Trigger Warning: How Modern Diet, Lifestyle, and Environment Pull the Trigger on Autosomal Dominant Polycystic Kidney Disease Progression
Abstract
1. Introduction
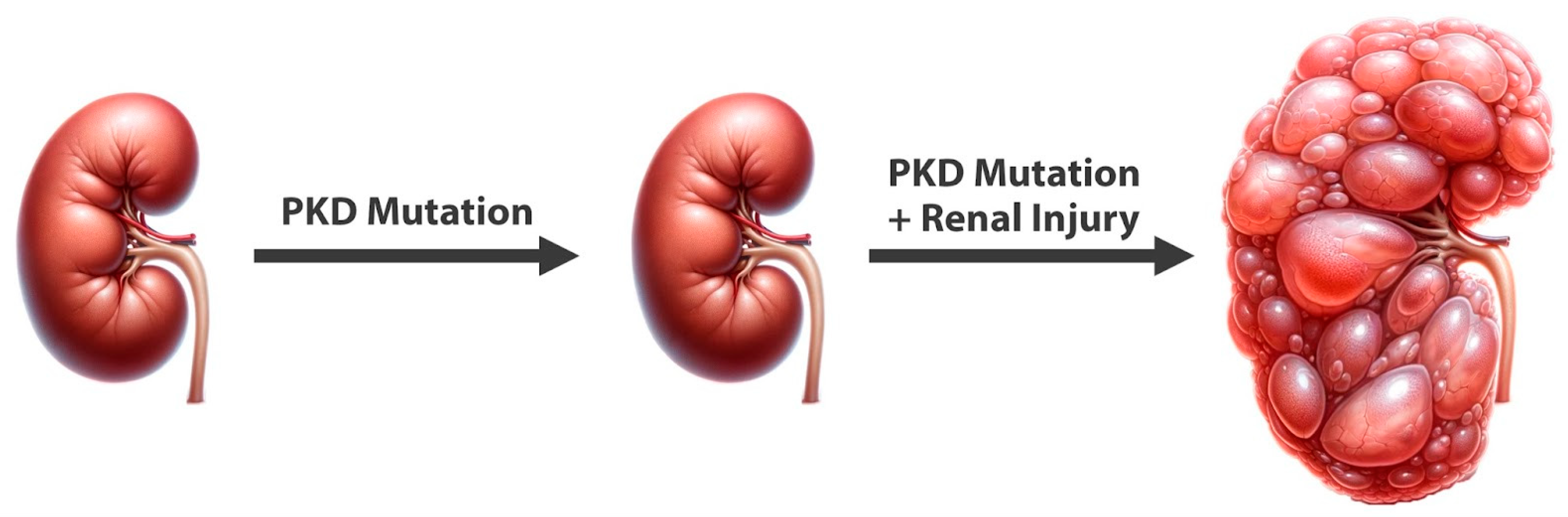
2. Genetic Foundations of ADPKD
3. Genes versus Triggers
4. Implications for Dietary Guidelines and Interventions
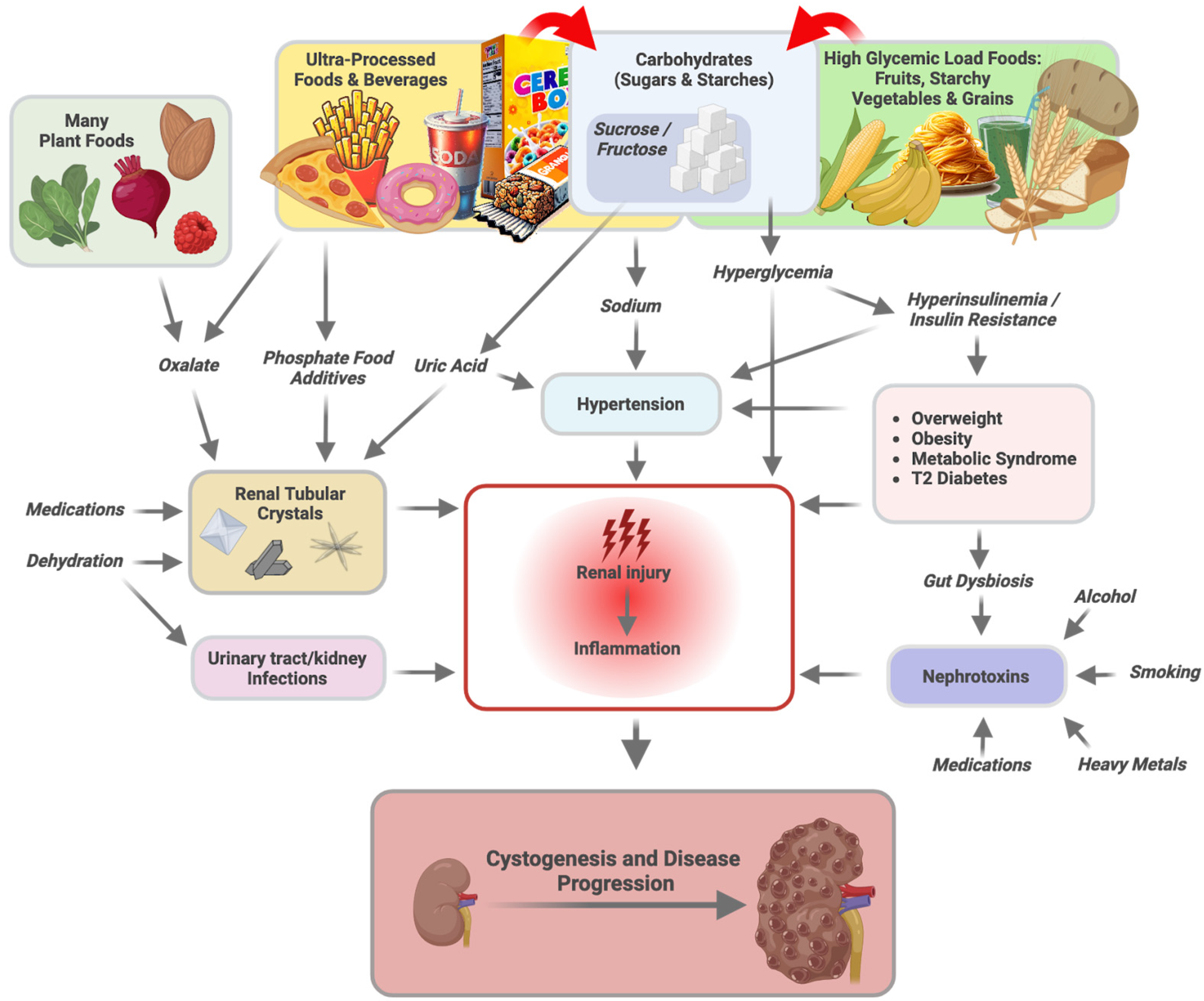
5. Carbohydrates
5.1. Carbohydrate Overconsumption, Persistent Hyperglycemia, and Renal Health
5.2. Hyperinsulinemia, Fructose, and Hypertension
5.3. Carbohydrate Restriction and Renal Health
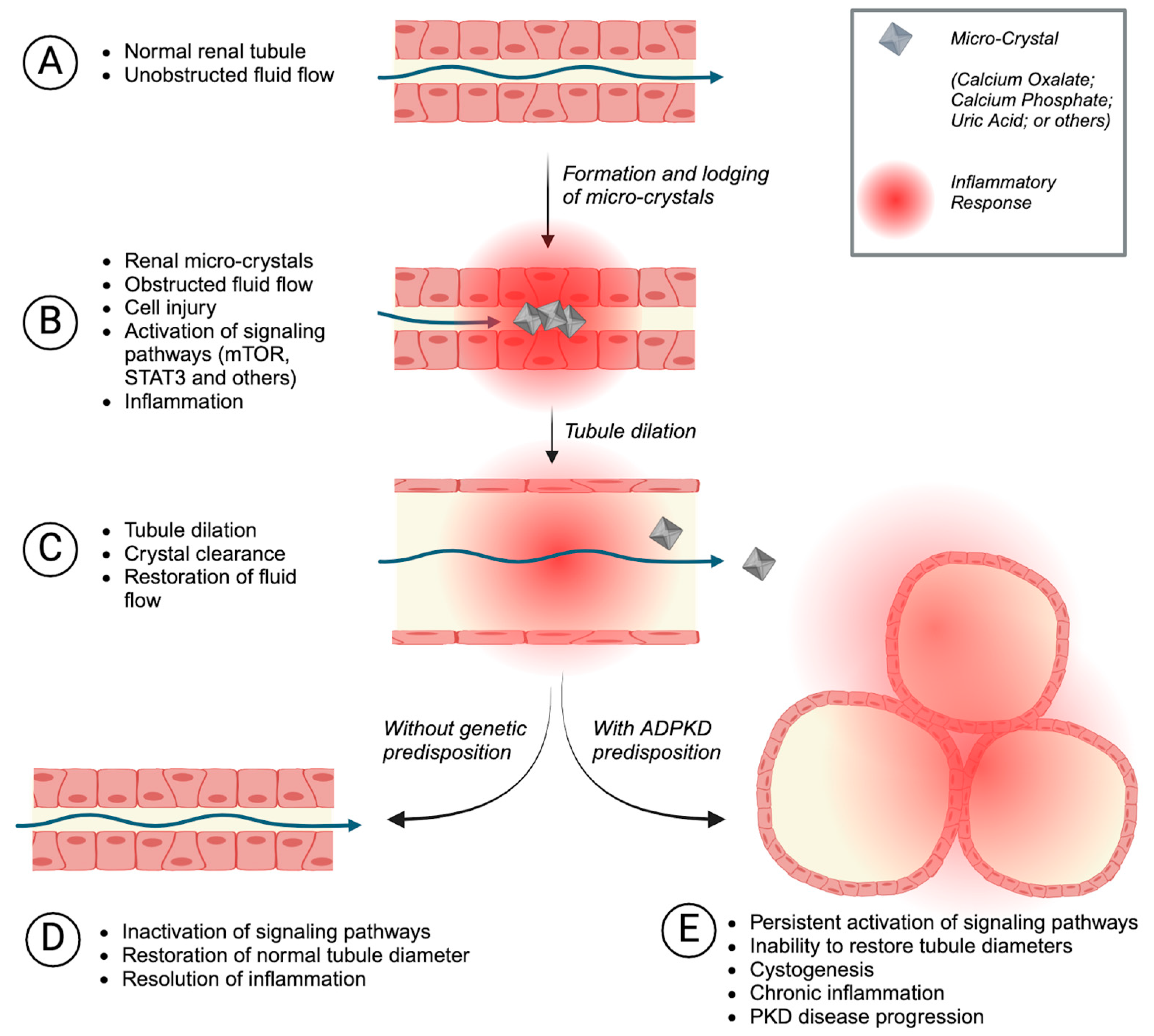
6. Tubular Microcrystals as Triggers of Cystogenesis
6.1. Oxalate
6.2. Phosphate
6.3. Uric Acid
6.4. Dehydration
6.5. Medications and Dietary Supplements
7. Sodium and Potassium
8. Increased Intestinal Permeability
9. Nephrotoxins
9.1. Medications
9.1.1. NSAIDs
9.1.2. Antibiotics
9.2. Psychoactive Substances
9.2.1. Alcohol
9.2.2. Nicotine/Smoking
9.3. Environmental Nephrotoxins
10. Probably Not a Trigger: Protein
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dobzhansky, T. Nothing in Biology Makes Sense except in the Light of Evolution. Am. Biol. Teach. 1973, 35, 125–129. [Google Scholar] [CrossRef]
