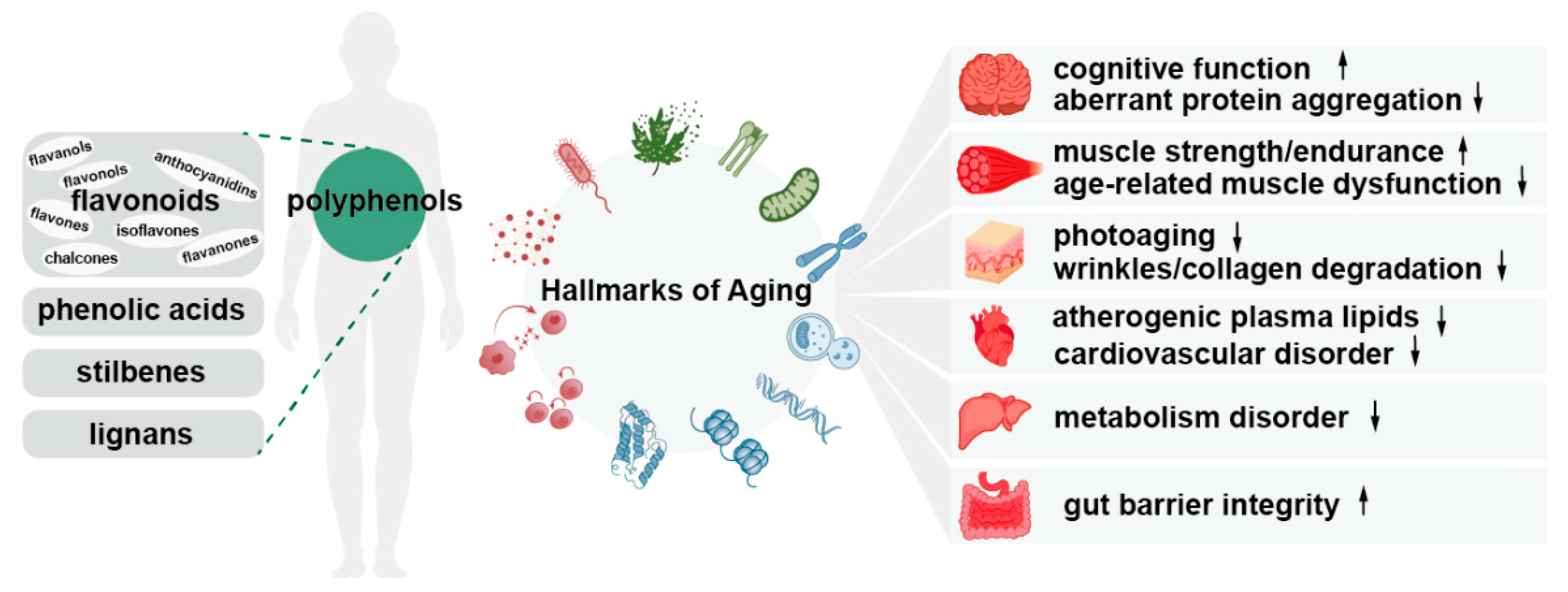
Dietary Polyphenols as Anti-Aging Agents: Targeting the Hallmarks of Aging
Abstract
1. Introduction
2. Biological Activities of Polyphenols
3. Natural Polyphenols for Anti-Aging Studies
3.1. Ellagic Acid (EA)
3.2. Gallic Acid (GA)
3.3. Rutin
3.4. Quercetin
3.5. Fisetin
3.6. Anthocyanins
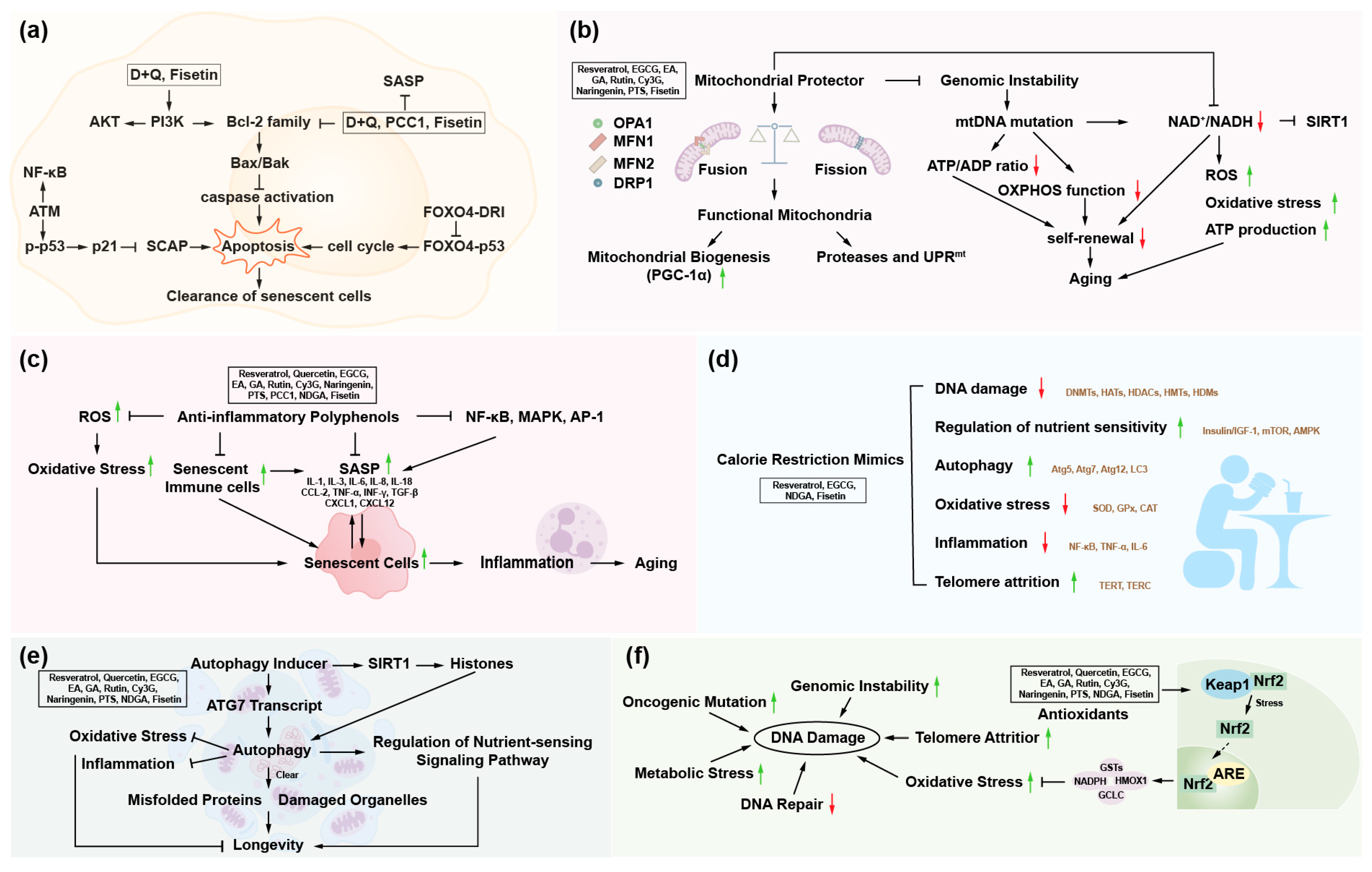
4. Polyphenols and Hallmarks of Aging
4.1. Polyphenols and Mitochondrial Dysfunction
4.2. Polyphenols and Epigenetic Alterations
4.3. Polyphenols and Disabled Macroautophagy
4.4. Polyphenols and Deregulated Nutrient-Sensing
4.5. Polyphenols and Chronic Inflammation
4.6. Polyphenols and Genomic Instability
4.7. Polyphenols and Dysbiosis
4.8. Polyphenols and Other Hallmarks of Aging
5. Conclusions
6. Outstanding Questions
- (1)
- What factors cause individual differences in the metabolism and bioavailability of polyphenols in the body? How can the intake of polyphenols be optimized to achieve the best anti-aging effect?
- (2)
- What are the synergy mechanisms and dynamic regulation of the anti-aging effect of polyphenols?
- (3)
- Regarding the effects of polyphenols in the human body, what is their long-term efficacy like? And what conclusions have been drawn from the relevant safety clinical trials?
- (4)
- The aging process involves the interaction of multiple organs and systems, but existing studies mainly focus on individual hallmarks of aging. What is the comprehensive impact of polyphenols on the aging of multiple organ systems throughout the body?
