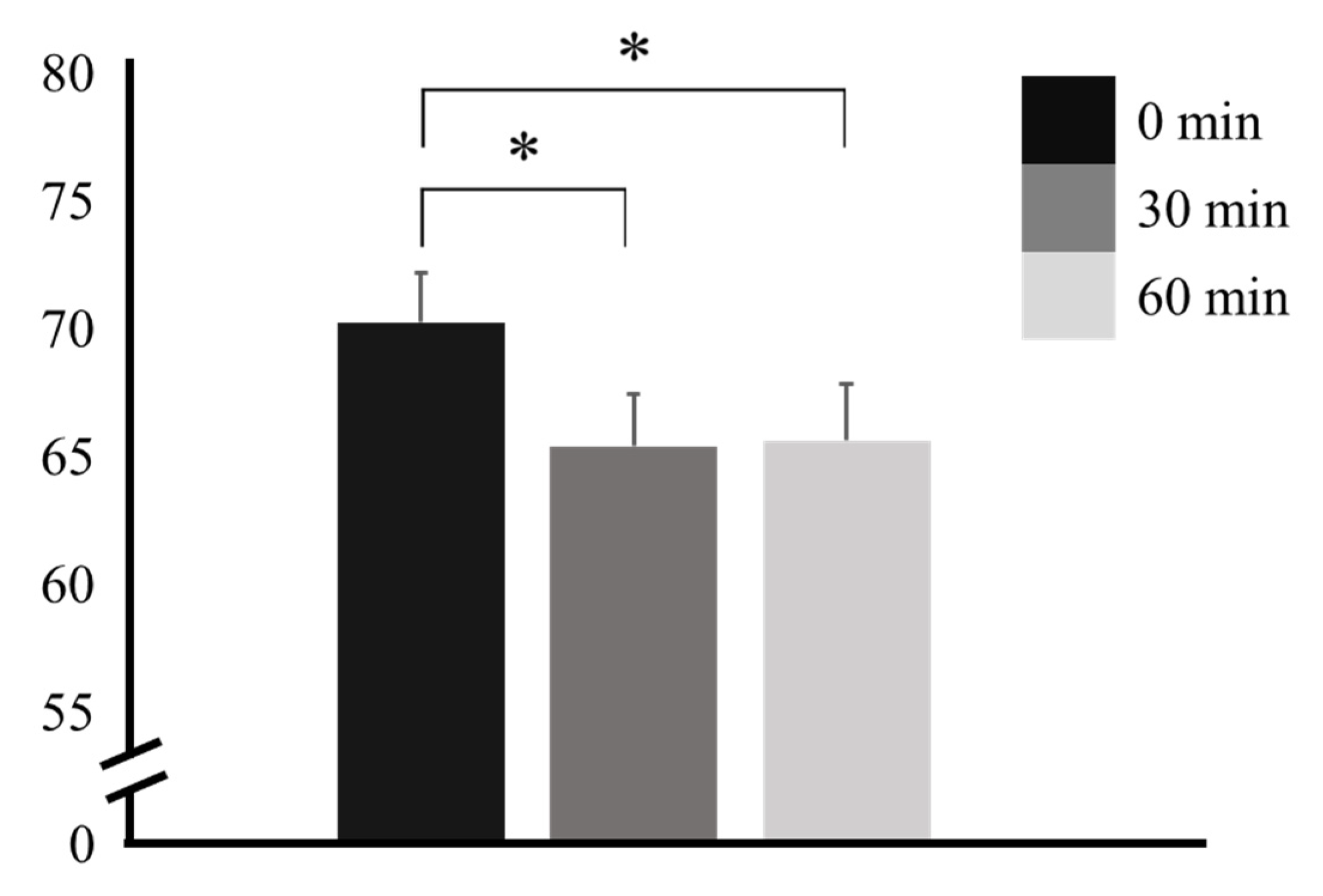
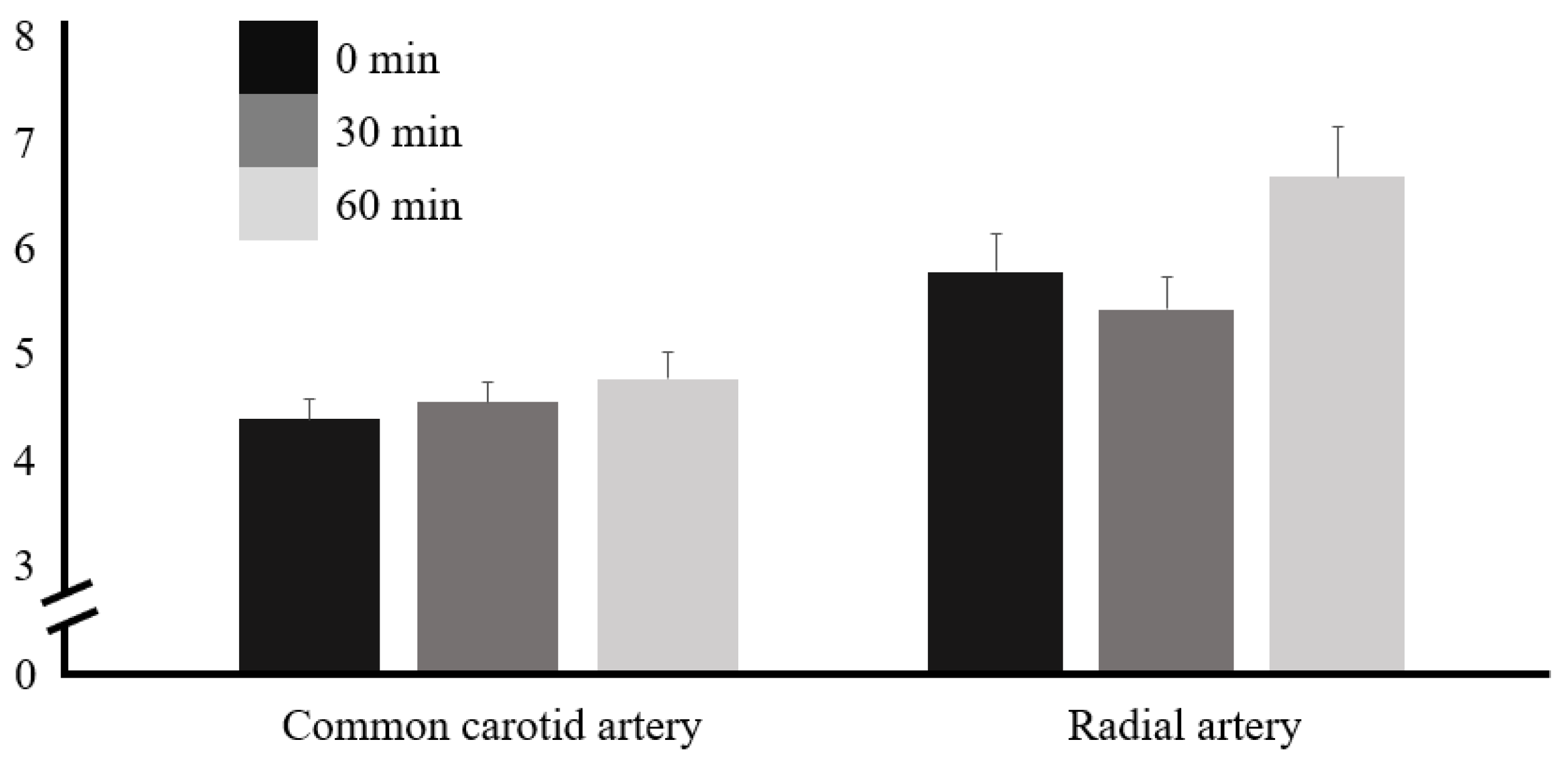
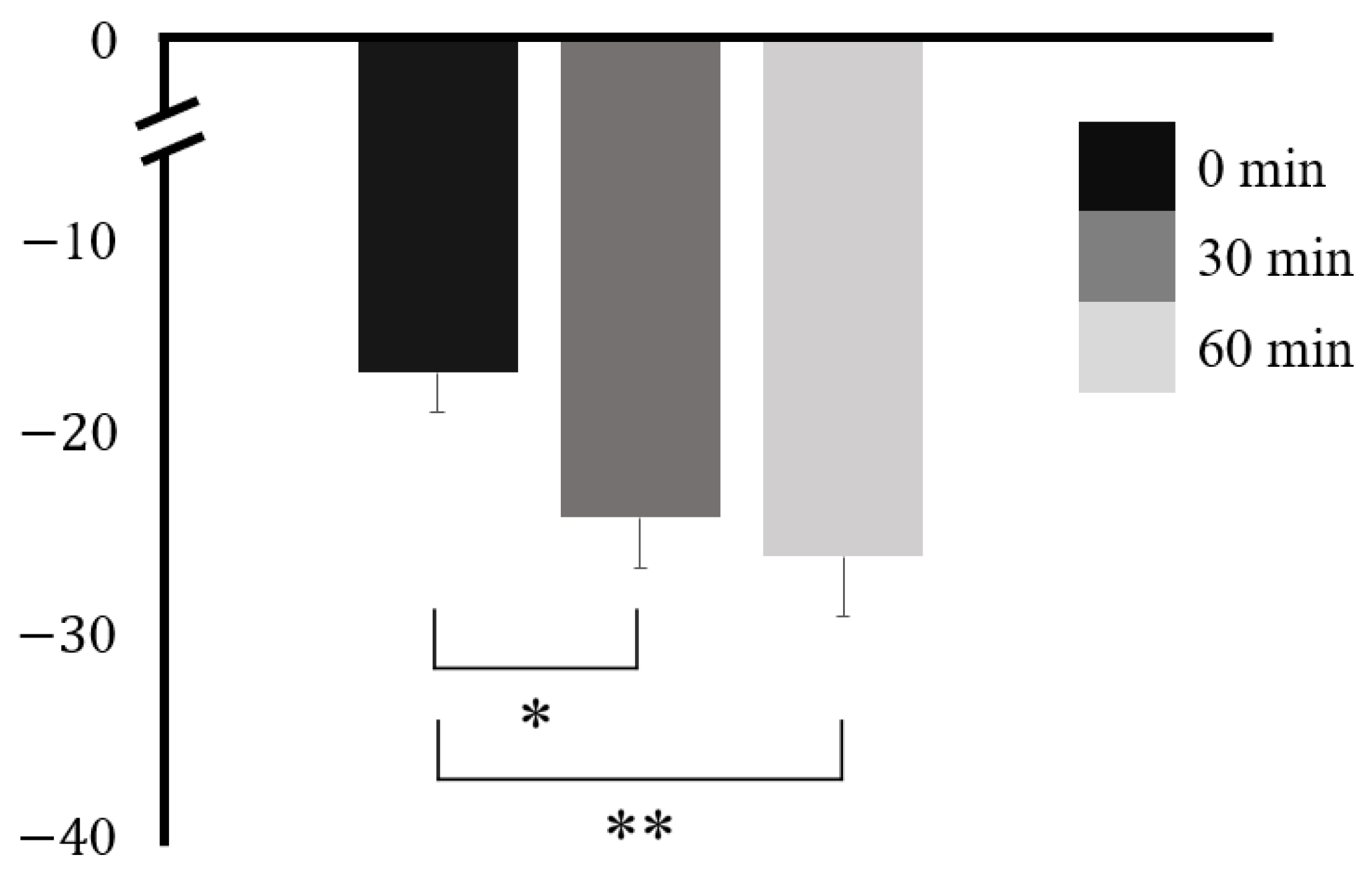
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heckman, M.A.; Weil, J.; Gonzalez de Mejia, E. Caffeine (1, 3, 7-trimethylxanthine) in Foods: A Comprehensive Review on Consumption, Functionality, Safety, and Regulatory Matters. J. Food Sci. 2010, 75, R77–R87. [Google Scholar] [CrossRef] [PubMed]
- Samoggia, A.; Riedel, B. Coffee consumption and purchasing behavior review: Insights for further research. Appetite 2018, 129, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Sökmen, B.; Armstrong, L.E.; Kraemer, W.J.; Casa, D.J.; Dias, J.C.; Judelson, D.A.; Maresh, C.M. Caffeine Use in Sports: Considerations for the Athlete. J. Strength Cond. Res. 2008, 22, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, M.; Shen, H.-Y.; Cherasse, Y.; Qu, W.-M.; Huang, Z.-L.; Bass, C.E.; Winsky-Sommerer, R.; Semba, K.; Fredholm, B.B.; Boison, D.; et al. Arousal Effect of Caffeine Depends on Adenosine A2A Receptors in the Shell of the Nucleus Accumbens. J. Neurosci. 2011, 31, 10067–10075. [Google Scholar] [CrossRef] [PubMed]
- Tabrizchi, R.; Bedi, S. Pharmacology of adenosine receptors in the vasculature. Pharmacol. Ther. 2001, 91, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Echeverri, D.; Montes, F.R.; Cabrera, M.; Galán, A.; Prieto, A. Caffeine’s Vascular Mechanisms of Action. Int. J. Vasc. Med. 2010, 2010, 834060. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Sebastião, A.M. Caffeine and Adenosine. J. Alzheimer’s Dis. 2010, 20, S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.; Singh, B.K.; Liehn, E.A.; Lim, S.Y.; Tikno, K.; Castano-Mayan, D.; Rattanasopa, C.; Nilcham, P.; Ghani, S.A.B.A.; Wu, Z.; et al. Caffeine prevents restenosis and inhibits vascular smooth muscle cell proliferation through the induction of autophagy. Autophagy 2022, 18, 2150–2160. [Google Scholar] [CrossRef]