- Ben-Dor, M.; Sirtoli, R.; Barkai, R. The evolution of the human trophic level during the Pleistocene. Am. J. Phys. Anthropol. 2021, 175, 27–56. [Google Scholar] [CrossRef]
- Psouni, E.; Janke, A.; Garwicz, M. Impact of Carnivory on Human Development and Evolution Revealed by a New Unifying Model of Weaning in Mammals. PLoS ONE 2012, 7, e32452. [Google Scholar] [CrossRef] [PubMed]
- O’KEefe, J.H.; O’KEefe, E.L.; Lavie, C.J.; Cordain, L. Debunking the vegan myth: The case for a plant-forward omnivorous whole-foods diet. Prog. Cardiovasc. Dis. 2022, 74, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Bastos, P.; Fontes; Eefe, O.; Lindeberg, S.; Cordain, L. The western diet and lifestyle and diseases of civilization. Res. Rep. Clin. Cardiol. 2011, 15, 2–8. [Google Scholar] [CrossRef]
- Eaton, S.B.; Konner, M. Paleolithic Nutrition. N. Engl. J. Med. 1985, 312, 283–289. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Chronic Kidney Disease in the United States. 2023. Available online: https://www.cdc.gov/kidneydisease/publications-resources/ckd-national-facts.html (accessed on 21 April 2024).