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
References
- Tu, W.-J.; Zeng, X.; Liu, Q. Aging tsunami coming: The main finding from China’s seventh national population census. Aging Clin. Exp. Res. 2022, 34, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, B.; Pothof, J.; Vijg, J.; Hoeijmakers, J.H.J. The central role of DNA damage in the ageing process. Nature 2021, 592, 695–703. [Google Scholar] [CrossRef]
- López-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Moskalev, A.; Guvatova, Z.; Lopes, I.A.; Beckett, C.W.; Kennedy, B.K.; De Magalhaes, J.P.; Makarov, A.A. Targeting aging mechanisms: Pharmacological perspectives. Trends Endocrinol. Metab. 2022, 33, 266–280. [Google Scholar] [CrossRef]
- Ma, T.; Tian, X.; Zhang, B.D.; Li, M.Q.; Wang, Y.; Yang, C.Y.; Wu, J.F.; Wei, X.Y.; Qu, Q.; Yu, Y.X.; et al. Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature 2022, 603, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Nadeeshani, H.; Li, J.; Ying, T.; Zhang, B.; Lu, J. Nicotinamide mononucleotide (NMN) as an anti-aging health product—Promises and safety concerns. J. Adv. Res. 2022, 37, 267–278. [Google Scholar] [CrossRef]
- Saccon, T.D.; Nagpal, R.; Yadav, H.; Cavalcante, M.B.; Nunes, A.D.C.; Schneider, A.; Gesing, A.; Hughes, B.; Yousefzadeh, M.; Tchkonia, T.; et al. Senolytic Combination of Dasatinib and Quercetin Alleviates Intestinal Senescence and Inflammation and Modulates the Gut Microbiome in Aged Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1895–1905. [Google Scholar] [CrossRef]
- Xu, T.T.; Li, H.; Dai, Z.; Lau, G.K.; Li, B.Y.; Zhu, W.L.; Liu, X.Q.; Liu, H.F.; Cai, W.W.; Huang, S.Q.; et al. Spermidine and spermine delay brain aging by inducing autophagy in SAMP8 mice. Aging 2020, 12, 6401–6414. [Google Scholar] [CrossRef]
- Luo, J.; Si, H.W.; Jia, Z.Q.; Liu, D.M. Dietary Anti-Aging Polyphenols and Potential Mechanisms. Antioxidants 2021, 10, 283. [Google Scholar] [CrossRef] [PubMed]
- Si, H.W.; Liu, D.M. Dietary antiaging phytochemicals and mechanisms associated with prolonged survival. J. Nutr. Biochem. 2014, 25, 581–591. [Google Scholar] [CrossRef]
- Li, J.; Liao, R.; Zhang, S.; Weng, H.; Liu, Y.; Tao, T.; Yu, F.; Li, G.; Wu, J. Promising remedies for cardiovascular disease: Natural polyphenol ellagic acid and its metabolite urolithins. Phytomedicine 2023, 116, 154867. [Google Scholar] [CrossRef]
- Benvenuto, M.; Albonici, L.; Focaccetti, C.; Ciuffa, S.; Fazi, S.; Cifaldi, L.; Miele, M.T.; De Maio, F.; Tresoldi, I.; Manzari, V.; et al. Polyphenol-Mediated Autophagy in Cancer: Evidence of In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2020, 21, 6635. [Google Scholar] [CrossRef]
- Moradi, S.Z.; Jalili, F.; Farhadian, N.; Joshi, T.; Wang, M.; Zou, L.; Cao, H.; Farzaei, M.H.; Xiao, J. Polyphenols and neurodegenerative diseases: Focus on neuronal regeneration. Crit. Rev. Food Sci. Nutr. 2022, 62, 3421–3436. [Google Scholar] [CrossRef]
- Islam, M.A.; Khandker, S.S.; Kotyla, P.J.; Hassan, R. Immunomodulatory Effects of Diet and Nutrients in Systemic Lupus Erythematosus (SLE): A Systematic Review. Front. Immunol. 2020, 11, 1477. [Google Scholar] [CrossRef]
- Long, Z.; Xiang, W.; He, Q.; Xiao, W.; Wei, H.; Li, H.; Guo, H.; Chen, Y.; Yuan, M.; Yuan, X.; et al. Efficacy and safety of dietary polyphenols in rheumatoid arthritis: A systematic review and meta-analysis of 47 randomized controlled trials. Front. Immunol. 2023, 14, 1024120. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, G.; Lonardo, M.S.; Cacciapuoti, N.; Muscariello, E.; Guida, B.; Faraonio, R.; Santillo, M.; Damiano, S. Dietary Polyphenols, Microbiome, and Multiple Sclerosis: From Molecular Anti-Inflammatory and Neuroprotective Mechanisms to Clinical Evidence. Int. J. Mol. Sci. 2023, 24, 7247. [Google Scholar] [CrossRef]
- Grabska-Kobylecka, I.; Szpakowski, P.; Krol, A.; Ksiazek-Winiarek, D.; Kobylecki, A.; Glabinski, A.; Nowak, D. Polyphenols and Their Impact on the Prevention of Neurodegenerative Diseases and Development. Nutrients 2023, 15, 3454. [Google Scholar] [CrossRef]
- Sandoval, V.; Sanz-Lamora, H.; Arias, G.; Marrero, P.F.; Haro, D.; Relat, J. Metabolic Impact of Flavonoids Consumption in Obesity: From Central to Peripheral. Nutrients 2020, 12, 2393. [Google Scholar] [CrossRef]
- Gazwi, H.S.S.; Hassan, M.S.; Ismail, H.A.; El-Naem, G.F.A.; Tony, S.K. The Hypoglycemic and Hypolipidemic Effects of Polyphenol-Rich Strawberry Juice on Diabetic Rats. Plant Foods Hum. Nutr. 2023, 78, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, X.J.; Chen, J.B.; Cao, J.P.; Li, X.; Sun, C.D. Citrus flavonoids and their antioxidant evaluation. Crit. Rev. Food Sci. 2022, 62, 3833–3854. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Singh, A.K.; Kumar, R.; Croley, C.R.; Pandey, A.K.; Coy-Barrera, E.; Kumar Patra, J.; Das, G.; Kerry, R.G.; Annunziata, G.; et al. Targeting Inflammation by Flavonoids: Novel Therapeutic Strategy for Metabolic Disorders. Int. J. Mol. Sci. 2019, 20, 4957. [Google Scholar] [CrossRef] [PubMed]
- Nomi, Y.; Iwasaki-Kurashige, K.; Matsumoto, H. Therapeutic Effects of Anthocyanins for Vision and Eye Health. Molecules 2019, 24, 3311. [Google Scholar] [CrossRef]
- Levenson, A.S. Metastasis-associated protein 1-mediated antitumor and anticancer activity of dietary stilbenes for prostate cancer chemoprevention and therapy. Semin. Cancer Biol. 2022, 80, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.Y.; Kim, M.Y.; Cho, J.Y. Antioxidant, Anti-Inflammatory, Anti-Menopausal, and Anti-Cancer Effects of Lignans and Their Metabolites. Int. J. Mol. Sci. 2022, 23, 15482. [Google Scholar] [CrossRef]
- Kozlowska, A.; Szostak-Wegierek, D. Flavonoids--food sources and health benefits. Rocz. Panstw. Zakl. Hig. 2014, 65, 79–85. [Google Scholar]
- Williamson, G.; Kay, C.D.; Crozier, A. The Bioavailability, Transport, and Bioactivity of Dietary Flavonoids: A Review from a Historical Perspective. Compr. Rev. Food Sci. F 2018, 17, 1054–1112. [Google Scholar] [CrossRef]
- Deng, Y.D.; Tu, Y.L.; Lao, S.H.; Wu, M.T.; Yin, H.T.; Wang, L.Q.; Liao, W.Z. The role and mechanism of citrus flavonoids in cardiovascular diseases prevention and treatment. Crit. Rev. Food Sci. 2022, 62, 7591–7614. [Google Scholar] [CrossRef]
- Xu, H.; Luo, J.; Huang, J.; Wen, Q. Flavonoids intake and risk of type 2 diabetes mellitus: A meta-analysis of prospective cohort studies. Medicine 2018, 97, e0686. [Google Scholar] [CrossRef] [PubMed]
- Jabczyk, M.; Nowak, J.; Hudzik, B.; Zubelewicz-Szkodzinska, B. Curcumin in Metabolic Health and Disease. Nutrients 2021, 13, 4440. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, Y.; Zhang, X.; Aa, J.; Wang, G.; Xie, Y. Curcumin regulates endogenous and exogenous metabolism via Nrf2-FXR-LXR pathway in NAFLD mice. Biomed. Pharmacother. 2018, 105, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Nisoli, E.; Tonello, C.; Cardile, A.; Cozzi, V.; Bracale, R.; Tedesco, L.; Falcone, S.; Valerio, A.; Cantoni, O.; Clementi, E.; et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 2005, 310, 314–317. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Zhang, H.H.; Zheng, J.; Hu, X.; Kong, W.; Hu, D.; Wang, S.X.; Zhang, P. Resveratrol attenuates high-fat diet-induced insulin resistance by influencing skeletal muscle lipid transport and subsarcolemmal mitochondrial beta-oxidation. Metabolism 2011, 60, 1598–1609. [Google Scholar] [CrossRef] [PubMed]
- De los Santos, S.; Reyes-Castro, L.A.; Coral-Vázquez, R.M.; Mendez, J.P.; Zambrano, E.; Canto, P. (-)-Epicatechin increases apelin/APLNR expression and modifies proteins involved in lipid metabolism of offspring descendants of maternal obesity. J. Nutr. Biochem. 2023, 117, 109350. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pae, M.; Meydani, S.N.; Wu, D. Green tea epigallocatechin-3-gallate modulates differentiation of naïve CD4+ T cells into specific lineage effector cells. J. Mol. Med. 2013, 91, 485–495. [Google Scholar] [CrossRef]
- Malaguarnera, L. Influence of Resveratrol on the Immune Response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef]
- Kang, H.K.; Ecklund, D.; Liu, M.; Datta, S.K. Apigenin, a non-mutagenic dietary flavonoid, suppresses lupus by inhibiting autoantigen presentation for expansion of autoreactive Th1 and Th17 cells. Arthritis Res. Ther. 2009, 11, R59. [Google Scholar] [CrossRef]