- Cavalcante, J.W.S.; Santos, P.R.M., Jr.; de Menezes, M.G.F.; Marques, H.O.; Cavalcante, L.P.; Pacheco, W.S. Influence of caffeine on blood pressure and platelet aggregation. Arq. Bras. Cardiol. 2000, 75, 102–105. [Google Scholar] [CrossRef]
- Mahmud, A.; Feely, J. Acute Effect of Caffeine on Arterial Stiffness and Aortic Pressure Waveform. Hypertension 2001, 38, 227–231. [Google Scholar] [CrossRef]
- Nichols, W.W.; O’Rourke, M.F.; Vlachopoulos, C. McDonald’s Blood Flow in Arteries. Theorectical, Experimental and Clinical Principles; Hodder Arnold: London, UK, 1998; pp. 54–401. [Google Scholar]
- Breithaupt-Grögler, K.; Belz, G.G. Epidemiology of the arterial stiffness. Pathol. Biol. 1999, 47, 604–613. [Google Scholar] [PubMed]
- McVeigh, G.E.; Morgan, D.J.; Finkelstein, S.M.; Lemay, L.A.; Cohn, J.N. Vascular abnormalities associated with long-term cigarette smoking identified by arterial waveform analysis. Am. J. Med. 1997, 102, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Smits, P.; Lenders, J.W.M.; Thien, T. Caffeine and theophylline attenuate adenosine-induced vasodilation in humans. Clin. Pharmacol. Ther. 1990, 48, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Peerapen, P.; Thongboonkerd, V. Caffeine in Kidney Stone Disease: Risk or Benefit? Adv. Nutr. Int. Rev. J. 2018, 9, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Rudelle, S.; Ferruzzi, M.G.; Cristiani, I.; Moulin, J.; Macé, K.; Acheson, K.J.; Tappy, L. Effect of a Thermogenic Beverage on 24-Hour Energy Metabolism in Humans. Obesity 2007, 15, 349–355. [Google Scholar] [CrossRef]