- Cordain, L.; Eades, M.R.; Eades, M.D. Hyperinsulinemic diseases of civilization: More than just Syndrome X. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003, 136, 95–112. [Google Scholar] [CrossRef]
- Cantarelli, L.; Valencia, M.G.; Alegria, L.L.; Fernandez, L.C.S.; Lopez, J.E.; Nicolas, F.G.; Casariego, G.J.N. Long-term effectiveness and safety of tolvaptan in autosomal dominant polycystic kidney disease. Med. Clin. 2024, 163, 1–7. [Google Scholar] [CrossRef]
- Raina, R.; Houry, A.; Rath, P.; Mangat, G.; Pandher, D.; Islam, M.; Khattab, A.G.; Kalout, J.K.; Bagga, S. Clinical Utility and Tolerability of Tolvaptan in the Treatment of Autosomal Dominant Polycystic Kidney Disease (ADPKD). Drug Health Patient Saf. 2022, 14, 147–159. [Google Scholar] [CrossRef]
- Schirutschke, H.; Gross, P.; Paliege, A.; Hugo, C. 10-Year Evaluation of Adherence and Satisfaction with Information about Tolvaptan in ADPKD: A Single-Center Pilot Study. Patient Prefer. Adherence 2021, 15, 1941–1952. [Google Scholar] [CrossRef]
- Lanktree, M.B.; Guiard, E.; Li, W.; Akbari, P.; Haghighi, A.; Iliuta, I.-A.; Shi, B.; Chen, C.; He, N.; Song, X.; et al. Intrafamilial Variability of ADPKD. Kidney Int. Rep. 2019, 4, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Roy, S.; Li, L.; Ma, M. Polycystic kidney disease: Novel insights into polycystin function. Trends Mol. Med. 2023, 29, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Saigusa, T.; Bell, P.D.; Yang, B.; Fu, L.; Privratsky, J.R.; Lu, X.; Ren, J.; Mei, C.; Crowley, S.D.; Holditch, S.J.; et al. Molecular Pathways and Therapies in Autosomal-Dominant Polycystic Kidney Disease. Physiology 2015, 30, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Fain, P.R.; McFann, K.K.; Taylor, M.R.; Tison, M.; Johnson, A.M.; Reed, B.; Schrier, R.W. Modifier genes play a significant role in the phenotypic expression of PKD1. Kidney Int. 2005, 67, 1256–1267. [Google Scholar] [CrossRef] [PubMed]
- Piontek, K.; Menezes, L.F.; A Garcia-Gonzalez, M.; Huso, D.L.; Germino, G.G. A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat. Med. 2007, 13, 1490–1495. [Google Scholar] [CrossRef]
- Weimbs, T. Polycystic kidney disease and renal injury repair: Common pathways, fluid flow, and the function of polycystin-1. Am. J. Physiol. Physiol. 2007, 293, F1423–F1432. [Google Scholar] [CrossRef]
- Sas, K.M.; Yin, H.; Fitzgibbon, W.R.; Baicu, C.F.; Zile, M.R.; Steele, S.L.; Amria, M.; Saigusa, T.; Funk, J.; Bunni, M.A.; et al. Hyperglycemia in the absence of cilia accelerates cystogenesis and induces renal damage. Am. J. Physiol. Physiol. 2015, 309, F79–F87. [Google Scholar] [CrossRef]
- Happé, H.; Leonhard, W.N.; van der Wal, A.; van de Water, B.; Leeuwen, I.S.L.-V.; Breuning, M.H.; de Heer, E.; Peters, D.J. Toxic tubular injury in kidneys from Pkd1-deletion mice accelerates cystogenesis accompanied by dysregulated planar cell polarity and canonical Wnt signaling pathways. Hum. Mol. Genet. 2009, 18, 2532–2542. [Google Scholar] [CrossRef]
- Takakura, A.; Contrino, L.; Zhou, X.; Bonventre, J.V.; Sun, Y.; Humphreys, B.D.; Zhou, J. Renal injury is a third hit promoting rapid development of adult polycystic kidney disease. Hum. Mol. Genet. 2009, 18, 2523–2531. [Google Scholar] [CrossRef]
- Torres, J.A.; Rezaei, M.; Broderick, C.; Lin, L.; Wang, X.; Hoppe, B.; Cowley, B.D.; Savica, V.; Torres, V.E.; Khan, S.; et al. Crystal deposition triggers tubule dilation that accelerates cystogenesis in polycystic kidney disease. J. Clin. Investig. 2019, 129, 4506–4522. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.; Godos, J.; Bonaccio, M.; Vitaglione, P.; Grosso, G. Ultra-Processed Foods and Nutritional Dietary Profile: A Meta-Analysis of Nationally Representative Samples. Nutrients 2021, 13, 3390. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z. Gut Microbiota: An Important Link between Western Diet and Chronic Diseases. Nutrients 2019, 11, 2287. [Google Scholar] [CrossRef] [PubMed]
- Whelan, K.; Bancil, A.S.; Lindsay, J.O.; Chassaing, B. Ultra-processed foods and food additives in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 406–427. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Ding, L.; Andoh, V.; Zhang, J.; Chen, L. The Mechanism of Hyperglycemia-Induced Renal Cell Injury in Diabetic Nephropathy Disease: An Update. Life 2023, 13, 539. [Google Scholar] [CrossRef]
- Reed, B.; Helal, I.; McFann, K.; Wang, W.; Yan, X.-D.; Schrier, R.W. The impact of type II diabetes mellitus in patients with autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 2011, 27, 2862–2865. [Google Scholar] [CrossRef]
- Nowak, K.L.; You, Z.; Gitomer, B.; Brosnahan, G.; Torres, V.E.; Chapman, A.B.; Perrone, R.D.; Steinman, T.I.; Abebe, K.Z.; Rahbari-Oskoui, F.F.; et al. Overweight and Obesity Are Predictors of Progression in Early Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2018, 29, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.L.; Steele, C.; Gitomer, B.; Wang, W.; Ouyang, J.; Chonchol, M.B. Overweight and Obesity and Progression of ADPKD. Clin. J. Am. Soc. Nephrol. 2021, 16, 908–915. [Google Scholar] [CrossRef]
- Nowak, K.L.; Moretti, F.; Bussola, N.; Steele, C.N.; Gregory, A.V.; Kline, T.L.; Ramanathan, S.; Trapletti, G.; Furlanello, C.; McCormick, L.; et al. Visceral Adiposity and Progression of ADPKD: A Cohort Study of Patients from the TEMPO 3:4 Trial. Am. J. Kidney Dis. 2024, 84, 275–285.e1. [Google Scholar] [CrossRef]
