A Risk Correlative Model for Sleep Disorders in Chinese Older Adults Based on Blood Micronutrient Levels: A Matched Case-Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Diagnosis of Sleep Disorders
2.3. Other Variables
2.4. Measurement of Micronutrient Levels
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of the Study Population
3.2. Comparison of the Micronutrient Levels between the Sleep Disorders and Controls
3.3. Multiple Micronutrient Status and Risk of Sleep Disorders
3.4. RF of Micronutrients on Sleep Disorders Impacts
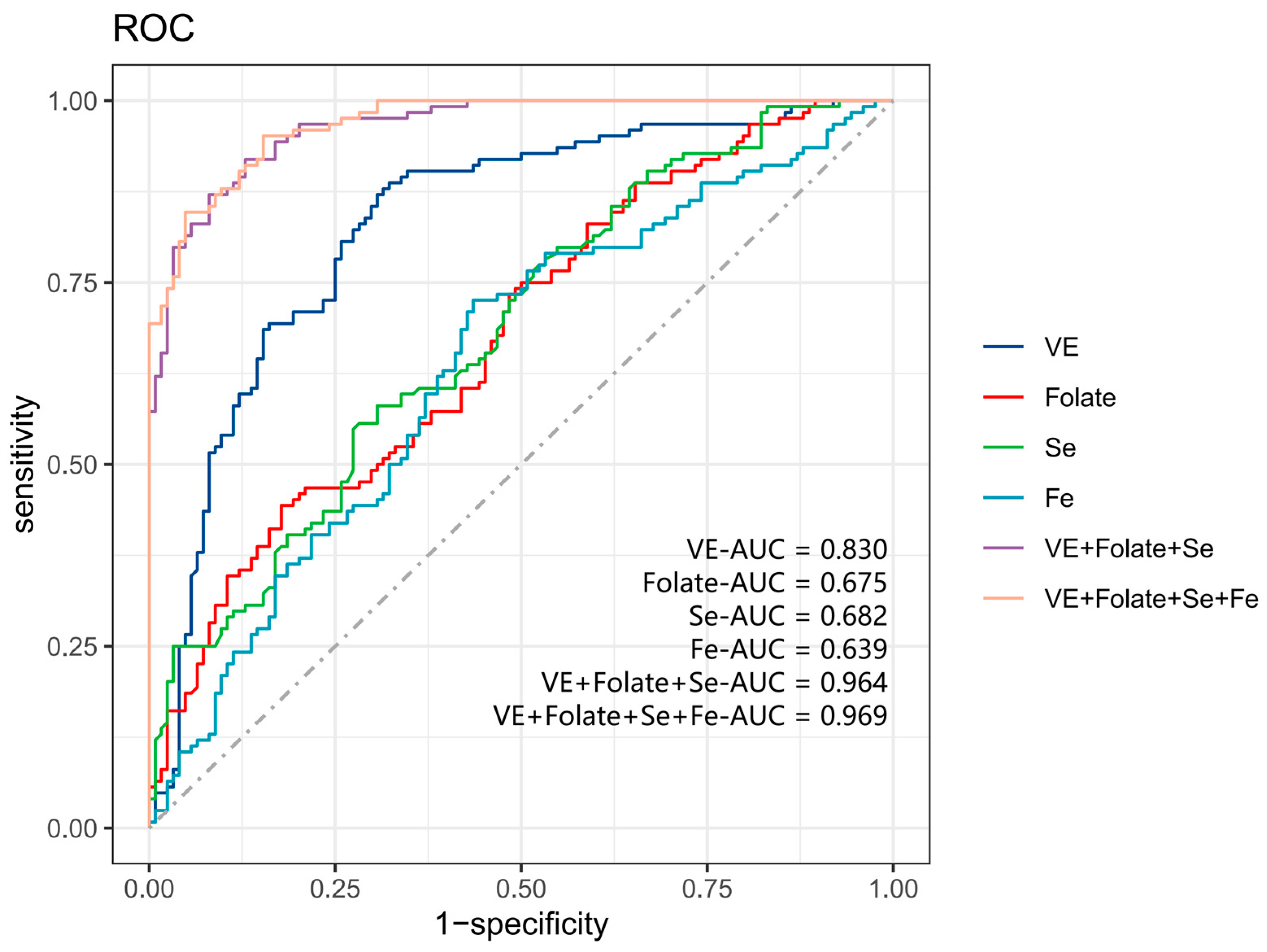
3.5. ROC Analysis of Micronutrients with Sleep Disorders
3.6. Nomogram Analysis of Micronutrients with Sleep Disorders
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosen, R.S.; Yarmush, M.L. Current Trends in Anti-Aging Strategies. Annu. Rev. Biomed. Eng. 2023, 25, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Yaremchuk, K. Sleep Disorders in the Elderly. Clin. Geriatr. Med. 2018, 34, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Porter, V.R.; Buxton, W.G.; Avidan, A.Y. Sleep, Cognition and Dementia. Curr. Psychiatry Rep. 2015, 17, 97. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.Y.; Cai, L.; Fan, L.M.; Zhao, M.; Cui, W.L.; Yang, J.T.; Golden, A.R. Association of socioeconomic factors and prevalence of hypertension with sleep disorder among the elderly in rural southwest China. Sleep Med. 2020, 71, 106–110. [Google Scholar] [CrossRef]
- Basnet, S.; Merikanto, I.; Lahti, T.; Männistö, S.; Laatikainen, T.; Vartiainen, E.; Partonen, T. Associations of common chronic non-communicable diseases and medical conditions with sleep-related problems in a population-based health examination study. Sleep Sci. 2016, 9, 249–254. [Google Scholar] [CrossRef]
- Baranwal, N.; Yu, P.K.; Siegel, N.S. Sleep physiology, pathophysiology, and sleep hygiene. Prog. Cardiovasc. Dis. 2023, 77, 59–69. [Google Scholar] [CrossRef]
- Norman, K.; Haß, U.; Pirlich, M. Malnutrition in Older Adults-Recent Advances and Remaining Challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef]
- Barceló, A.; Barbé, F.; de la Peña, M.; Vila, M.; Pérez, G.; Piérola, J.; Durán, J.; Agustí, A.G. Antioxidant status in patients with sleep apnoea and impact of continuous positive airway pressure treatment. Eur. Respir. J. 2006, 27, 756–760. [Google Scholar] [CrossRef]
- Zhao, M.; Tuo, H.; Wang, S.; Zhao, L. The Effects of Dietary Nutrition on Sleep and Sleep Disorders. Mediat. Inflamm. 2020, 2020, 3142874. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Xue, Q.; Zhang, J. The association between serum total folic acid concentration and severe difficulty falling asleep in US adults: NHANES 2005–2008. Front. Neurol. 2023, 14, 1225403. [Google Scholar] [CrossRef]