- Ruxton, C.H.S. The impact of caffeine on mood, cognitive function, performance and hydration: A review of benefits and risks. Nutr. Bull. 2008, 33, 15–25. [Google Scholar] [CrossRef]
- Pohler, H. Caffeine Intoxication and Addiction. J. Nurse Pract. 2010, 6, 49–52. [Google Scholar] [CrossRef]
- Drake, C.; Roehrs, T.; Shambroom, J.; Roth, T. Caffeine Effects on Sleep Taken 0, 3, or 6 Hours before Going to Bed. J. Clin. Sleep Med. 2013, 9, 1195–1200. [Google Scholar] [CrossRef]
- Waring, W.S.; Goudsmit, J.; Marwick, J.; Webb, D.J.; Maxwell, S.R. Acute caffeine intake influences central more than peripheral blood pressure in young adults. Am. J. Hypertens. 2003, 16, 919–924. [Google Scholar] [CrossRef]
- Bailey, R.L.; Saldanha, L.G.; Gahche, J.J.; Dwyer, J.T. Estimating caffeine intake from energy drinks and dietary supplements in the United States. Nutr. Rev. 2014, 72, 9–13. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the safety of caffeine. EFSA J. 2015, 13, 4102. [Google Scholar] [CrossRef]
- Lee, J.-S.; Park, H.-S.; Han, S.; Tana, G.; Chang, M.-J. Study on relationship between caffeine intake level and metabolic syndrome and related diseases in Korean adults: 2013~2016 Korea National Health and Nutrition Examination Survey. J. Nutr. Health 2019, 52, 227–241. [Google Scholar] [CrossRef]
- Lim, H.S.; Hwang, J.Y.; Choi, J.C.; Kim, M. Assessment of caffeine intake in the Korean population. Food Addit. Contam. Part A 2015, 32, 1786–1798. [Google Scholar] [CrossRef] [PubMed]