- Pyo, I.S.; Yun, S.Y.; Yoon, Y.E.; Choi, J.W.; Lee, S.J. Mechanisms of Aging and the Preventive Effects of Resveratrol on Age-Related Diseases. Molecules 2020, 25, 4649. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jia, R.; Celi, P.; Zhuo, Y.; Ding, X.; Zeng, Q.; Bai, S.; Xu, S.; Yin, H.; Lv, L.; et al. Resveratrol Alleviating the Ovarian Function Under Oxidative Stress by Alternating Microbiota Related Tryptophan-Kynurenine Pathway. Front. Immunol. 2022, 13, 911381. [Google Scholar] [CrossRef]
- Han, D.W.; Lee, M.H.; Kim, H.H.; Hyon, S.H.; Park, J.C. Epigallocatechin-3-gallate regulates cell growth, cell cycle and phosphorylated nuclear factor-κB in human dermal fibroblasts. Acta Pharmacol. Sin. 2011, 32, 637–646. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef]
- Ismaeel, A.; McDermott, M.M.; Joshi, J.K.; Sturgis, J.C.; Zhang, D.X.; Ho, K.J.; Sufit, R.; Ferrucci, L.; Peterson, C.A.; Kosmac, K. Cocoa flavanols, Nrf2 activation, and oxidative stress in peripheral artery disease: Mechanistic findings in muscle based on outcomes from a randomized trial. Am. J. Physiol-Cell Ph 2024, 326, C589–C605. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Medoro, A.; Ali, S.; Passarella, D.; Intrieri, M.; Scapagnini, G. Dietary Flavonoids and Adult Neurogenesis: Potential Implications for Brain Aging. Curr. Neuropharmacol. 2023, 21, 651–668. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary polyphenol impact on gut health and microbiota. Crit. Rev. Food Sci. 2021, 61, 690–711. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between Gut Microbiota and Curcumin: A New Key of Understanding for the Health Effects of Curcumin. Nutrients 2020, 12, 2499. [Google Scholar] [CrossRef]
- Chen, P.T.; Chen, Z.T.; Hou, W.C.; Yu, L.C.; Chen, R.P. Polyhydroxycurcuminoids but not curcumin upregulate neprilysin and can be applied to the prevention of Alzheimer’s disease. Sci. Rep. 2016, 6, 29760. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ghosh, S.S.; Ghosh, S. Curcumin improves intestinal barrier function: Modulation of intracellular signaling, and organization of tight junctions. Am. J. Physiol-Cell Ph 2017, 312, C438–C445. [Google Scholar] [CrossRef]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.H.; Huang, S.M.; Li, T.T.; Li, N.; Han, D.D.; Zhang, B.; Xu, Z.J.Z.; Zhang, S.Y.; Pang, J.M.; Wang, S.L.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 2021, 9, 184. [Google Scholar] [CrossRef]
- Del Bo, C.; Bernardi, S.; Cherubini, A.; Porrini, M.; Gargari, G.; Hidalgo-Liberona, N.; González-Domíniguez, R.; Zamora-Ros, R.; Peron, G.; Marino, M.; et al. A polyphenol-rich dietary pattern improves intestinal permeability, evaluated as serum zonulin levels, in older subjects: The MaPLE randomised controlled trial. Clin. Nutr. 2021, 40, 3006–3018. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Joma, N.; Bielawski, P.B.; Saini, A.; Kakkar, A.; Maysinger, D. Nanocarriers for natural polyphenol senotherapeutics. Aging Cell 2024, 23, e14178. [Google Scholar] [CrossRef] [PubMed]
- Yessenkyzy, A.; Saliev, T.; Zhanaliyeva, M.; Masoud, A.R.; Umbayev, B.; Sergazy, S.; Krivykh, E.; Gulyayev, A.; Nurgozhin, T. Polyphenols as Caloric-Restriction Mimetics and Autophagy Inducers in Aging Research. Nutrients 2020, 12, 1344. [Google Scholar] [CrossRef] [PubMed]
- Harper, S.A.; Bassler, J.R.; Peramsetty, S.; Yang, Y.; Roberts, L.M.; Drummer, D.; Mankowski, R.T.; Leeuwenburgh, C.; Ricart, K.; Patel, R.P.; et al. Resveratrol and exercise combined to treat functional limitations in late life: A pilot randomized controlled trial. Exp. Gerontol. 2021, 143, 111111. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, X.; Chen, K.; Lang, H.; Zhang, Y.; Hou, P.; Ran, L.; Zhou, M.; Zheng, J.; Yi, L.; et al. Resveratrol prevents sarcopenic obesity by reversing mitochondrial dysfunction and oxidative stress via the PKA/LKB1/AMPK pathway. Aging 2019, 11, 2217–2240. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, R.; Nakashima, R.; Yano, M.; Iwahara, N.; Asakura, S.; Nojima, I.; Saga, Y.; Kunimoto, R.; Horio, Y.; Kuno, A. Resveratrol, a SIRT1 activator, attenuates aging-associated alterations in skeletal muscle and heart in mice. J. Pharmacol. Sci. 2023, 152, 112–122. [Google Scholar] [CrossRef]
- Gerhardt, E.; Graber, S.; Szego, E.M.; Moisoi, N.; Martins, L.M.; Outeiro, T.F.; Kermer, P. Idebenone and resveratrol extend lifespan and improve motor function of HtrA2 knockout mice. PLoS ONE 2011, 6, e28855. [Google Scholar] [CrossRef]
- Wood, J.G.; Rogina, B.; Lavu, S.; Howitz, K.; Helfand, S.L.; Tatar, M.; Sinclair, D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 2004, 430, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekara, K.T.; Shakarad, M.N. Aloe vera or resveratrol supplementation in larval diet delays adult aging in the fruit fly, Drosophila melanogaster. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 965–971. [Google Scholar] [CrossRef]
- Zhu, H.; Li, X.; Qiao, M.; Sun, X.; Li, G. Resveratrol Alleviates Inflammation and ER Stress Through SIRT1/NRF2 to Delay Ovarian Aging in a Short-Lived Fish. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.; Franco, F.N.; Caldeira, C.A.; de Araujo, G.R.; Vieira, A.; Chaves, M.M. Resveratrol has its antioxidant and anti-inflammatory protective mechanisms decreased in aging. Arch. Gerontol. Geriatr. 2023, 107, 104895. [Google Scholar] [CrossRef]
- Bellaver, B.; Souza, D.G.; Souza, D.O.; Quincozes-Santos, A. Resveratrol increases antioxidant defenses and decreases proinflammatory cytokines in hippocampal astrocyte cultures from newborn, adult and aged Wistar rats. Toxicol. In Vitro 2014, 28, 479–484. [Google Scholar] [CrossRef]
- Allen, E.N.; Potdar, S.; Tapias, V.; Parmar, M.; Mizuno, C.S.; Rimando, A.; Cavanaugh, J.E. Resveratrol and pinostilbene confer neuroprotection against aging-related deficits through an ERK1/2-dependent mechanism. J. Nutr. Biochem. 2018, 54, 77–86. [Google Scholar] [CrossRef]
- Amakura, Y.; Okada, M.; Tsuji, S.; Tonogai, Y. High-performance liquid chromatographic determination with photodiode array detection of ellagic acid in fresh and processed fruits. J. Chromatogr. A 2000, 896, 87–93. [Google Scholar] [CrossRef]
- Xian, W.Y.; Deng, Y.; Yang, Y.Z.; Tan, Z.L.; Chen, C.L.; Li, W.; Yang, R.L. Ameliorative Effect of Ellagic Acid on Aging in Rats with the Potential Mechanism Relying on the Gut Microbiota and Urolithin A-Producing Ability. J. Agric. Food Chem. 2023, 71, 7396–7407. [Google Scholar] [CrossRef]
- Naiini, M.R.; Shahouzehi, B.; Khaksari, M.; Azizi, S.; Naghibi, N.; Nazari-Robati, M. Ellagic acid reduces hepatic lipid contents through regulation of SIRT1 and AMPK in old rats. Arch. Physiol. Biochem. 2023, 10, 1–8. [Google Scholar] [CrossRef]
- Liu, Q.S.; Li, S.R.; Li, K.Q.; Li, X.; Yin, X.Y.; Pang, Z.R. Ellagic acid improves endogenous neural stem cells proliferation and neurorestoration through Wnt/β-catenin signaling in vivo and in vitro. Mol. Nutr. Food Res. 2017, 61, 1600587. [Google Scholar] [CrossRef]
- Dong, H.; Cao, Y.X.; Zou, K.; Shao, Q.; Liu, R.H.; Zhang, Y.; Pan, L.Z.; Ning, B. Ellagic acid promotes osteoblasts differentiation via activating SMAD2/3 pathway and alleviates bone mass loss in OVX mice. Chem-Biol. Interact. 2024, 388, 110852. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.Y.; Choi, J.S.; Kang, S.W.; Lee, Y.J.; Park, J.; Kang, Y.H. Dietary compound ellagic acid alleviates skin wrinkle and inflammation induced by UV-B irradiation. Exp. Dermatol. 2010, 19, E182–E190. [Google Scholar] [CrossRef]
- Bai, S.J.; Yu, Y.R.; An, L.; Wang, W.B.; Fu, X.Q.; Chen, J.; Ma, J.F. Ellagic Acid Increases Stress Resistance via Insulin/IGF-1 Signaling Pathway in Caenorhabditis elegans. Molecules 2022, 27, 6168. [Google Scholar] [CrossRef]
- Kharat, P.; Sarkar, P.; Mouliganesh, S.; Tiwary, V.; Priya, V.B.R.; Sree, N.Y.; Annapoorna, H.V.; Saikia, D.K.; Mahanta, K.; Thirumurugan, K. Ellagic acid prolongs the lifespan of Drosophila melanogaster. Geroscience 2020, 42, 271–285. [Google Scholar] [CrossRef]