- Deng, M.G.; Liu, F.; Liang, Y.; Chen, Y.; Nie, J.Q.; Chai, C.; Wang, K. Associations of serum zinc, copper, and selenium with sleep disorders in the American adults: Data from NHANES 2011–2016. J. Affect. Disord. 2023, 323, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, C.; Lu, L.; Knutson, K.L.; Carnethon, M.R.; Fly, A.D.; Luo, J.; Haas, D.M.; Shikany, J.M.; Kahe, K. Association of magnesium intake with sleep duration and sleep quality: Findings from the CARDIA study. Sleep 2022, 45, zsab276. [Google Scholar] [CrossRef] [PubMed]
- Cherasse, Y.; Urade, Y. Dietary Zinc Acts as a Sleep Modulator. Int. J. Mol. Sci. 2017, 18, 2334. [Google Scholar] [CrossRef]
- Leung, W.; Singh, I.; McWilliams, S.; Stockler, S.; Ipsiroglu, O.S. Iron deficiency and sleep—A scoping review. Sleep Med. Rev. 2020, 51, 101274. [Google Scholar] [CrossRef]
- Zhao, L.; Li, S.; Zhu, Y.; Wang, T.; Su, Y.; Shi, Z.; Zhang, Y.; Zhao, Y. Association between Selenium Intake and Optimal Sleep Duration: A National Longitudinal Study. Nutrients 2023, 15, 397. [Google Scholar] [CrossRef]
- Huang, K.; Fang, H.; Yu, D.; Guo, Q.; Xu, X.; Ju, L.; Cai, S.; Yang, Y.; Wei, X.; Zhao, L. Usual Intake of Micronutrients and Prevalence of Inadequate Intake among Chinese Adults: Data from CNHS 2015–2017. Nutrients 2022, 14, 4714. [Google Scholar] [CrossRef]
- Luo, X.; Tang, M.; Wei, X.; Peng, Y. Association between magnesium deficiency score and sleep quality in adults: A population-based cross-sectional study. J. Affect. Disord. 2024, 358, 105–112. [Google Scholar] [CrossRef]
- Rémi, J.; Pollmächer, T.; Spiegelhalder, K.; Trenkwalder, C.; Young, P. Sleep-Related Disorders in Neurology and Psychiatry. Dtsch. Arztebl. Int. 2019, 116, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Fishbain, D.A.; Cole, B.; Lewis, J.E.; Gao, J. What is the evidence for chronic pain being etiologically associated with the DSM-IV category of sleep disorder due to a general medical condition? A structured evidence-based review. Pain Med. 2010, 11, 158–179. [Google Scholar] [CrossRef]
- Hu, J.; Szymczak, S. A review on longitudinal data analysis with random forest. Brief. Bioinform. 2023, 24, bbad002. [Google Scholar] [CrossRef]
- Muscaritoli, M. The Impact of Nutrients on Mental Health and Well-Being: Insights from the Literature. Front. Nutr. 2021, 8, 656290. [Google Scholar] [CrossRef]