- Barone, J.; Roberts, H. Caffeine consumption. Food Chem. Toxicol. 1996, 34, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Seifert, S.M.; Schaechter, J.L.; Hershorin, E.R.; Lipshultz, S.E. Health Effects of Energy Drinks on Children, Adolescents, and Young Adults. Pediatrics 2011, 127, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Massey, L.K.; Whiting, S.J. Caffeine, Urinary Calcium, Calcium Metabolism and Bone. J. Nutr. 1993, 123, 1611–1614. [Google Scholar] [CrossRef] [PubMed]
- Alsabri, S.G.; Mari, W.O.; Younes, S.; Elsadawi, M.A.; Oroszi, T.L. Kinetic and Dynamic Description of Caffeine. J. Caffeine Adenosine Res. 2018, 8, 3–9. [Google Scholar] [CrossRef]
- Calvo, J.L.; Fei, X.; Domínguez, R.; Pareja-Galeano, H. Caffeine and Cognitive Functions in Sports: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 868. [Google Scholar] [CrossRef]
- Ruíz-Moreno, C.; Lara, B.; de Souza, D.B.; Gutiérrez-Hellín, J.; Romero-Moraleda, B.; Cuéllar-Rayo, Á.; Del Coso, J. Acute caffeine intake increases muscle oxygen saturation during a maximal incremental exercise test. Br. J. Clin. Pharmacol. 2020, 86, 861–867. [Google Scholar] [CrossRef]
- Gonzaga, L.A.; Vanderlei, L.C.M.; Gomes, R.L.; Valenti, V.E. Caffeine affects autonomic control of heart rate and blood pressure recovery after aerobic exercise in young adults: A crossover study. Sci. Rep. 2017, 7, 14091. [Google Scholar] [CrossRef]
- Souza, D.B.; Del Coso, J.; Casonatto, J.; Polito, M.D. Acute effects of caffeine-containing energy drinks on physical performance: A systematic review and meta-analysis. Eur. J. Nutr. 2017, 56, 13–27. [Google Scholar] [CrossRef] [PubMed]
- McClaran, S.R.; Wetter, T.J. Low doses of caffeine reduce heart rate during submaximal cycle ergometry. J. Int. Soc. Sports Nutr. 2007, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.J.; Knowlton, R.G.; Brown, D.D. Caffeine Affects Heart Rate and Blood Pressure Response to Prolonged Walking. J. Cardiopulm. Rehabil. 1992, 12, 418–422. [Google Scholar] [CrossRef]