- Araújo, J.; Cai, J.; Stevens, J. Prevalence of Optimal Metabolic Health in American Adults: National Health and Nutrition Examination Survey 2009–2016. Metab. Syndr. Relat. Disord. 2019, 17, 46–52. [Google Scholar] [CrossRef]
- Augustin, L.S.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Björck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E.; et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Hu, H.; Zharikov, S.; Tuttle, K.R.; Short, R.A.; Glushakova, O.; Ouyang, X.; Feig, D.I.; Block, E.R.; Herrera-Acosta, J.; et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am. J. Physiol. Physiol. 2006, 290, F625–F631. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Perez-Pozo, S.E.; Lillo, J.L.; Grases, F.; Schold, J.D.; Kuwabara, M.; Sato, Y.; Hernando, A.A.; Garcia, G.; Jensen, T.; et al. Fructose increases risk for kidney stones: Potential role in metabolic syndrome and heat stress. BMC Nephrol. 2018, 19, 315. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W.; Abebe, K.Z.; Perrone, R.D.; Torres, V.E.; Braun, W.E.; Steinman, T.I.; Winklhofer, F.T.; Brosnahan, G.; Czarnecki, P.G.; Hogan, M.C.; et al. Blood Pressure in Early Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2014, 371, 2255–2266. [Google Scholar] [CrossRef] [PubMed]
- Ertuglu, L.A.; Elijovich, F.; Laffer, C.L.; Kirabo, A. Salt-Sensitivity of Blood Pressure and Insulin Resistance. Front. Physiol. 2021, 12, 793924. [Google Scholar] [CrossRef]
- Soleimani, M.; Barone, S.; Luo, H.; Zahedi, K. Pathogenesis of Hypertension in Metabolic Syndrome: The Role of Fructose and Salt. Int. J. Mol. Sci. 2023, 24, 4294. [Google Scholar] [CrossRef]
- Manrique, C.; Lastra, G.; Gardner, M.; Sowers, J.R. The Renin Angiotensin Aldosterone System in Hypertension: Roles of Insulin Resistance and Oxidative Stress. Med. Clin. N. Am. 2009, 93, 569–582. [Google Scholar] [CrossRef]
- Brands, M.W. Role of Insulin-Mediated Antinatriuresis in Sodium Homeostasis and Hypertension. Hypertension 2018, 72, 1255–1262. [Google Scholar] [CrossRef]
- Muniyappa, R.; Sowers, J.R. Role of insulin resistance in endothelial dysfunction. Rev. Endocr. Metab. Disord. 2013, 14, 5–12. [Google Scholar] [CrossRef]
- Potenza, M.A.; Marasciulo, F.L.; Chieppa, D.M.; Brigiani, G.S.; Formoso, G.; Quon, M.J.; Montagnani, M. Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am. J. Physiol. Circ. Physiol. 2005, 289, H813–H822. [Google Scholar] [CrossRef]
- Johnson, R.J.; Sanchez-Lozada, L.G.; Nakagawa, T. The Effect of Fructose on Renal Biology and Disease. J. Am. Soc. Nephrol. 2010, 21, 2036–2039. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Lanaspa, M.A.; Sanchez-Lozada, L.G.; Tolan, D.; Nakagawa, T.; Ishimoto, T.; Andres-Hernando, A.; Rodriguez-Iturbe, B.; Stenvinkel, P. The fructose survival hypothesis for obesity. Philos. Trans. R. Soc. B Biol. Sci. 2023, 378, 20220230. [Google Scholar] [CrossRef] [PubMed]
- Cabral, P.D.; Hong, N.J.; Khan, A.H.; Ortiz, P.A.; Beierwaltes, W.H.; Imig, J.D.; Garvin, J.L. Fructose Stimulates Na/H Exchange Activity and Sensitizes the Proximal Tubule to Angiotensin II. Hypertension 2014, 63, e68–e73. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.M.; Dulloo, A.G.; Yepuri, G.; Montani, J.-P. Fructose ingestion acutely elevates blood pressure in healthy young humans. Am. J. Physiol. Integr. Comp. Physiol. 2008, 294, R730–R737. [Google Scholar] [CrossRef]
- Jalal, D.I.; Smits, G.; Johnson, R.J.; Chonchol, M. Increased Fructose Associates with Elevated Blood Pressure. J. Am. Soc. Nephrol. 2010, 21, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Andres-Hernando, A.; Jensen, T.J.; Kuwabara, M.; Orlicky, D.J.; Cicerchi, C.; Li, N.; Roncal-Jimenez, C.A.; Garcia, G.E.; Ishimoto, T.; Maclean, P.S.; et al. Vasopressin mediates fructose-induced metabolic syndrome by activating the V1b receptor. J. Clin. Investig. 2021, 6, e140848. [Google Scholar] [CrossRef]
- Student, J.; Sowers, J.; Lockette, W. THIRSTY FOR FRUCTOSE: Arginine Vasopressin, Fructose, and the Pathogenesis of Metabolic and Renal Disease. Front. Cardiovasc. Med. 2022, 9, 883365. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Ward, C.J.; Harris, P.C.; Torres, V.E. Vasopressin Directly Regulates Cyst Growth in Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2007, 19, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Grantham, J.J.; Higashihara, E.; Perrone, R.D.; Krasa, H.B.; Ouyang, J.; Czerwiec, F.S.; et al. Tolvaptan in Patients with Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2012, 367, 2407–2418. [Google Scholar] [CrossRef]
- Xu, L.; Hu, G.; Qiu, J.; Fan, Y.; Ma, Y.; Miura, T.; Kohzuki, M.; Ito, O. High Fructose-Induced Hypertension and Renal Damage Are Exaggerated in Dahl Salt-Sensitive Rats via Renal Renin-Angiotensin System Activation. J. Am. Heart Assoc. 2021, 10, e016543. [Google Scholar] [CrossRef]
- Xu, L.; Hu, G.; Qiu, J.; Miura, T.; Yamakoshi, S.; Namai-Takahashi, A.; Kohzuki, M.; Ito, O. Exercise Training Prevents High Fructose-Induced Hypertension and Renal Damages in Male Dahl Salt-Sensitive Rats. Med. Sci. Sports Exerc. 2023, 55, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Gersch, M.S.; Mu, W.; Cirillo, P.; Reungjui, S.; Zhang, L.; Roncal, C.; Sautin, Y.Y.; Johnson, R.J.; Nakagawa, T. Fructose, but not dextrose, accelerates the progression of chronic kidney disease. Am. J. Physiol. Physiol. 2007, 293, F1256–F1261. [Google Scholar] [CrossRef] [PubMed]