- Rahimi, V.B.; Askari, V.R.; Mousavi, S.H. Ellagic acid reveals promising anti-aging effects against D-galactose-induced aging on human neuroblastoma cell line, SH-SY5Y: A mechanistic study. Biomed. Pharmacother. 2018, 108, 1712–1724. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, C.; Stutts, J.; Clatterbuck, K.; Nosoudi, N. Effect of ellagic acid and retinoic acid on collagen and elastin production by human dermal fibroblasts. Bio-Med. Mater. Eng. 2023, 34, 473–480. [Google Scholar] [CrossRef]
- Andreux, P.A.; Blanco-Bose, W.; Ryu, D.; Burdet, F.; Ibberson, M.; Aebischer, P.; Auwerx, J.; Singh, A.; Rinsch, C. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat. Metab. 2019, 1, 595–603. [Google Scholar] [CrossRef]
- Zhang, M.W.; Tang, X.; Mao, B.Y.; Zhang, Q.X.; Zhao, J.X.; Chen, W.; Cui, S.M. Inhibition of the NF-κB and mTOR targets by urolithin A attenuates d-galactose-induced aging in mice. Food Funct. 2023, 14, 10375–10386. [Google Scholar] [CrossRef]
- Ballesteros-Alvarez, J.; Nguyen, W.; Sivapatham, R.; Rane, A.; Andersen, J.K. Urolithin A reduces amyloid-beta load and improves cognitive deficits uncorrelated with plaque burden in a mouse model of Alzheimer’s disease. Geroscience 2023, 45, 1095–1113. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.; Mouchiroud, L.; Andreux, P.A.; Katsyuba, E.; Moullan, N.; Nicolet-dit-Félix, A.A.; Williams, E.G.; Jha, P.; Lo Sasso, G.; Huzard, D.; et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat. Med. 2016, 22, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chen, F.C.; Lei, J.X.; Li, Q.L.; Zhou, B.H. Activation of the miR-34a-Mediated SIRT1/mTOR Signaling Pathway by Urolithin A Attenuates d-Galactose-Induced Brain Aging in Mice. Neurotherapeutics 2019, 16, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Lei, J.X.; Chen, F.C.; Zhou, B.H. Ameliorative effect of urolithin A on d-gal-induced liver and kidney damage in aging mice: Its antioxidative, anti-inflammatory and antiapoptotic properties. RSC Adv. 2020, 10, 8027–8038. [Google Scholar] [CrossRef]
- Guo, L.; Cao, J.H.; Wei, T.T.; Li, J.H.; Feng, Y.K.; Wang, L.P.; Sun, Y.; Chai, Y.R. Gallic acid attenuates thymic involution in the D-galactose induced accelerated aging mice. Immunobiology 2020, 225, 151870. [Google Scholar] [CrossRef]
- Rahimifard, M.; Baeeri, M.; Bahadar, H.; Moini-Nodeh, S.; Khalid, M.; Haghi-Aminjan, H.; Mohammadian, H.; Abdollahi, M. Therapeutic Effects of Gallic Acid in Regulating Senescence and Diabetes; an In Vitro Study. Molecules 2020, 25, 5875. [Google Scholar] [CrossRef]
- Mori, T.; Koyama, N.; Yokoo, T.; Segawa, T.; Maeda, M.; Sawmiller, D.; Tan, J.; Town, T. Gallic acid is a dual α/β-secretase modulator that reverses cognitive impairment and remediates pathology in Alzheimer mice. J. Biol. Chem. 2020, 295, 16251–16266. [Google Scholar] [CrossRef]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Thirumurugan, K. Longevity-promoting efficacies of rutin in high fat diet fed. Biogerontology 2020, 21, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, L.M.; Machado, M.L.; da Silva, A.F.; Baptista, F.B.O.; da Silveira, T.L.; Soares, F.A.A.; Arantes, L.P. Rutin protects Huntington’s disease through the insulin/IGF1 (IIS) signaling pathway and autophagy activity: Study in model. Food Chem. Toxicol. 2020, 141, 111323. [Google Scholar] [CrossRef] [PubMed]
- Saafan, S.M.; Mohamed, S.A.; Noreldin, A.E.; El Tedawy, F.A.; Elewa, Y.H.A.; Fadly, R.S.; Al Jaouni, S.K.; El-Far, A.H.; Alsenosy, A.A. Rutin attenuates d-galactose-induced oxidative stress in rats’ brain and liver: Molecular docking and experimental approaches. Food Funct. 2023, 14, 5728–5751. [Google Scholar] [CrossRef]
- Li, T.Y.; Chen, S.F.; Feng, T.; Dong, J.; Li, Y.Y.; Li, H. Rutin protects against aging-related metabolic dysfunction. Food Funct. 2016, 7, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Bescós, P.; Jiménez-Aliaga, K.L.; Benedí, J.; Martín-Aragón, S. A Diet Containing Rutin Ameliorates Brain Intracellular Redox Homeostasis in a Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 4863. [Google Scholar] [CrossRef]
- Cui, Z.F.; Zhao, X.T.; Amevor, F.K.; Du, X.X.; Wang, Y.; Li, D.Y.; Shu, G.; Tian, Y.F.; Zhao, X.L. Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Front. Immunol. 2022, 13, 943321. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Tuday, E.; Allen, S.; Kim, J.; Trott, D.W.; Holland, W.L.; Donato, A.J.; Lesniewski, L.A. Senolytic drugs, dasatinib and quercetin, attenuate adipose tissue inflammation, and ameliorate metabolic function in old age. Aging Cell 2023, 22, e13767. [Google Scholar] [CrossRef] [PubMed]
- Novais, E.J.; Tran, V.A.; Johnston, S.N.; Darris, K.R.; Roupas, A.J.; Sessions, G.A.; Shapiro, I.M.; Diekman, B.O.; Risbud, M.V. Long-term treatment with senolytic drugs Dasatinib and Quercetin ameliorates age-dependent intervertebral disc degeneration in mice. Nat. Commun. 2021, 12, 5213. [Google Scholar] [CrossRef]
- Ruggiero, A.D.; Vemuri, R.; Blawas, M.; Long, M.S.; DeStephanis, D.; Williams, A.G.; Chen, H.Y.; Justice, J.N.; Macauley, S.I.; Day, S.M.; et al. Long-term dasatinib plus quercetin effects on aging outcomes and inflammation in nonhuman primates: Implications for senolytic clinical trial design. Geroscience 2023, 45, 2785–2803. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Khajevand-Khazaei, M.R.; Ziaee, P.; Motevalizadeh, S.A.; Rohani, M.; Afshin-Majd, S.; Baluchnejadmojarad, T.; Roghani, M. Naringenin ameliorates learning and memory impairment following systemic lipopolysaccharide challenge in the rat. Eur. J. Pharmacol. 2018, 826, 114–122. [Google Scholar] [CrossRef]
- Chen, G.P.; Zeng, L.; Yan, F.; Liu, J.L.; Qin, M.Q.; Wang, F.F.; Zhang, X. Long-term oral administration of naringenin counteracts aging-related retinal degeneration regulation of mitochondrial dynamics and autophagy. Front. Pharmacol. 2022, 13, 919905. [Google Scholar] [CrossRef]
- Piragine, E.; De Felice, M.; Germelli, L.; Brinkmann, V.; Flori, L.; Martini, C.; Calderone, V.; Ventura, N.; Da Pozzo, E.; Testai, L. The Citrus flavanone naringenin prolongs the lifespan in C. elegans and slows signs of brain aging in mice. Exp. Gerontol. 2024, 194, 112495. [Google Scholar] [CrossRef]
- Chen, W.; Chu, Q.; Ye, X.; Sun, Y.H.; Liu, Y.Y.; Jia, R.Y.; Li, Y.L.; Tu, P.C.; Tang, Q.; Yu, T.; et al. Canidin-3-glucoside prevents nano-plastics induced toxicity activating autophagy and promoting discharge. Environ. Pollut. 2021, 274, 116524. [Google Scholar] [CrossRef]
- Sanjay; Shin, J.H.; Park, M.; Lee, H.J. Cyanidin-3-Glucoside Regulates the M1/M2 Polarization of Microglia via PPARγ and Aβ42 Phagocytosis Through TREM2 in an Alzheimer’s Disease Model. Mol. Neurobiol. 2022, 59, 5135–5148. [Google Scholar] [CrossRef]
- Baek, H.; Sanjay; Park, M.; Lee, H.J. Cyanidin-3-O-glucoside protects the brain and improves cognitive function in APPswe/PS1ΔE9 transgenic mice model. J. Neuroinflamm. 2023, 20, 268. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.N.; Zhang, J.L.; Qin, M.L. Protective effect of cyanidin 3-O-glucoside on beta-amyloid peptide-induced cognitive impairment in rats. Neurosci. Lett. 2013, 534, 285–288. [Google Scholar] [CrossRef]
- Jaiswal, V.; Park, M.; Lee, H.J. Comparative Transcriptome Analysis of the Expression of Antioxidant and Immunity Genes in the Spleen of a Cyanidin 3-O-Glucoside-Treated Alzheimer’s Mouse Model. Antioxidants 2021, 10, 1435. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, G.X.; Wang, Y.W.; Li, W.; Yi, S.L.; Wang, K.; Fan, L.; Tang, J.J.; Chen, R.N. Multi-Omics Integration in Mice with Parkinson’s Disease and the Intervention Effect of Cyanidin-3-Glucoside. Front. Aging Neurosci. 2022, 14, 877078. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Z.; Shan, S.; Wu, A.B.; Zhao, C.; Ye, X.; Zheng, X.D.; Zhu, R.Y. Cyanidin-3-O-glucoside promotes stress tolerance and lifespan extension of exposed to polystyrene via DAF-16 pathway. Mech. Ageing Dev. 2022, 207, 111723. [Google Scholar] [CrossRef]
- Zhou, H.J.; Liu, S.A.; Zhang, N.Y.; Fang, K.H.; Zong, J.B.; An, Y.; Chang, X.T. Downregulation of Sirt6 by CD38 promotes cell senescence and aging. Aging 2022, 14, 9730–9757. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Fu, Q.; Li, Z.; Liu, H.; Wang, Y.; Lin, X.; He, R.; Zhang, X.; Ju, Z.; Campisi, J.; et al. The flavonoid procyanidin C1 has senotherapeutic activity and increases lifespan in mice. Nat. Metab. 2021, 3, 1706–1726. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Chen, X.; Yang, Z.; Chen, J.; Zhu, W.; Li, Y.; Wen, Y.; Deng, C.; Gu, C.; et al. Senolytic and senomorphic agent procyanidin C1 alleviates structural and functional decline in the aged retina. Proc. Natl. Acad. Sci. USA 2024, 121, e2311028121. [Google Scholar] [CrossRef] [PubMed]