- Mosqueda-Garcia, R.; Tseng, C.-J.; Biaggioni, I.; Robertson, R.M.; Robertson, D. Effects of caffeine on baroreflex activity in humans. Clin. Pharmacol. Ther. 1990, 48, 568–574. [Google Scholar] [CrossRef]
- Dix, L.M.; van Bel, F.; Baerts, W.; Lemmers, P.M. Effects of caffeine on the preterm brain: An observational study. Early Hum. Dev. 2018, 120, 17–20. [Google Scholar] [CrossRef]
- Özkan, B.; Yüksel, N.; Anık, Y.; Altıntaş, O.; Demirci, A.; Çağlar, Y. The Effect of Caffeine on Retrobulbar Hemodynamics. Curr. Eye Res. 2008, 33, 804–809. [Google Scholar] [CrossRef]
- Yu, S.; McEniery, C.M. Central Versus Peripheral Artery Stiffening and Cardiovascular Risk. Arter. Thromb. Vasc. Biol. 2020, 40, 1028–1033. [Google Scholar] [CrossRef]
- Sato, K.; Sanders, K.M.; Gerthoffer, W.T.; Publicover, N.G. Sources of calcium utilized in cholinergic responses in canine colonic smooth muscle. Am. J. Physiol. Physiol. 1994, 267, C1666–C1673. [Google Scholar] [CrossRef]
- Kramer, R.H.; Mokkapatti, R.; Levitan, E.S. Effects of caffeine on intracellular calcium, calcium current and calcium-dependent potassium current in anterior pituitary GH3 cells. Pflügers Arch. Eur. J. Physiol. 1994, 426, 12–20. [Google Scholar] [CrossRef]
- Mansia, G.; De Backer, G.; Dominiczak, A.; Cifkova, R.; Fagard, R.; Germano, G.; Grassi, G.; Heagerty, A.M.; Kjeldsen, S.E.; Laurent, S.; et al. 2007 ESH-ESC Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Blood Press 2007, 16, 135–232. [Google Scholar] [CrossRef]