- Andres-Hernando, A.; Orlicky, D.J.; Cicerchi, C.; Kuwabara, M.; Garcia, G.E.; Nakagawa, T.; Sanchez-Lozada, L.G.; Johnson, R.J.; Lanaspa, M.A. High Fructose Corn Syrup Accelerates Kidney Disease and Mortality in Obese Mice with Metabolic Syndrome. Biomolecules 2023, 13, 780. [Google Scholar] [CrossRef] [PubMed]
- Lustig, R.H.; Schmidt, L.A.; Brindis, C.D. The toxic truth about sugar. Nature 2012, 482, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Chebib, F.T.; Nowak, K.L.; Chonchol, M.B.; Bing, K.; Ghanem, A.; Rahbari-Oskoui, F.F.; Dahl, N.K.; Mrug, M. Polycystic Kidney Disease Diet: What is Known and What is Safe. Clin. J. Am. Soc. Nephrol. 2024, 19, 664–682. [Google Scholar] [CrossRef]
- Bruci, A.; Tuccinardi, D.; Tozzi, R.; Balena, A.; Santucci, S.; Frontani, R.; Mariani, S.; Basciani, S.; Spera, G.; Gnessi, L.; et al. Very Low-Calorie Ketogenic Diet: A Safe and Effective Tool for Weight Loss in Patients with Obesity and Mild Kidney Failure. Nutrients 2020, 12, 333. [Google Scholar] [CrossRef]
- Rojas-Morales, P.; León-Contreras, J.C.; Sánchez-Tapia, M.; Silva-Palacios, A.; Cano-Martínez, A.; González-Reyes, S.; Jiménez-Osorio, A.S.; Hernández-Pando, R.; Osorio-Alonso, H.; Sánchez-Lozada, L.G.; et al. A ketogenic diet attenuates acute and chronic ischemic kidney injury and reduces markers of oxidative stress and inflammation. Life Sci. 2022, 289, 120227. [Google Scholar] [CrossRef]
- Sethi, S.; Wakeham, D.; Ketter, T.; Hooshmand, F.; Bjornstad, J.; Richards, B.; Westman, E.; Krauss, R.M.; Saslow, L. Ketogenic Diet Intervention on Metabolic and Psychiatric Health in Bipolar and Schizophrenia: A Pilot Trial. Psychiatry Res. 2024, 335, 115866. [Google Scholar] [CrossRef]
- Rojas-Morales, P.; Pedraza-Chaverri, J.; Tapia, E. Ketone bodies for kidney injury and disease. Adv. Redox Res. 2021, 2, 100009. [Google Scholar] [CrossRef]
- Poplawski, M.M.; Mastaitis, J.W.; Isoda, F.; Grosjean, F.; Zheng, F.; Mobbs, C.V. Reversal of Diabetic Nephropathy by a Ketogenic Diet. PLoS ONE 2011, 6, e18604. [Google Scholar] [CrossRef]
- Athinarayanan, S.J.; Roberts, C.G.P.; Vangala, C.; Shetty, G.K.; McKenzie, A.L.; Weimbs, T.; Volek, J.S. The case for a ketogenic diet in the management of kidney disease. BMJ Open Diabetes Res. Care 2024, 12, e004101. [Google Scholar] [CrossRef] [PubMed]
- Weimbs, T.; Saville, J.; Kalantar-Zadeh, K. Ketogenic metabolic therapy for chronic kidney disease—The pro part. Clin. Kidney J. 2023, 17, sfad273. [Google Scholar] [CrossRef] [PubMed]
- Cordain, L.; Eaton, S.; Miller, J.B.; Mann, N.; Hill, K. The paradoxical nature of hunter-gatherer diets: Meat-based, yet non-atherogenic. Eur. J. Clin. Nutr. 2002, 56, S42–S52. [Google Scholar] [CrossRef] [PubMed]
- Cordain, L.; Miller, J.B.; Eaton, S.B.; Mann, N.; Holt, S.H.; Speth, J.D. Plant-animal subsistence ratios and macronutrient energy estimations in worldwide hunter-gatherer diets. Am. J. Clin. Nutr. 2000, 71, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Epstein, F.H.; Brenner, B.M.; Meyer, T.W.; Hostetter, T.H. Dietary Protein Intake and the Progressive Nature of Kidney Disease. N. Engl. J. Med. 1982, 307, 652–659. [Google Scholar] [CrossRef]
- Cooper, I.D.; Kyriakidou, Y.; Edwards, K.; Petagine, L.; Seyfried, T.N.; Duraj, T.; Soto-Mota, A.; Scarborough, A.; Jacome, S.L.; Brookler, K.; et al. Ketosis Suppression and Ageing (KetoSAge): The Effects of Suppressing Ketosis in Long Term Keto-Adapted Non-Athletic Females. Int. J. Mol. Sci. 2023, 24, 15621. [Google Scholar] [CrossRef]
- Cooper, I.D.; Kyriakidou, Y.; Petagine, L.; Edwards, K.; Soto-Mota, A.; Brookler, K.; Elliott, B.T. Ketosis Suppression and Ageing (KetoSAge) Part 2: The Effect of Suppressing Ketosis on Biomarkers Associated with Ageing, HOMA-IR, Leptin, Osteocalcin, and GLP-1, in Healthy Females. Biomedicines 2024, 12, 1553. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Reeves, W.B.; Awad, A.S. Pathophysiology of diabetic kidney disease: Impact of SGLT2 inhibitors. Nat. Rev. Nephrol. 2021, 17, 319–334. [Google Scholar] [CrossRef]
- Tomita, I.; Kume, S.; Sugahara, S.; Osawa, N.; Yamahara, K.; Yasuda-Yamahara, M.; Takeda, N.; Chin-Kanasaki, M.; Kaneko, T.; Mayoux, E.; et al. SGLT2 Inhibition Mediates Protection from Diabetic Kidney Disease by Promoting Ketone Body-Induced mTORC1 Inhibition. Cell Metab. 2020, 32, 404–419.e6. [Google Scholar] [CrossRef]
- Kim, H.A.; Na Jang, H.; Kong, S.H.; Lee, Y.; Choi, S.H.; Cho, Y.M.; Jang, H.C.; Oh, T.J. Ketonuria as an Indicator of Improvement of Renal Function in Patients with Type 2 Diabetes Receiving SGLT2 Inhibitor Treatment. Endocrinol. Metab. 2024, 39, 653–658. [Google Scholar] [CrossRef]
- Perkovic, V.; Tuttle, K.R.; Rossing, P.; Mahaffey, K.W.; Mann, J.F.; Bakris, G.; Baeres, F.M.; Idorn, T.; Bosch-Traberg, H.; Lausvig, N.L.; et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2024, 391, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Kipp, K.R.; Rezaei, M.; Lin, L.; Dewey, E.C.; Weimbs, T.; Kruger, S.L.; Schimmel, M.F.; Parker, N.; Shillingford, J.M.; Leamon, C.P.; et al. A mild reduction of food intake slows disease progression in an orthologous mouse model of polycystic kidney disease. Am. J. Physiol. Renal. Physiol. 2016, 310, F726–F731. [Google Scholar] [CrossRef] [PubMed]