- Strong, R.; Miller, R.A.; Astle, C.M.; Floyd, R.A.; Flurkey, K.; Hensley, K.L.; Javors, M.A.; Leeuwenburgh, C.; Nelson, J.F.; Ongini, E.; et al. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell 2008, 7, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Rimando, A.; Pallas, M.; Camins, A.; Porquet, D.; Reeves, J.; Shukitt-Hale, B.; Smith, M.A.; Joseph, J.A.; Casadesus, G. Low-dose pterostilbene, but not resveratrol, is a potent neuromodulator in aging and Alzheimer’s disease. Neurobiol. Aging 2012, 33, 2062–2071. [Google Scholar] [CrossRef] [PubMed]
- La Spina, M.; Sansevero, G.; Biasutto, L.; Zoratti, M.; Peruzzo, R.; Berardi, N.; Sale, A.; Azzolini, M. Pterostilbene Improves Cognitive Performance in Aged Rats: An in Vivo Study. Cell Physiol. Biochem. 2019, 52, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.A.; Fisher, D.R.; Cheng, V.; Rimando, A.M.; Shukitt-Hale, B. Cellular and behavioral effects of stilbene resveratrol analogues: Implications for reducing the deleterious effects of aging. J. Agric. Food Chem. 2008, 56, 10544–10551. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.Y.; Yan, Y.M.; Jiang, Y.; Meng, X.F. Ellagic Acid and Its Anti-Aging Effects on Central Nervous System. Int. J. Mol. Sci. 2022, 23, 10937. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, Y.Z.; Ding, Y.H.; Wang, J.; Geng, J.; Yang, H.; Ren, J.; Tang, J.Y.; Gao, J. Neuroprotective effects of gallic acid against hypoxia/reoxygenation-induced mitochondrial dysfunctions in vitro and cerebral ischemia/reperfusion injury in vivo. Brain Res. 2014, 1589, 126–139. [Google Scholar] [CrossRef]
- Wang, L.L.; Tian, X. Epigallocatechin-3-Gallate Protects against Homocysteine-Induced Brain Damage in Rats. Planta Med. 2018, 84, 34–41. [Google Scholar] [CrossRef]
- Hwang, E.; Park, S.Y.; Lee, H.J.; Lee, T.Y.; Sun, Z.W.; Yi, T.H. Gallic Acid Regulates Skin Photoaging in UVB-exposed Fibroblast and Hairless Mice. Phytother. Res. 2014, 28, 1778–1788. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, D.; Chitnis, A.; Talekar, A.; Mulay, P.; Makkar, M.; James, J.; Thirumurugan, K. Hormetic efficacy of rutin to promote longevity in. Biogerontology 2017, 18, 397–411. [Google Scholar] [CrossRef]
- Liu, H.X.; Xu, Q.X.; Wufuer, H.; Li, Z.; Sun, R.; Jiang, Z.R.; Dou, X.F.; Fu, Q.; Campisi, J.; Sun, Y. Rutin is a potent senomorphic agent to target senescent cells and can improve chemotherapeutic efficacy. Aging Cell 2024, 23, e13921. [Google Scholar] [CrossRef] [PubMed]
- Bazyar, H.; Javid, A.Z.; Ahangarpour, A.; Zaman, F.; Hosseini, S.A.; Zohoori, V.; Aghamohammadi, V.; Yazdanfar, S.; Cheshmeh, M.G.D. The effects of rutin supplement on blood pressure markers, some serum antioxidant enzymes, and quality of life in patients with type 2 diabetes mellitus compared with placebo. Front. Nutr. 2023, 10, 1214420. [Google Scholar] [CrossRef] [PubMed]
- Oluranti, O.I.; Alabi, B.A.; Michael, O.S.; Ojo, A.O.; Fatokun, B.P. Rutin prevents cardiac oxidative stress and inflammation induced by bisphenol A and dibutyl phthalate exposure via NRF-2/NF-κB pathway. Life Sci. 2021, 284, 119878. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.S.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Yannakoulia, M.; Ntanasi, E.; Anastasiou, C.A.; Scarmeas, N. Frailty and nutrition: From epidemiological and clinical evidence to potential mechanisms. Metabolism 2017, 68, 64–76. [Google Scholar] [CrossRef]
- Oei, S.; Millar, C.L.; Lily, T.N.N.; Mukamal, K.J.; Kiel, D.P.; Lipsitz, L.A.; Hannan, M.T.; Sahni, S. Higher intake of dietary flavonols, specifically dietary quercetin, is associated with lower odds of frailty onset over 12 years of follow-up among adults in the Framingham Heart Study. Am. J. Clin. Nutr. 2023, 118, 27–33. [Google Scholar] [CrossRef]
- Maher, P. Modulation of multiple pathways involved in the maintenance of neuronal function during aging by fisetin. Genes. Nutr. 2009, 4, 297–307. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin is a senotherapeutic that extends health and lifespan. Ebiomedicine 2018, 36, 18–28. [Google Scholar] [CrossRef]
- Takaya, K.; Asou, T.; Kishi, K. Fisetin, a potential skin rejuvenation drug that eliminates senescent cells in the dermis. Biogerontology 2024, 25, 161–175. [Google Scholar] [CrossRef]
- Ding, H.G.; Li, Y.; Chen, S.L.; Wen, Y.; Zhang, S.Y.; Luo, E.S.; Li, X.S.; Zhong, W.H.; Zeng, H.K. Fisetin ameliorates cognitive impairment by activating mitophagy and suppressing neuroinflammation in rats with sepsis-associated encephalopathy. Cns Neurosci. Ther. 2022, 28, 247–258. [Google Scholar] [CrossRef]
- Maher, P.; Akaishi, T.; Abe, K. Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proc. Natl. Acad. Sci. USA 2006, 103, 16568–16573. [Google Scholar] [CrossRef]
- Hair, R.; Sakaki, J.R.; Chun, O.K. Anthocyanins, Microbiome and Health Benefits in Aging. Molecules 2021, 26, 537. [Google Scholar] [CrossRef]
- Sharma, P.; McClees, S.F.; Afaq, F. Pomegranate for Prevention and Treatment of Cancer: An Update. Molecules 2017, 22, 177. [Google Scholar] [CrossRef]
- Li, J.; Zhao, R.T.; Zhao, H.; Chen, G.Y.; Jiang, Y.H.; Lyu, X.L.; Wu, T. Reduction of Aging-Induced Oxidative Stress and Activation of Autophagy by Bilberry Anthocyanin Supplementation via the AMPK-mTOR Signaling Pathway in Aged Female Rats. J. Agric. Food Chem. 2019, 67, 7832–7843. [Google Scholar] [CrossRef]
- Correia, P.; Araújo, P.; Ribeiro, C.; Oliveira, H.; Pereira, A.R.; Mateus, N.; de Freitas, V.; Bras, N.F.; Gameiro, P.; Coelho, P.; et al. Anthocyanin-Related Pigments: Natural Allies for Skin Health Maintenance and Protection. Antioxidants 2021, 10, 1038. [Google Scholar] [CrossRef]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef]
- Annesley, S.J.; Fisher, P.R. Mitochondria in Health and Disease. Cells 2019, 8, 680. [Google Scholar] [CrossRef]
- Park, C.B.; Larsson, N.G. Mitochondrial DNA mutations in disease and aging. J. Cell Biol. 2011, 193, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Luo, Y.J.; Chen, X.J. Quercetin attenuates mitochondrial dysfunction and biogenesis via upregulated AMPK/SIRT1 signaling pathway in OA rats. Biomed. Pharmacother. 2018, 103, 1585–1591. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, C.; Dai, S.; Liu, Y.; Zhang, F.; Peng, C.; Li, Y. Quercetin Protects Ethanol-Induced Hepatocyte Pyroptosis via Scavenging Mitochondrial ROS and Promoting PGC-1alpha-Regulated Mitochondrial Homeostasis in L02 Cells. Oxid. Med. Cell Longev. 2022, 2022, 4591134. [Google Scholar] [CrossRef] [PubMed]
- Mizuguchi, Y.; Hatakeyama, H.; Sueoka, K.; Tanaka, M.; Goto, Y.I. Low dose resveratrol ameliorates mitochondrial respiratory dysfunction and enhances cellular reprogramming. Mitochondrion 2017, 34, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, X.; Fan, W.; Pandovski, S.; Tian, Y.; Dillin, A. Inter-tissue communication of mitochondrial stress and metabolic health. Life Metab. 2023, 2, load001. [Google Scholar] [CrossRef] [PubMed]
- Nargund, A.M.; Pellegrino, M.W.; Fiorese, C.J.; Baker, B.M.; Haynes, C.M. Mitochondrial Import Efficiency of ATFS-1 Regulates Mitochondrial UPR Activation. Science 2012, 337, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, X.Y.; Chen, P.; Liu, L.M.; Xin, N.; Tian, Y.; Dillin, A. The Mitochondrial Unfolded Protein Response Is Mediated Cell-Non-autonomously by Retromer-Dependent Wnt Signaling. Cell 2018, 174, 870–883.e17. [Google Scholar] [CrossRef]
- Suárez-Rivero, J.M.; Pastor-Maldonado, C.J.; Romero-González, A.; Gómez-Fernandez, D.; Povea-Cabello, S.; Alvarez-Córdoba, M.; Villalón-García, I.; Talaverón-Rey, M.; Suárez-Carrillo, A.; Munuera-Cabeza, M.; et al. Pterostilbene in Combination with Mitochondrial Cofactors Improve Mitochondrial Function in Cellular Models of Mitochondrial Diseases. Front. Pharmacol. 2022, 13, 862085. [Google Scholar] [CrossRef]