- Costa, R.; Rocha, C.; Santos, H. Cardiovascular and Cerebrovascular Response to RedBull® Energy Drink Intake in Young Adults. Anatol. J. Cardiol. 2023, 27, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, S.S.; Namath, A.G.; Tuxhorn, I.E.; Lewis, S.J.; Galán, R.F. Decreased heart rate and enhanced sinus arrhythmia during interictal sleep demonstrate autonomic imbalance in generalized epilepsy. J. Neurophysiol. 2016, 115, 1988–1999. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.; Taylor, W.R. Is increased arterial stiffness a cause or consequence of atherosclerosis? Atherosclerosis 2016, 249, 226–227. [Google Scholar] [CrossRef] [PubMed]
- Terai, N.; Spoerl, E.; Pillunat, L.E.; Stodtmeister, R. The effect of caffeine on retinal vessel diameter in young healthy subjects. Acta Ophthalmol. 2012, 90, e524–e528. [Google Scholar] [CrossRef] [PubMed]
- Lunt, M.J.; Ragab, S.; Birch, A.A.; Schley, D.; Jenkinson, D.F. Comparison of caffeine-induced changes in cerebral blood flow and middle cerebral artery blood velocity shows that caffeine reduces middle cerebral artery diameter. Physiol. Meas. 2004, 25, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P. Epidemiology of hypertension. Lancet 1994, 344, 101–106. [Google Scholar] [CrossRef]
- Mehta, A.; Jain, A.C.; Mehta, M.C.; Billie, M. Caffeine and cardiac arrhythmias. An experimental study in dogs with review of literature. Acta Cardiol. 1997, 52, 273–283. [Google Scholar]
- Addicott, M.A.; Yang, L.L.; Peiffer, A.M.; Burnett, L.R.; Burdette, J.H.; Chen, M.Y.; Hayasaka, S.; Kraft, R.A.; Maldjian, J.A.; Laurienti, P.J. The effect of daily caffeine use on cerebral blood flow: How much caffeine can we tolerate? Hum. Brain Mapp. 2009, 30, 3102–3114. [Google Scholar] [CrossRef]
- Stroke Intensive Care Units: Objectives and Results.|Stroke. Available online: https://www.ahajournals.org/doi/abs/10.1161/01.STR.10.3.235 (accessed on 23 December 2023).