- Sales, L.V.; Bruin, V.M.; D’Almeida, V.; Pompéia, S.; Bueno, O.F.; Tufik, S.; Bittencourt, L. Cognition and biomarkers of oxidative stress in obstructive sleep apnea. Clinics 2013, 68, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Thongchumnum, W.; Vallibhakara, S.A.; Sophonsritsuk, A.; Vallibhakara, O. Effect of Vitamin E Supplementation on Chronic Insomnia Disorder in Postmenopausal Women: A Prospective, Double-Blinded Randomized Controlled Trial. Nutrients 2023, 15, 1187. [Google Scholar] [CrossRef] [PubMed]
- Menezo, Y.; Elder, K.; Clement, A.; Clement, P. Folic Acid, Folinic Acid, 5 Methyl TetraHydroFolate Supplementation for Mutations That Affect Epigenesis through the Folate and One-Carbon Cycles. Biomolecules 2022, 12, 197. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Fan, R.; Song, L.; Wang, S.; You, M.; Cai, M.; Wu, Y.; Li, Y.; Xu, M. Association of methyl donor nutrients dietary intake and sleep disorders in the elderly revealed by the intestinal microbiome. Food Funct. 2024, 15, 6335–6346. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yu, L.; Li, W.; Zhang, H.; Huang, X.; Chen, W.; Wang, D. Association of vitamins with hearing loss, vision disorder and sleep problem in the US general population. Environ. Sci. Pollut. Res. Int. 2023, 30, 53876–53886. [Google Scholar] [CrossRef]
- Du, M.; Liu, J.; Han, N.; Zhao, Z.; Yang, J.; Xu, X.; Luo, S.; Wang, H. Maternal sleep quality during early pregnancy, risk factors and its impact on pregnancy outcomes: A prospective cohort study. Sleep Med. 2021, 79, 11–18. [Google Scholar] [CrossRef]
- Chen, P.C.; Guo, C.H.; Tseng, C.J.; Wang, K.C.; Liu, P.J. Blood trace minerals concentrations and oxidative stress in patients with obstructive sleep apnea. J. Nutr. Health Aging 2013, 17, 639–644. [Google Scholar] [CrossRef]
- Laleli Koc, B.; Elmas, B.; Tugrul Ersak, D.; Erol, S.A.; Kara, O.; Sahin, D. Evaluation of Serum Selenium Level, Quality of Sleep, and Life in Pregnant Women with Restless Legs Syndrome. Biol. Trace. Elem. Res. 2023, 201, 1143–1150. [Google Scholar] [CrossRef]
- Ji, X.; Grandner, M.A.; Liu, J. The relationship between micronutrient status and sleep patterns: A systematic review. Public Health Nutr. 2017, 20, 687–701. [Google Scholar] [CrossRef]
- Arab, A.; Rafie, N.; Amani, R.; Shirani, F. The Role of Magnesium in Sleep Health: A Systematic Review of Available Literature. Biol. Trace. Elem. Res. 2023, 201, 121–128. [Google Scholar] [CrossRef]
- Yang, H.; Lu, S.; Yang, L. Clinical prediction models for the early diagnosis of obstructive sleep apnea in stroke patients: A systematic review. Syst. Rev. 2024, 13, 38. [Google Scholar] [CrossRef]