- Jardim, F.R.; de Rossi, F.T.; Nascimento, M.X.; Barros, R.G.D.; Borges, P.A.; Prescilio, I.C.; de Oliveira, M.R. Resveratrol and Brain Mitochondria: A Review. Mol. Neurobiol. 2018, 55, 2085–2101. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Seo, H.; Cho, M.; Kim, J.; Chung, H.S.; Lee, I.; Kim, M.J. Rutin inhibits DRP1-mediated mitochondrial fission and prevents ethanol-induced hepatotoxicity in HepG2 cells and zebrafish. Anim. Cells Syst. 2021, 25, 74–81. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Qu, J.; Liu, G.H.; Belmonte, J.C.I. The ageing epigenome and its rejuvenation. Nat. Rev. Mol. Cell Bio 2020, 21, 137–150. [Google Scholar] [CrossRef]
- Sen, P.; Shah, P.P.; Nativio, R.; Berger, S.L. Epigenetic Mechanisms of Longevity and Aging. Cell 2016, 166, 822–839. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Hayano, M.; Griffin, P.T.; Amorim, J.A.; Bonkowski, M.S.; Apostolides, J.K.; Salfati, E.L.; Blanchette, M.; Munding, E.M.; Bhakta, M.; et al. Loss of epigenetic information as a cause of mammalian aging. Cell 2023, 186, 305–326. [Google Scholar] [CrossRef] [PubMed]
- Lowe, R.; Barton, C.; Jenkins, C.A.; Ernst, C.; Forman, O.; Fernandez-Twinn, D.S.; Bock, C.; Rossiter, S.J.; Faulkes, C.G.; Ozanne, S.E.; et al. Ageing-associated DNA methylation dynamics are a molecular readout of lifespan variation among mammalian species. Genome Biol. 2018, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.P.; Hung, P.F.; Ku, W.Y.; Chang, C.Y.; Wu, B.H.; Wu, M.H.; Yao, J.Y.; Yang, J.R.; Lee, C.H. The inhibitory activity of gallic acid against DNA methylation: Application of gallic acid on epigenetic therapy of human cancers. Oncotarget 2018, 9, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Griñán-Ferré, C.; Bellver-Sanchis, A.; Izquierdo, V.; Corpas, R.; Roig-Soriano, J.; Chillón, M.; Andres-Lacueva, C.; Somogyvári, M.; Soti, C.; Sanfeliu, C.; et al. The pleiotropic neuroprotective effects of resveratrol in cognitive decline and Alzheimer’s disease pathology: From antioxidant to epigenetic therapy. Ageing Res. Rev. 2021, 67, 101271. [Google Scholar] [CrossRef] [PubMed]
- Cione, E.; La Torre, C.; Cannataro, R.; Caroleo, M.C.; Plastina, P.; Gallelli, L. Quercetin, Epigallocatechin Gallate, Curcumin, and Resveratrol: From Dietary Sources to Human MicroRNA Modulation. Molecules 2020, 25, 63. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.L.; Yang, S.Y.; Cai, L.L.; Qin, L.Q.; Li, B.Y.; Wan, Z.X. Effects of Quercetin Intervention on Cognition Function in APP/PS1 Mice was Affected by Vitamin D Status. Mol. Nutr. Food Res. 2018, 62, 1800621. [Google Scholar] [CrossRef]
- Boesch-Saadatmandi, C.; Wagner, A.E.; Wolffram, S.; Rimbach, G. Effect of quercetin on inflammatory gene expression in mice liver—role of redox factor 1, miRNA-122 and miRNA-125b. Pharmacol. Res. 2012, 65, 523–530. [Google Scholar] [CrossRef]
- Zhang, X.; Abels, E.R.; Redzic, J.S.; Margulis, J.; Finkbeiner, S.; Breakefield, X.O. Potential Transfer of Polyglutamine and CAG-Repeat RNA in Extracellular Vesicles in Huntington’s Disease: Background and Evaluation in Cell Culture. Cell Mol. Neurobiol. 2016, 36, 459–470. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef]
- Wang, L.M.; Klionsky, D.J.; Shen, H.M. The emerging mechanisms and functions of microautophagy. Nat. Rev. Mol. Cell Bio 2023, 24, 186–203. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Liu, Y.; Huang, C.; Fan, S.J. Targeting stress granules in neurodegenerative diseases: A focus on biological function and dynamics disorders. Biofactors 2024, 50, 422–438. [Google Scholar] [CrossRef]
- Rusmini, P.; Cortese, K.; Crippa, V.; Cristofani, R.; Cicardi, M.E.; Ferrari, V.; Vezzoli, G.; Tedesco, B.; Meroni, M.; Messi, E.; et al. Trehalose induces autophagy via lysosomal-mediated TFEB activation in models of motoneuron degeneration. Autophagy 2019, 15, 631–651. [Google Scholar] [CrossRef] [PubMed]
- Henderson, M.X.; Trojanowski, J.Q.; Lee, V.M.Y. α-Synuclein pathology in Parkinson’s disease and related α-synucleinopathies. Neurosci. Lett. 2019, 709, 134316. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, J.; Pakrashi, S.; Sarbajna, A.; Dutta, M.; Bandyopadhyay, J. Quercetin Attenuates Copper-Induced Apoptotic Cell Death and Endoplasmic Reticulum Stress in SH-SY5Y Cells by Autophagic Modulation. Biol. Trace Elem. Res. 2022, 200, 5022–5041. [Google Scholar] [CrossRef]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
- Xiong, R.; Zhou, X.G.; Tang, Y.; Wu, J.M.; Sun, Y.S.; Teng, J.F.; Pan, R.; Law, B.Y.; Zhao, Y.; Qiu, W.Q.; et al. Lychee seed polyphenol protects the blood-brain barrier through inhibiting Abeta(25–35)-induced NLRP3 inflammasome activation via the AMPK/mTOR/ULK1-mediated autophagy in bEnd.3 cells and APP/PS1 mice. Phytother. Res. 2021, 35, 954–973. [Google Scholar] [CrossRef]
- Holczer, M.; Besze, B.; Zambo, V.; Csala, M.; Banhegyi, G.; Kapuy, O. Epigallocatechin-3-Gallate (EGCG) Promotes Autophagy-Dependent Survival via Influencing the Balance of mTOR-AMPK Pathways upon Endoplasmic Reticulum Stress. Oxid. Med. Cell Longev. 2018, 2018, 6721530. [Google Scholar] [CrossRef]
- Pineda-Ramirez, N.; Aguilera, P. Resveratrol as an inductor of autophagy: Is there a unique pathway of activation? Neural Regen. Res. 2021, 16, 101–103. [Google Scholar] [CrossRef]
- Ruetenik, A.; Barrientos, A. Dietary restriction, mitochondrial function and aging: From yeast to humans. Biochim. Biophys. Acta 2015, 1847, 1434–1447. [Google Scholar] [CrossRef]
- Hwangbo, D.S.; Lee, H.Y.; Abozaid, L.S.; Min, K.J. Mechanisms of Lifespan Regulation by Calorie Restriction and Intermittent Fasting in Model Organisms. Nutrients 2020, 12, 1194. [Google Scholar] [CrossRef]
- Waziry, R.; Ryan, C.P.; Corcoran, D.L.; Huffman, K.M.; Kobor, M.S.; Kothari, M.; Graf, G.H.; Kraus, V.B.; Kraus, W.E.; Lin, D.T.S.; et al. Effect of long-term caloric restriction on DNA methylation measures of biological aging in healthy adults from the CALERIE trial. Nat. Aging 2023, 3, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Carroll, B.; Korolchuk, V.I. Nutrient sensing, growth and senescence. FEBS J. 2018, 285, 1948–1958. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, O.; Youm, Y.; Shchukina, I.; Ryu, S.; Sidorov, S.; Ravussin, A.; Nguyen, K.; Aladyeva, E.; Predeus, A.N.; Smith, S.R.; et al. Caloric restriction in humans reveals immunometabolic regulators of health span. Science 2022, 375, 671–677. [Google Scholar] [CrossRef]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef]
- Kocaadam, B.; Sanlier, N. Curcumin, an active component of turmeric, and its effects on health. Crit. Rev. Food Sci. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- Chen, P.; Chen, F.C.; Lei, J.X.; Zhou, B.H. Pomegranate polyphenol punicalagin improves learning memory deficits, redox homeostasis, and neuroinflammation in aging mice. Phytother. Res. 2023, 37, 3655–3674. [Google Scholar] [CrossRef]
- Angele-Martinez, C.; Ameer, F.S.; Raval, Y.S.; Huang, G.; Tzeng, T.J.; Anker, J.N.; Brumaghim, J.L. Polyphenol effects on CuO-nanoparticle-mediated DNA damage, reactive oxygen species generation, and fibroblast cell death. Toxicol. In Vitro 2022, 78, 105252. [Google Scholar] [CrossRef]
- Dobrzynska, M.M.; Gajowik, A. Protection and Mitigation by Resveratrol of DNA Damage Induced in Irradiated Human Lymphocytes In Vitro. Radiat. Res. 2022, 197, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Priya, B.; Ravi, S.; Kirubakaran, S. Targeting ATM and ATR for cancer therapeutics: Inhibitors in clinic. Drug Discov. Today 2023, 28, 103662. [Google Scholar] [CrossRef]
- Thilakarathna, W.; Rupasinghe, H.P.V. Microbial metabolites of proanthocyanidins reduce chemical carcinogen-induced DNA damage in human lung epithelial and fetal hepatic cells in vitro. Food Chem. Toxicol. 2019, 125, 479–493. [Google Scholar] [CrossRef]
- Zeng, X.; Li, X.; Li, X.; Wei, C.; Shi, C.; Hu, K.; Kong, D.; Luo, Q.; Xu, Y.; Shan, W.; et al. Fecal microbiota transplantation from young mice rejuvenates aged hematopoietic stem cells by suppressing inflammation. Blood 2023, 141, 1691–1707. [Google Scholar] [CrossRef]
- Parker, A.; Romano, S.; Ansorge, R.; Aboelnour, A.; Le Gall, G.; Savva, G.M.; Pontifex, M.G.; Telatin, A.; Baker, D.; Jones, E.; et al. Fecal microbiota transfer between young and aged mice reverses hallmarks of the aging gut, eye, and brain. Microbiome 2022, 10, 68. [Google Scholar] [CrossRef]