- Salinet, A.S.; Panerai, R.B.; Robinson, T.G. The Longitudinal Evolution of Cerebral Blood Flow Regulation after Acute Ischaemic Stroke. Cerebrovasc. Dis. Extra 2014, 4, 186–197. [Google Scholar] [CrossRef]

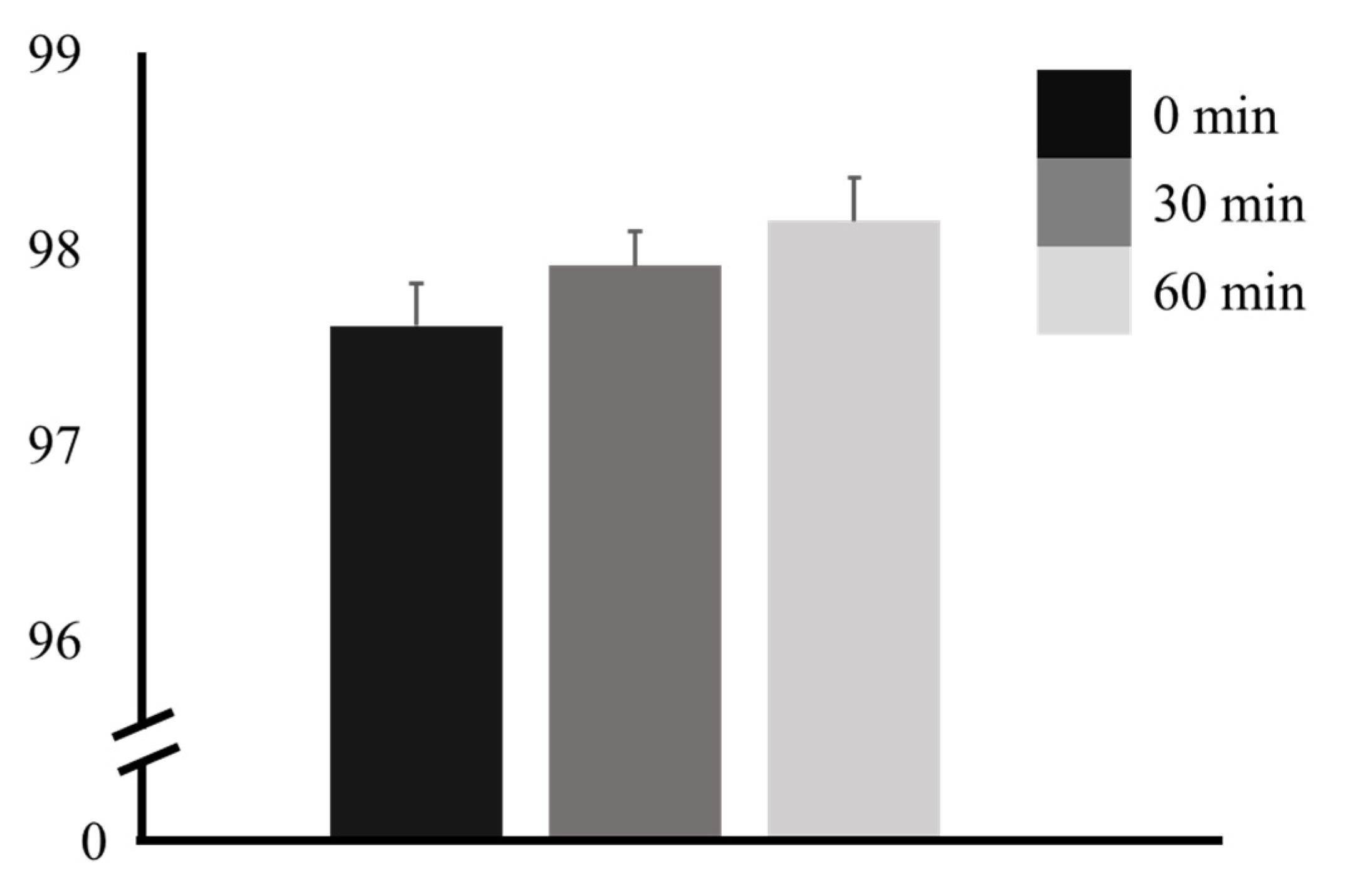

| Time | F | p | Factor 1 | Factor 2 | Mean Difference ± SE (Factor 1 − Factor 2) | t | pTukey |
|---|---|---|---|---|---|---|---|
| 0 min | 2.40 | 0.121 | 0 min | 30 min | −0.308 ± 0.227 | −1.35 | 0.379 |
| 30 min | 60 min | −0.538 ± 0.538 | −1.71 | 0.221 | |||
| 60 min | 30 min | 60 min | −0.231 ± 0.213 | −1.30 | 0.411 |
| Time | F | p | Factor 1 | Factor 2 | Mean Difference ± SE (Factor 1 − Factor 2) | t | pTukey |
|---|---|---|---|---|---|---|---|
| 0 min | 6.19 | 0.004 * | 0 min | 30 min | 4.846 ± 1.58 | 3.068 | 0.014 * |
| 30 min | 60 min | 4.615 ± 1.70 | 2.714 | 0.031 * | |||
| 60 min | 30 min | 60 min | −0.231 ± 1.36 | −0.169 | 0.984 |
| Vessel | Time | F | p | Factor 1 | Factor 2 | Mean Difference ± SE (Factor 1 − Factor 2) | t | pTukey |
|---|---|---|---|---|---|---|---|---|
| CCA | 0 min | 37.5 | <0.001 * | 0 min | 30 min | 18.70 ± 2.64 | 7.09 | <0.001 * |
| 30 min | 60 min | 21.03 ± 3.09 | 6.81 | <0.001 * | ||||
| 60 min | 30 min | 60 min | 2.32 ± 2.18 | 1.06 | 0.544 | |||
| RA | 0 min | 21.9 | <0.001 * | 0 min | 30 min | 10.93 ± 2.47 | 4.42 | <0.001 * |
| 30 min | 60 min | 13.33 ± 2.06 | 6.46 | <0.001 * | ||||
| 60 min | 30 min | 60 min | 2.40 ± 1.86 | −1.30 | 0.412 |
| Vessel | Time | F | p | Factor 1 | Factor 2 | Mean Difference ± SE (Factor 2 − Factor 1) | t | pnc | pTukey |
|---|---|---|---|---|---|---|---|---|---|
| CCA | 0 min | 1.16 | 0.323 | 0 min | 30 min | −0.166 ± 0.200 | −0.832 | 0.413 | 0.687 |
| 30 min | 60 min | −0.381 ± 0.235 | −1.619 | 0.118 | 0.256 | ||||