- Warner, G.; Hein, K.Z.; Nin, V.; Edwards, M.; Chini, C.C.; Hopp, K.; Harris, P.C.; Torres, V.E.; Chini, E.N. Food Restriction Ameliorates the Development of Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2015, 27, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.A.; Torres, J.A.; Kruger, S.L.; Kruger, S.L.; Broderick, C.; Broderick, C.; Amarlkhagva, T.; Amarlkhagva, T.; Agrawal, S.; Agrawal, S.; et al. Ketosis Ameliorates Renal Cyst Growth in Polycystic Kidney Disease. Cell Metab. 2019, 30, 1007–1023.e5. [Google Scholar] [CrossRef]
- Torres, J.A.; Holznecht, N.; Asplund, D.A.; Amarlkhagva, T.; Kroes, B.C.; Rebello, J.; Agrawal, S.; Weimbs, T. A combination of β-hydroxybutyrate and citrate ameliorates disease progression in a rat model of polycystic kidney disease. Am. J. Physiol. Physiol. 2024, 326, F352–F368. [Google Scholar] [CrossRef]
- Torres, J.A.; Holznecht, N.; Asplund, D.A.; Kroes, B.C.; Amarlkhagva, T.; Haeffner, M.M.; Sharpe, E.H.; Koestner, S.; Strubl, S.; Schimmel, M.F.; et al. β-Hydroxybutyrate Recapitulates the Beneficial Effects of Ketogenic Metabolic Therapy in Polycystic Kidney Disease. iScience 2024, 27, 110773. [Google Scholar] [CrossRef]
- Bruen, D.M.; Kingaard, J.J.; Munits, M.; Paimanta, C.S.; Torres, J.A.; Saville, J.; Weimbs, T. Ren.Nu, a Dietary Program for Individuals with Autosomal-Dominant Polycystic Kidney Disease Implementing a Sustainable, Plant-Focused, Kidney-Safe, Ketogenic Approach with Avoidance of Renal Stressors. Kidney Dial. 2022, 2, 183–203. [Google Scholar] [CrossRef]
- Wang, L.; Chen, P.; Xiao, W. β-hydroxybutyrate as an Anti-Aging Metabolite. Nutrients 2021, 13, 3420. [Google Scholar] [CrossRef]
- Møller, N. Ketone Body, 3-Hydroxybutyrate: Minor Metabolite—Major Medical Manifestations. J. Clin. Endocrinol. Metab. 2020, 105, 2884–2892. [Google Scholar] [CrossRef]
- Strubl, S.; Oehm, S.; Torres, J.A.; Grundmann, F.; Haratani, J.; Decker, M.; Vuong, S.; Bhandal, A.K.; Methot, N.; Haynie-Cion, R.; et al. Ketogenic dietary interventions in autosomal dominant polycystic kidney disease—A retrospective case series study: First insights into feasibility, safety and effects. Clin. Kidney J. 2021, 15, 1079–1092. [Google Scholar] [CrossRef]
- Cukoski, S.; Lindemann, C.H.; Arjune, S.; Todorova, P.; Brecht, T.; Kühn, A.; Oehm, S.; Strubl, S.; Becker, I.; Kämmerer, U.; et al. Feasibility and impact of ketogenic dietary interventions in polycystic kidney disease: KETO-ADPKD—A randomized controlled trial. Cell Rep. Med. 2023, 4, 101283. [Google Scholar] [CrossRef] [PubMed]
- Knol, M.G.E.; Bais, T.; Geertsema, P.; Connelly, M.A.; Bakker, S.J.L.; Gansevoort, R.T.; van Gastel, M.D.A.; DIPAK Consortium. Higher beta-hydroxybutyrate ketone levels associated with a slower kidney function decline in ADPKD. Nephrol. Dial. Transplant. 2023, 39, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, F.; Rashid, H.U.; Yesmine, S.; Monjur, F.; Chatterjee, T.K. Preliminary analysis of the effect of Stevia (Stevia rebaudiana) in patients with chronic kidney disease (stage I to stage III). Contemp. Clin. Trials Commun. 2018, 12, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Noitem, R.; Yuajit, C.; Soodvilai, S.; Muanprasat, C.; Chatsudthipong, V. Steviol slows renal cyst growth by reducing AQP2 expression and promoting AQP2 degradation. Biomed. Pharmacother. 2018, 101, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Yuajit, C.; Muanprasat, C.; Gallagher, A.-R.; Fedeles, S.V.; Kittayaruksakul, S.; Homvisasevongsa, S.; Somlo, S.; Chatsudthipong, V. Steviol retards renal cyst growth through reduction of CFTR expression and inhibition of epithelial cell proliferation in a mouse model of polycystic kidney disease. Biochem. Pharmacol. 2014, 88, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Hanna, C.; Potretzke, T.A.; Chedid, M.; Rangel, L.J.; Arroyo, J.; Zubidat, D.; Tebben, P.J.; Cogal, A.G.; Torres, V.E.; Harris, P.C.; et al. Kidney Cysts in Hypophosphatemic Rickets with Hypercalciuria: A Case Series. Kidney Med. 2022, 4, 100419. [Google Scholar] [CrossRef]
- Hanna, C.; Potretzke, T.A.; Cogal, A.G.; Mkhaimer, Y.G.; Tebben, P.J.; Torres, V.E.; Lieske, J.C.; Harris, P.C.; Sas, D.J.; Milliner, D.S.; et al. High Prevalence of Kidney Cysts in Patients with CYP24A1 Deficiency. Kidney Int. Rep. 2021, 6, 1895–1903. [Google Scholar] [CrossRef]
- Blijdorp, C.J.; Severs, D.; Musterd-Bhaggoe, U.M.; Gansevoort, R.T.; Zietse, R.; Hoorn, E.J.; DIPAK Consortium; Drenth, J.P.H.; de Fijter, J.W.; Losekoot, M.; et al. Serum bicarbonate is associated with kidney outcomes in autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 2020, 36, 2248–2255. [Google Scholar] [CrossRef] [PubMed]
- Nishiura, J.A.A.L.; Neves, R.F.; Eloi, S.R.; Cintra, S.M.; Ajzen, S.A.; Heilberg, I.P. Evaluation of Nephrolithiasis in Autosomal Dominant Polycystic Kidney Disease Patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 838–844. [Google Scholar] [CrossRef]
- Sakhaee, K.; Maalouf, N.M.; Sinnott, B. Kidney Stones 2012: Pathogenesis, Diagnosis, and Management. J. Clin. Endocrinol. Metab. 2012, 97, 1847–1860. [Google Scholar] [CrossRef]
- Rocha, D.R.; Xue, L.; Sousa, H.M.G.; Matos, A.C.C.; Hoorn, E.J.; Salih, M.; Heilberg, I.P. Urinary Citrate Is Associated with Kidney Outcomes in Early Polycystic Kidney Disease. Kidney360 2022, 3, 2110–2115. [Google Scholar] [CrossRef] [PubMed]
- E Torres, V.; Harris, P.C.; Pirson, Y. Autosomal dominant polycystic kidney disease. Lancet 2007, 369, 1287–1301. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Krambeck, A.E.; Rule, A.D. Determining the true burden of kidney stone disease. Nat. Rev. Nephrol. 2020, 16, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Gui, Z.; Yu, L.; Chen, Y.; Zhang, M.; He, J.; Hao, Y. Study from the United States: Increased prevalence of kidney stones in patients with high weight-adjusted waist index. Front. Nutr. 2024, 10, 1171775. [Google Scholar] [CrossRef]