- Barcena, C.; Valdes-Mas, R.; Mayoral, P.; Garabaya, C.; Durand, S.; Rodriguez, F.; Fernandez-Garcia, M.T.; Salazar, N.; Nogacka, A.M.; Garatachea, N.; et al. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat. Med. 2019, 25, 1234–1242. [Google Scholar] [CrossRef]
- Chen, L.; Zhernakova, D.V.; Kurilshikov, A.; Andreu-Sanchez, S.; Wang, D.; Augustijn, H.E.; Vich Vila, A.; Lifelines Cohort, S.; Weersma, R.K.; Medema, M.H.; et al. Influence of the microbiome, diet and genetics on inter-individual variation in the human plasma metabolome. Nat. Med. 2022, 28, 2333–2343. [Google Scholar] [CrossRef]
- Mou, Y.; Du, Y.; Zhou, L.; Yue, J.; Hu, X.; Liu, Y.; Chen, S.; Lin, X.; Zhang, G.; Xiao, H.; et al. Gut Microbiota Interact with the Brain through Systemic Chronic Inflammation: Implications on Neuroinflammation, Neurodegeneration, and Aging. Front. Immunol. 2022, 13, 796288. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kumar, R.; Sharma, A.; Goel, A.; Padwad, Y. Long-term consumption of green tea EGCG enhances murine health span by mitigating multiple aspects of cellular senescence in mitotic and post-mitotic tissues, gut dysbiosis, and immunosenescence. J. Nutr. Biochem. 2022, 107, 109068. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Su, Z.; Mackenzie, G.G. Chlorogenic acid combined with epigallocatechin-3-gallate mitigates D-galactose-induced gut aging in mice. Food Funct. 2023, 14, 2684–2697. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wu, X.; Qiu, L.; Wan, R.; Zhu, X.; Chen, S.; Yang, X.; Liu, X.; Wu, J. Quercetin influences intestinal dysbacteriosis and delays alveolar epithelial cell senescence by regulating PTEN/PI3K/AKT signaling in pulmonary fibrosis. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 4809–4822. [Google Scholar] [CrossRef]
- Yan, C.J.; Zhou, Z. Ellagic acid and pentagalloylglucose are potential inhibitors of prion protein fibrillization. Int. J. Biol. Macromol. 2021, 172, 371–380. [Google Scholar] [CrossRef]

| Polyphenols | Source | Age-Related Conditions | Model | Dosage | Anti-Aging Activity and Proposed Anti-Aging Mechanism |
|---|---|---|---|---|---|
Resveratrol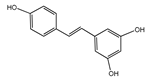 | Grapes, red wine, peanuts, and blueberries | Age-related motor deficits | Older adults | 500 mg/day, 1000 mg/day | A pilot randomized controlled trial indicates that the combined application of exercise training and resveratrol in elderly people with functional limitations could improve skeletal muscle mitochondrial function and exercise-related physical function indicators [58]. |
| Sarcopenic obesity | Sprague-Dawley (SD) rats | 0.4% of diet | Resveratrol could ameliorate mitochondrial dysfunction and oxidative stress, thereby improving protein metabolism and helping to prevent sarcopenic obesity in the elderly [59]. | ||
| Aging | ddY mice (derived from a non-inbred strain carrying a retrovirus causing significant mortality with age) | 0.4 g/kg of diet | Resveratrol could reduce the level of acetylated proteins in muscles and restore autophagic activity, thereby alleviating age-related sarcopenia and cardiomyocyte hypertrophy [60]. | ||
| Aging | HtrA2 KO mice (the absence of serine/threonine protease HtrA2 causes a PD phenotype) | 25 mg/kg body weight (BW) | Resveratrol treatment can extend the lifespan of HtrA2 KO mice and delay the deterioration of the motor phenotype by attenuating apoptosis at the level of Bax [61]. | ||
| Aging | Caenorhabditis elegans (C. elegans) | 100 mM | Resveratrol, as a Sirtuin activator, can simulate calorie restriction and extend the lifespan of C. elegans [62]. | ||
| Aging | Drosophila melanogaster | 200 μM | Supplementation of resveratrol to larval can effectively eliminate ROS, thereby extending the adult lifespan of fruit flies and not reducing their reproductive capacity [63]. | ||
| Ovarian aging | N. guentheri | 200 μg/g food | Resveratrol could delay ovarian aging by alleviating inflammation and ER stress through the SIRT1/NRF2 pathway [64]. | ||
| Aging | S. cerevisiae | 10, 100, 500 μM | Resveratrol could mimic calorie restriction by stimulating Sir2, increase DNA stability, and extend lifespan by 70% in yeast [65]. | ||
| Aging | Human mononuclear cells (PBMCs) | 5 µM | Compared with the elderly, resveratrol exerts better antioxidant and anti-inflammatory effects in PBMCs of middle-aged individuals [66]. | ||
| Brain aging | Hippocampal astrocytes | 10 μM | Resveratrol could increase the antioxidant defense capacity and reduce pro-inflammatory cytokines in hippocampal astrocyte cultures of rats of all ages, improving NDs [67]. | ||
| Age-related motor deficits | SH-SY5Y cells | 1 or 5 μM | Resveratrol alleviates age-related motor deficits by promoting the survival of dopaminergic neurons and the activation of the ERK1/2 pathway [68]. | ||
Ellagic acid  | Berries, nuts, tea, and medicinal plants [69] | Aging | D-gal-induced aging rats | 120 mg/kg BW | EA improved oxidative damage and inflammation in D-gal-induced aging rats [70]. |
| Hepatic lipid metabolism disorders induced by aging | 25-month-old rats | 30 mg/kg BW | EA affected lipid metabolism in the aged liver via the sirt1/AMPK/sreBP-1c/PPAr-α pathway for age-related metabolic disorders [71]. | ||
| Brain aging | SD rats | 10, 30, 90 mg/kg BW | EA could increase the proliferation of brain neural stem cells and protect brain cell activity through the Wnt/β-catenin signaling pathway to help treat nerve dysfunction, NDs, and aging [72]. | ||
| Osteoporosis | C57BL/6 mice, bone mesenchymal stem cells | Mice: 10, 50 mg/kg BW; cell: 0 μM–30 μM | EA could promote bone formation by activating the SMAD2/3 signaling pathway to ameliorate aging-induced osteoporosis [73]. | ||
| Skin aging | Male SKH-1 hairless mice, human dermal fibroblasts | Mice: 10 μmol/L, cell: 1–10 μmol/L | EA could reduce wrinkles and UV-induced skin inflammation to improve photoaging by decreasing UV-B-induced collagen degradation and inflammatory reactions [74]. | ||
| Aging | C. elegans | 50 μM | EA reduced the injury caused by UV radiation and enhanced stress resistance to extend the lifespan of C. elegans [75]. | ||
| Aging | Drosophila melanogaster | 100 μM and 200 μM | EA can up-regulate the expression of dFOXO, CAT, and SOD, thereby extending lifespan [76]. | ||
| Aging | SH-SY5Y cells | 0.1–1 μM | Lower concentrations of EA could provide better anti-aging benefits than higher concentrations of EA and metformin, presumably via the PPAR-γ/ HO-1 signaling pathway [77]. | ||
| Skin sagging and wrinkling | Human dermal fibroblasts | 2 μg/mL | EA improved skin extracellular matrix production of elastin and collagen and improved skin fine wrinkles [78]. | ||
Urolithin A  | Gut microbiota metabolite of EA | Aging | Healthy elderly male and female | 250, 500, 1000 and 2000 mg | UA could promote mitochondrial autophagy and improve muscle health in old animals and in preclinical models of aging [79]. |
| Aging | D-gal-induced aging mice | 3, 15 mg/kg BW | Uro-A from the colon can prevent D-gal-induced aging in mice by blocking NF-κB and mTOR targets, improving motor and cognitive abilities [80]. | ||
| Alzheimer’s disease (AD) | 3xTg-AD mice, B6129SF2/J mice | 5 mg/kg BW | UA could improve the cognition of 3xTg-AD mice and prolong longevity in normal aging mice by inducing autophagy and increasing amyloid-β (Aβ) clearance in neuronal cells [81]. | ||
| Aging | C. elegans, C57BL/6 mice | C. elegans: 50 μM, mice: 50 mg/kg BW | Urolithin A could induce mitophagy and improve mitochondrial and muscle function in C. elegans and rodents, leading to life extension [82]. | ||
| Brain aging | H2O2-induced PC12 cell, D-gal-induced aging mice | Cell: 10, 30, 50 μg/mL, Mice: 50, 100, 150 mg/kg body weight | Urolithin A can exert neuroprotective effects and delay brain aging by activating miR-34a-mediated SIRT1/mTOR signaling pathway [83]. | ||
| Aging | D-gal-induced aging mice | 50, 100, 150 mg/kg BW | UA could attenuate D-gal-induced liver injury in aged mice via antioxidant, anti-inflammatory, and anti-apoptotic properties [84]. | ||