| 60 min | 30 min | 60 min | −0.215 ± 0.307 | −0.700 | 0.490 | 0.766 | |||
| RA | 0 min | 3.55 | 0.036 * | 0 min | 30 min | 0.347 ± 0.431 | 0.805 | 0.428 | 0.703 |
| 30 min | 60 min | −0.864 ± 0.439 | −1.970 | 0.060 | 0.140 | ||||
| 60 min | 30 min | 60 min | −1.212 ± 0.529 | −2.291 | 0.031 * | 0.076 |
| Time | F | p | Factor 1 | Factor 2 | Mean Difference ± SE (Factor 2 − Factor 1) | t | pTukey |
|---|---|---|---|---|---|---|---|
| 0 min | 8.81 | <0.001 * | 0 min | 30 min | 7.30 ± 1.94 | 3.76 | 0.003 * |
| 30 min | 60 min | 9.29 ± 2.99 | 3.11 | 0.012 * | |||
| 60 min | 30 min | 60 min | 1.99 ± 1.90 | 1.05 | 0.554 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.-B.; Kim, J.-H.; Song, C.-H.; Park, C.; Kang, C.-K. Diagnostic Ultrasound-Based Investigation of Central vs. Peripheral Arterial Changes Consequent to Low-Dose Caffeine Ingestion. Nutrients 2024, 16, 228. https://doi.org/10.3390/nu16020228
Jin Y-B, Kim J-H, Song C-H, Park C, Kang C-K. Diagnostic Ultrasound-Based Investigation of Central vs. Peripheral Arterial Changes Consequent to Low-Dose Caffeine Ingestion. Nutrients. 2024; 16(2):228. https://doi.org/10.3390/nu16020228
Chicago/Turabian StyleJin, Yu-Bin, Jeong-Hyeon Kim, Chae-Hyeon Song, Chansol Park, and Chang-Ki Kang. 2024. "Diagnostic Ultrasound-Based Investigation of Central vs. Peripheral Arterial Changes Consequent to Low-Dose Caffeine Ingestion" Nutrients 16, no. 2: 228. https://doi.org/10.3390/nu16020228
APA StyleJin, Y.-B., Kim, J.-H., Song, C.-H., Park, C., & Kang, C.-K. (2024). Diagnostic Ultrasound-Based Investigation of Central vs. Peripheral Arterial Changes Consequent to Low-Dose Caffeine Ingestion. Nutrients, 16(2), 228. https://doi.org/10.3390/nu16020228