- Fargue, S.; Milliner, D.S.; Knight, J.; Olson, J.B.; Lowther, W.T.; Holmes, R.P. Hydroxyproline Metabolism and Oxalate Synthesis in Primary Hyperoxaluria. J. Am. Soc. Nephrol. 2018, 29, 1615–1623. [Google Scholar] [CrossRef]
- Demoulin, N.; Aydin, S.; Gillion, V.; Morelle, J.; Jadoul, M. Pathophysiology and Management of Hyperoxaluria and Oxalate Nephropathy: A Review. Am. J. Kidney Dis. 2022, 79, 717–727. [Google Scholar] [CrossRef]
- Crivelli, J.J.; Mitchell, T.; Knight, J.; Wood, K.D.; Assimos, D.G.; Holmes, R.P.; Fargue, S. Contribution of Dietary Oxalate and Oxalate Precursors to Urinary Oxalate Excretion. Nutrients 2020, 13, 62. [Google Scholar] [CrossRef]
- Kumar, P.; Patel, M.; Thomas, V.; Knight, J.; Holmes, R.P.; Mitchell, T. Dietary Oxalate Induces Urinary Nanocrystals in Humans. Kidney Int. Rep. 2020, 5, 1040–1051. [Google Scholar] [CrossRef]
- Garland, V.; Herlitz, L.; Regunathan-Shenk, R. Diet-induced oxalate nephropathy from excessive nut and seed consumption. BMJ Case Rep. 2020, 13, e237212. [Google Scholar] [CrossRef]
- Chen, C.-L.; Fang, H.-C.; Chou, K.-J.; Wang, J.-S.; Chung, H.-M. Acute Oxalate Nephropathy After Ingestion of Star Fruit. Am. J. Kidney Dis. 2001, 37, 418–422. [Google Scholar] [CrossRef]
- Makkapati, S.; D’aGati, V.D.; Balsam, L. “Green Smoothie Cleanse” Causing Acute Oxalate Nephropathy. Am. J. Kidney Dis. 2018, 71, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Getting, J.E.; Gregoire, J.R.; Phul, A.; Kasten, M.J. Oxalate Nephropathy Due to ‘Juicing’: Case Report and Review. Am. J. Med. 2013, 126, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.; Hanchanale, V.S.; Myatt, A.; Somani, B.; Nabi, G.; Biyani, C.S. Citrate salts for preventing and treating calcium containing kidney stones in adults. Cochrane Database Syst. Rev. 2015, 2015, CD010057. [Google Scholar] [CrossRef] [PubMed]
- Abcar, A.; Hever, A.; Momi, J.S.; Sim, J.J. Acute Phosphate Nephropathy. Perm. J. 2009, 13, 48–50. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shiizaki, K.; Tsubouchi, A.; Miura, Y.; Seo, K.; Kuchimaru, T.; Hayashi, H.; Iwazu, Y.; Miura, M.; Battulga, B.; Ohno, N.; et al. Calcium phosphate microcrystals in the renal tubular fluid accelerate chronic kidney disease progression. J. Clin. Investig. 2021, 131, e145693. [Google Scholar] [CrossRef]
- Joo, W.C.; Lee, S.W.; Yang, D.H.; Han, J.Y.; Kim, M.-J. A case of biopsy-proven chronic kidney disease on progression from acute phosphate nephropathy. Kidney Res. Clin. Pract. 2012, 31, 124–127. [Google Scholar] [CrossRef]
- Ritz, E.; Hahn, K.; Ketteler, M.; Kuhlmann, M.K.; Mann, J. Phosphate Additives in Food—A Health Risk. Dtsch. Ärzteblatt Int. 2012, 109, 49–55. [Google Scholar] [CrossRef]
- D’aLessandro, C.; Piccoli, G.B.; Cupisti, A. The “phosphorus pyramid”: A visual tool for dietary phosphate management in dialysis and CKD patients. BMC Nephrol. 2015, 16, 9. [Google Scholar] [CrossRef]
- Sato, M.; Kataoka, H.; Ushio, Y.; Manabe, S.; Watanabe, S.; Akihisa, T.; Makabe, S.; Yoshida, R.; Iwasa, N.; Mitobe, M.; et al. High Serum Phosphate Level as a Risk Factor to Determine Renal Prognosis in Autosomal Dominant Polycystic Kidney Disease: A Retrospective Study. Medicines 2020, 7, 13. [Google Scholar] [CrossRef]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef]
- Sánchez-Lozada, L.G.; Madero, M.; Mazzali, M.; Feig, D.I.; Nakagawa, T.; Lanaspa, M.A.; Kanbay, M.; Kuwabara, M.; Rodriguez-Iturbe, B.; Johnson, R.J. Sugar, salt, immunity and the cause of primary hypertension. Clin. Kidney J. 2023, 16, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, A.A.; Antenor, J.A.; Kumar, V.; Roncal, C.; Garcia, G.E.; Andres-Hernando, A.; Lanaspa, M.A.; Johnson, R.J. Uric Acid: A Friend in the Past, a Foe in the Present. Integr. Med. Nephrol. Androl. 2022, 9, 8. [Google Scholar] [CrossRef]
- Zhu, Y.; Pandya, B.J.; Choi, H.K. Comorbidities of Gout and Hyperuricemia in the US General Population: NHANES 2007–2008. Am. J. Med. 2012, 125, 679–687.e1. [Google Scholar] [CrossRef] [PubMed]
- Riese, R.J.; Sakhaee, K. Uric Acid Nephrolithiasis: Pathogenesis and Treatment. J. Urol. 1992, 148, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Park, H.C.; Kim, H.; Jo, H.A.; Huh, H.; Jang, J.Y.; Kang, A.-Y.; Kim, S.H.; Cheong, H.I.; Kang, D.-H.; et al. Hyperuricemia and deterioration of renal function in autosomal dominant polycystic kidney disease. BMC Nephrol. 2014, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Helal, I.; McFann, K.; Reed, B.; Yan, X.-D.; Schrier, R.W.; Fick-Brosnahan, G.M. Serum uric acid, kidney volume and progression in autosomal-dominant polycystic kidney disease. Nephrol. Dial. Transplant. 2012, 28, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, I.H.; Santos, A.G.; Bianchini, J.M.; Santos, L.G.B.; Martini, M.C.R.; Silva, V.d.S.; Martin, L.C. Predictors of autosomal dominant polycystic kidney disease progression: A Brazilian single-center cohort. Braz. J. Nephrol. 2024, 46, e20230040. [Google Scholar] [CrossRef]
- Kocyigit, I.; Yilmaz, M.I.; Orscelik, O.; Sipahioglu, M.H.; Unal, A.; Eroglu, E.; Kalay, N.; Tokgoz, B.; Axelsson, J.; Oymak, O. Serum Uric Acid Levels and Endothelial Dysfunction in Patients with Autosomal Dominant Polycystic Kidney Disease. Nephron Clin. Pract. 2013, 123, 157–164. [Google Scholar] [CrossRef]