- Xu, R.; Miao, L.; Ni, J.; Ding, Y.; Song, Y.; Yang, C.; Zhu, B.; Jiang, R. Risk factors and prediction model of sleep disturbance in patients with maintenance hemodialysis: A single center study. Front. Neurol. 2022, 13, 955352. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Choi, S.J.; Lee, S.; Wijaya, R.H.; Kim, J.H.; Joo, E.Y.; Kim, J.K. Predicting the Risk of Sleep Disorders Using a Machine Learning-Based Simple Questionnaire: Development and Validation Study. J. Med. Internet. Res. 2023, 25, e46520. [Google Scholar] [CrossRef] [PubMed]
- Luginbühl, M.; Gaugler, S. The application of fully automated dried blood spot analysis for liquid chromatography-tandem mass spectrometry using the CAMAG DBS-MS 500 autosampler. Clin. Biochem. 2020, 82, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Tonoli, D.; Varesio, E.; Hopfgartner, G. The use of mass spectrometry to analyze dried blood spots. Mass Spectrom Rev. 2016, 35, 361–438. [Google Scholar] [CrossRef]
- Martial, L.C.; Aarnoutse, R.E.; Mulder, M.; Schellekens, A.; Brüggemann, R.J.M.; Burger, D.M.; Schene, A.H.; Batalla, A. Dried Blood Spot sampling in psychiatry: Perspectives for improving therapeutic drug monitoring. Eur. Neuropsychopharmacol. 2017, 27, 205–216. [Google Scholar] [CrossRef]
- Malsagova, K.; Kopylov, A.; Stepanov, A.; Butkova, T.; Izotov, A.; Kaysheva, A. Dried Blood Spot in Laboratory: Directions and Prospects. Diagnostics 2020, 10, 248. [Google Scholar] [CrossRef]
- Meesters, R.J.; Hooff, G.P. State-of-the-art dried blood spot analysis: An overview of recent advances and future trends. Bioanalysis 2013, 5, 2187–2208. [Google Scholar] [CrossRef]



| Characteristics | Total Sleep Disorders (n = 366) | Matched Sleep Disorders (n = 124) | Control (n = 124) | p1 Value | p2 Value |
|---|---|---|---|---|---|
| Age (years) | 68.60 ± 4.52 | 69.1 ± 4.3 | 69.1 ± 4.3 | 0.400 | 1.000 |
| Sex, male (%) | 135 (36.90) | 51 (41.1) | 51 (41.1) | 0.284 | 1.000 |
| Education levels, n (%) | 0.144 | 0.157 | |||
| Illiteracy | 241 (65.8) | 61 (49.2) | 46 (37.1) | ||
| Primary school | 88 (24.0) | 29 (23.4) | 36 (29.0) | ||
| Secondary school and above | 37 (10.1) | 34 (27.4) | 42 (33.9) | ||
| Smoking status, n (%) | 0.748 | 0.221 | |||
| Non-smoker | 239 (65.3) | 79 (63.7) | 89 (71.8) | ||
| Ex-smoker and smoker | 127 (34.7) | 45 (36.3) | 35 (28.2) | ||
| Alcohol drinking, n (%) | 0.952 | 0.353 | |||
| Yes | 299 (78.1) | 23 (18.5) | 30 (24.2) | ||
| No | 67 (21.9) | 101 (81.5) | 94 (75.8) | ||