Gallic acid 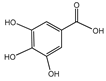 | Rheum palmatum, Eucalyptus robusta, Cornus officinalis | Age-associated thymic involution | D-gal-treated mice | 200, 250, 500 mg/kg body weight | GA administration may ameliorate age-related thymic degeneration and enhance immune function in the elderly by stimulating FoxN1 expression, increasing proliferating cells, and decreasing apoptotic cells [85]. |
| Aging | H2O2-induced rat’s embryonic fibroblast cells | 1000 μM | GA could postpone aging through its antioxidative stress potential and modulation of mitochondrial complexes’ activities [86]. | ||
| AD | APP/PS1 transgenic AD mouse model | 20 mg/kg body weight | EA could inhibit neuroinflammation and stabilize brain oxidative stress [87]. | ||
Rutin | Fagopyrum esculentum, Ruta graveolens, and Sophora japonica [88] | Aging | Drosophila melanogaster | 200 μM and 400 μM | Rutin could improve the resistance of male and female Drosophila melanogaster fed with a high-fat diet, increase the expression of age-related genes, and extend longevity [89]. |
| Huntington’s disease | C. elegans | 15, 30, 60, 120 μM | Rutin could inhibit polyglutamine protein aggregation in muscle, decrease neuronal death, and extend longevity through antioxidant, autophagy, and insulin/IGF1 signaling pathways [90]. | ||
| Aging | D-gal-treated mice | 50 mg/kg body weight | Rutin could enhance the biochemical indicators of aging rats by exerting antioxidant effects and regulating apoptosis-related proteins to inhibit cell apoptosis [91]. | ||
| Age-related metabolic dysfunction | Twenty-month-old rats | 25, 50 mg/kg body weight | Rutin suppresses aging-associated mitochondrial dysfunction, endoplasmic reticulum stress, and oxidative stress, thus improving the response to age-related metabolic dysfunction [92]. | ||
| AD | TgAPP mice (a model for AD) | 30 mg/kg body weight | Rutin could raise GSH/GSSG levels, reduce MDA levels, and inhibit APP expression and BACE1 activity, resulting in anti-AD and anti-aging benefits [93]. | ||
Quercetin | Grapes, peaches, onions, garlic [94] | Age-related metabolic dysfunction | Twenty-one-month-old mice | Dasatinib (5 mg/kg BW) and quercetin (50 mg/kg BW) | Dasatinib + quercetin (D + Q) lowers inflammation in adipose tissue and enhances systemic metabolic function in older people [95]. |
| Age-dependent progression of disc degeneration | C57BL/6 mice | Dasatinib (5 mg/kg BW) and quercetin (50 mg/kg BW) | D + Q can target senescent cells non-invasively and lessen the impact of age-dependent degeneration [96]. | ||
| Aging | Macaca fascicularis | Dasatinib (5 mg/kg BW) and quercetin (50 mg/kg BW) | D + Q could prevent aging by strengthening the gut barrier, boosting immunity, and combating inflammation [97]. | ||
| Aging | C57BL/6 mice | Dasatinib (5 mg/kg BW) and quercetin (50 mg/kg BW) | D + Q could lead to selective elimination of senescent cells and the release of senescence-associated pro-inflammatory cytokines [98]. | ||
| Intestinal senescence | BALB/c mice | Dasatinib (5 mg/kg BW) and quercetin (50 mg/kg BW) | Long-term D + Q treatment could decrease the gene expression of senescence and inflammation to alleviate intestinal senescence [9]. | ||
Naringenin | Ribes meyeri | Aging | C57BL/6 mice | 25, 50, 100 mg/kg BW | Naringenin could reduce neuronal damage and mitigate systemic inflammation induced by LPS by modulating the expression of NF-κB/TNFα/COX-2/iNOS/TLR4/GFAP [99]. |
| Retinal degeneration | C57BL/6 mice | 100 mg/kg BW | Oral administration of naringenin could regulate mitochondrial dynamics and autophagy to counteract aging-related retinal degeneration [100]. | ||
| Aging | C. elegans, 6-month-old C57BL/6J mice | C. elegans: 100 μM, mice: 100 mg/kg BW | Naringenin could extend the lifespan of C. elegans and slow brain aging in mice by increasing the expression of SIRT enzymes, promoting the activity of metabolic enzymes, and upregulating the expression of anti-aging markers [101]. | ||
Cyanidin-3-O-glucoside (Cy3G) | Vegetables and berries [102] | AD | HMC3 cell, APPswe/PS1ΔE9 mice (a model for AD) | cell: 50 μM, mice: 30 mg/kg BW | Cy3G could eliminate accumulated β-amyloid and modulate microglial polarization by activating PPARγ and promoting Aβ42 phagocytosis through TREM2 overexpression [103]. |
| AD | APPswe/PS1ΔE9 mice | 30 mg/kg BW | Cy3G could reduce lysosome-associated protein expression, increase autophagy, and modulate the PI3K/Akt/GSK3β signaling pathway to protect neurons and enhance cognitive performance in AD mice [104]. | ||
| AD | SD rats received Aβ in the hippocampus | 10 mg/kg BW | Cy3G could attenuate Aβ-induced tau protein hyperphosphorylation and GSK-3β hyperactivation, possibly rescuing Aβ-induced cognitive deficits through GSK-3β/tau variation [105]. | ||
| AD | APPswe/PS1ΔE9 mice | 30 mg/kg BW | Cy3G may exert therapeutic effects on AD through antioxidant and immunomodulatory mechanisms [106]. | ||
| Parkinson’s disease (PD) | MPTP-induced C57BL/6J | 10, 20, 40 mg/kg BW | Cy3G could play a role in the treatment of PD by modulating the structure and metabolism of gut microbiota [107]. | ||
| Aging | C. elegans | 12.5, 25, 50 μg/mL | Cy3G could enhance resistance and extend the lifespan of polystyrene-exposed C. elegans through the DAF-16 pathway [108]. | ||
| Aging | H9c2 cells | 1 mM | Cy3G could decrease CD38 expression, increase Sirt6 expression in tissues, and restore NAD+ and NK cell levels to exert anti-aging effects through CD38-Sirt6 signaling [109]. | ||
Procyanidin C1 (PCC1) | Grape seed extract | Aging | PSC27 cells, WI38 cells, HUVEC cells; C57BL/6J mice | Cell: 1, 5, 10 μg/mL Mice: 20 mg/kg BW | Low concentrations of PCC1 can inhibit the formation of SASP, while at higher concentrations, it may selectively kill senescent cells by promoting the production of ROS and mitochondrial dysfunction [110]. |
| Aged retina | Aged mice | 8 mg/kg of diet | Long-term PCC1 treatment could relieve function and structural impairment in the aged retina and reduce the accumulation of senescent cells and secretion of SASP [111]. | ||
Nordihydroguaiaretic acid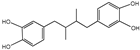 | Larrea tridentata | Aging | UM-HET3 mice (designed to model the aging process and age-related diseases) | 2.5, 5 g/kg of diet | Nordihydroguaiaretic acid could increase the survival rate of male mice but does not change the survival rate of female mice, which might be explained by gender differences in steady-state levels or drug metabolism [112]. |
Pterostilbene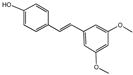 | Blueberries | AD | Senescence accelerated mouse prone 8 (SAMP8) mice | 120 mg/kg of diet | Pterostilbene could increase the expression of peroxisome proliferator-activated receptor (PPAR) α, improve cognitive function and cellular stress regulation ability, and alleviate AD symptoms [113]. |
| AD | 18-month-old rats | 22.5 mg/kg BW | Pterostilbene could increase the expression of postsynaptic density protein 95 and improve the cognitive ability of elderly rats with mild cognitive impairment AD [114]. | ||
| AD | 19-month-old male Fischer | 40, 160 mg/kg of diet | Pterostilbene can effectively reverse cognitive behavioral deficits and dopamine release and improve age-related cognitive degeneration [115]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Fang, M.; Tu, X.; Mo, X.; Zhang, L.; Yang, B.; Wang, F.; Kim, Y.-B.; Huang, C.; Chen, L.; et al. Dietary Polyphenols as Anti-Aging Agents: Targeting the Hallmarks of Aging. Nutrients 2024, 16, 3305. https://doi.org/10.3390/nu16193305
Liu Y, Fang M, Tu X, Mo X, Zhang L, Yang B, Wang F, Kim Y-B, Huang C, Chen L, et al. Dietary Polyphenols as Anti-Aging Agents: Targeting the Hallmarks of Aging. Nutrients. 2024; 16(19):3305. https://doi.org/10.3390/nu16193305
Chicago/Turabian StyleLiu, Ying, Minglv Fang, Xiaohui Tu, Xueying Mo, Lu Zhang, Binrui Yang, Feijie Wang, Young-Bum Kim, Cheng Huang, Liang Chen, and et al. 2024. "Dietary Polyphenols as Anti-Aging Agents: Targeting the Hallmarks of Aging" Nutrients 16, no. 19: 3305. https://doi.org/10.3390/nu16193305
APA StyleLiu, Y., Fang, M., Tu, X., Mo, X., Zhang, L., Yang, B., Wang, F., Kim, Y.-B., Huang, C., Chen, L., & Fan, S. (2024). Dietary Polyphenols as Anti-Aging Agents: Targeting the Hallmarks of Aging. Nutrients, 16(19), 3305. https://doi.org/10.3390/nu16193305