| Marital status, n (%) | 0.476 | 0.683 | |||
| Married or cohabited | 330 (90.2) | 109 (87.9) | 112 (90.3) | ||
| Single, separated, or widowed | 36 (9.8) | 15 (12.1) | 12 (9.7) | ||
| BMI (kg/m2) | 25.18 ± 3.17 | 25.61 ± 3.66 | 25.96 ± 3.56 | 0.21 | 0.433 |
| Waist circumference (cm) | 87.82 ± 9.02 | 88.99 ± 10.07 | 90.36 ± 8.38 | 0.224 | 0.252 |
| Micronutrients | Sleep Disorders (n = 124) | Control (n = 124) | p Value |
|---|---|---|---|
| VA (ng/mL) | 310.13 ± 128.85 | 366.74 ± 140.32 | <0.001 |
| VD (ng/mL) | 35.70 ± 13.70 | 36.90 ± 12.90 | 0.482 |
| VE (μg/mL) | 2.56 ± 0.59 | 3.61 ± 1.17 | <0.001 |
| VB1 (ng/mL) | 0.86 ± 0.66 | 0.82 ± 0.48 | 0.774 |
| VB2 (ng/mL) | 3.65 ± 1.75 | 4.03 ± 3.32 | 0.942 |
| VB3 (μg/mL) | 8.64 ± 2.34 | 8.90 ± 2.21 | 0.442 |
| VB5 (μg/mL) | 47.50 ± 22.74 | 54.15 ± 50.64 | 0.995 |
| VB6 (ng/mL) | 9.74 ± 6.43 | 10.60 ± 6.29 | 0.181 |
| Folate (ng/mL) | 4.51 ± 2.35 | 6.02 ± 2.24 | <0.001 |
| Mg (μg/mL) | 36.99 ± 7.59 | 39.63 ± 6.11 | 0.002 |
| Cu (ng/mL) | 794.14 ± 155.56 | 966.34 ± 690.63 | 0.002 |
| Fe (μg/mL) | 362.83 ± 77.36 | 401.37 ± 89.24 | <0.001 |
| Zn (μg/mL) | 7.51 ± 2.60 | 8.88 ± 7.62 | 0.294 |
| Se (ng/mL) | 95.70 ± 21.83 | 113.03 ± 26.30 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.; Chen, X.; Zhang, L.; Wang, Z.; Duan, H.; Wu, Q.; Yan, R.; Wang, D.; Li, Z.; He, R.; et al. A Risk Correlative Model for Sleep Disorders in Chinese Older Adults Based on Blood Micronutrient Levels: A Matched Case-Control Study. Nutrients 2024, 16, 3306. https://doi.org/10.3390/nu16193306
Cheng C, Chen X, Zhang L, Wang Z, Duan H, Wu Q, Yan R, Wang D, Li Z, He R, et al. A Risk Correlative Model for Sleep Disorders in Chinese Older Adults Based on Blood Micronutrient Levels: A Matched Case-Control Study. Nutrients. 2024; 16(19):3306. https://doi.org/10.3390/nu16193306
Chicago/Turabian StyleCheng, Cheng, Xukun Chen, Liyang Zhang, Zehao Wang, Huilian Duan, Qi Wu, Ruiting Yan, Di Wang, Zhongxia Li, Ruikun He, and et al. 2024. "A Risk Correlative Model for Sleep Disorders in Chinese Older Adults Based on Blood Micronutrient Levels: A Matched Case-Control Study" Nutrients 16, no. 19: 3306. https://doi.org/10.3390/nu16193306
APA StyleCheng, C., Chen, X., Zhang, L., Wang, Z., Duan, H., Wu, Q., Yan, R., Wang, D., Li, Z., He, R., Li, Z., Chen, Y., Ma, F., Du, Y., Li, W., & Huang, G. (2024). A Risk Correlative Model for Sleep Disorders in Chinese Older Adults Based on Blood Micronutrient Levels: A Matched Case-Control Study. Nutrients, 16(19), 3306. https://doi.org/10.3390/nu16193306