- Brosnahan, G.M.; You, Z.; Wang, W.; Gitomer, B.Y.; Chonchol, M. Serum Uric Acid and Progression of Autosomal Dominant Polycystic Kidney Disease: Results from the HALT PKD Trials. Curr. Hypertens. Rev. 2021, 17, 228–237. [Google Scholar] [CrossRef]
- Iglesias, A.L.; Pardo, M.B.; Magariños, C.R.; Pértega, S.; Castro, D.S.; Falcón, T.G.; Rodríguez-Carmona, A.; Fontán, M.P. Association of urinary excretion rates of uric acid with biomarkers of kidney injury in patients with advanced chronic kidney disease. PLoS ONE 2024, 19, e0304105. [Google Scholar] [CrossRef]
- Bargagli, M.; Dhayat, N.A.; Anderegg, M.; Semmo, M.; Huynh-Do, U.; Vogt, B.; Ferraro, P.M.; Fuster, D.G. Urinary Lithogenic Risk Profile in ADPKD Patients Treated with Tolvaptan. Clin. J. Am. Soc. Nephrol. 2020, 15, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Nagao, S.; Nishii, K.; Katsuyama, M.; Kurahashi, H.; Marunouchi, T.; Takahashi, H.; Wallace, D.P. Increased Water Intake Decreases Progression of Polycystic Kidney Disease in the PCK Rat. J. Am. Soc. Nephrol. 2006, 17, 2220–2227. [Google Scholar] [CrossRef] [PubMed]
- Sagar, P.S.; Zhang, J.; Luciuk, M.; Mannix, C.; Wong, A.T.Y.; Rangan, G.K. Increased water intake reduces long-term renal and cardiovascular disease progression in experimental polycystic kidney disease. PLoS ONE 2019, 14, e0209186. [Google Scholar] [CrossRef] [PubMed]
- Dev, H.; Zhu, C.; Barash, I.; Blumenfeld, J.D.; He, X.; RoyChoudhury, A.; Wu, A.; Prince, M.R. Feasibility of Water Therapy for Slowing Autosomal Dominant Polycystic Kidney Disease Progression. Kidney360 2024, 5, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Rangan, G.K.; Wong, A.T.; Munt, A.; Zhang, J.Q.; Saravanabavan, S.; Louw, S.; Allman-Farinelli, M.; Badve, S.V.; Boudville, N.; Chan, J.; et al. Prescribed Water Intake in Autosomal Dominant Polycystic Kidney Disease. NEJM Evid. 2022, 1, EVIDoa2100021. [Google Scholar] [CrossRef]
- Le Louët, H.; Pitts, P.J. Twenty-First Century Global ADR Management: A Need for Clarification, Redesign, and Coordinated Action. Ther. Innov. Regul. Sci. 2022, 57, 100–103. [Google Scholar] [CrossRef]
- Chenchula, S.; Atal, S.; Uppugunduri, C.R.S. A review of real-world evidence on preemptive pharmacogenomic testing for preventing adverse drug reactions: A reality for future health care. Pharmacogenom. J. 2024, 24, 9. [Google Scholar] [CrossRef]
- Matlaga, B.R.; Shah, O.D.; Assimos, D.G. Drug-Induced Urinary Calculi. Rev. Urol. 2003, 5, 227–231. [Google Scholar]
- Dorfman, L.E.; Smith, J.P. Sulfonamide Crystalluria: A Forgotten Disease. J. Urol. 1970, 104, 482–483. [Google Scholar] [CrossRef]
- Tantranont, N.; Luque, Y.; Hsiao, M.; Haute, C.; Gaber, L.; Barrios, R.; Adrogue, H.E.; Niasse, A.; Truong, L.D. Vancomycin-Associated Tubular Casts and Vancomycin Nephrotoxicity. Kidney Int. Rep. 2021, 6, 1912–1922. [Google Scholar] [CrossRef]
- Wu, D.S.-H.; Stoller, M.L. Indinavir urolithiasis. Curr. Opin. Urol. 2000, 10, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Llanos, M.; Kwon, A.; Herlitz, L.; Shafi, T.; Cohen, S.; Gebreselassie, S.K.; Sawaf, H.; Bobart, S.A. The Clinical and Pathological Characteristics of Patients with Oxalate Nephropathy. Kidney360 2023, 5, 65–72. [Google Scholar] [CrossRef]
- Bargagli, M.; Ferraro, P.M.; Vittori, M.; Lombardi, G.; Gambaro, G.; Somani, B. Calcium and Vitamin D Supplementation and Their Association with Kidney Stone Disease: A Narrative Review. Nutrients 2021, 13, 4363. [Google Scholar] [CrossRef] [PubMed]
- Frassetto, L.; Morris, J.R.C.; Sellmeyer, D.E.; Todd, K.; Sebastian, A. Diet, evolution and aging. Eur. J. Nutr. 2001, 40, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Keith, D.S.; Torres, V.E.; Johnson, C.M.; Holley, K.E. Effect of Sodium Chloride, Enalapril, and Losartan on the Development of Polycystic Kidney Disease in Han:SPRD Rats. Am. J. Kidney Dis. 1994, 24, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Cowley, B.; Grantham, J.; Muessel, M.; Kraybill, A.; Gattone, V. Modification of disease progression in rats with inherited polycystic kidney disease. Am. J. Kidney Dis. 1996, 27, 865–879. [Google Scholar] [CrossRef]
- Torres, V.E.; Chapman, A.B.; Perrone, R.D.; Bae, K.T.; Abebe, K.Z.; Bost, J.E.; Miskulin, D.C.; Steinman, T.I.; Braun, W.E.; Winklhofer, F.T.; et al. Analysis of baseline parameters in the HALT polycystic kidney disease trials. Kidney Int. 2012, 81, 577–585. [Google Scholar] [CrossRef]
- Torres, V.E.; Grantham, J.J.; Chapman, A.B.; Mrug, M.; Bae, K.T.; King, B.F.; Wetzel, L.H.; Martin, D.; Lockhart, M.E.; Bennett, W.M.; et al. Potentially Modifiable Factors Affecting the Progression of Autosomal Dominant Polycystic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 640–647. [Google Scholar] [CrossRef]
- Kramers, B.J.; Koorevaar, I.W.; Drenth, J.P.; de Fijter, J.W.; Neto, A.G.; Peters, D.J.; Vart, P.; Wetzels, J.F.; Zietse, R.; Gansevoort, R.T.; et al. Salt, but not protein intake, is associated with accelerated disease progression in autosomal dominant polycystic kidney disease. Kidney Int. 2020, 98, 989–998. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Matsushita, K.; Sang, Y.; Brunskill, N.J.; Carrero, J.J.; Chodick, G.; Hasegawa, T.; Heerspink, H.L.; Hirayama, A.; Landman, G.W.D.; et al. Serum potassium and adverse outcomes across the range of kidney function: A CKD Prognosis Consortium meta-analysis. Eur. Heart J. 2018, 39, 1535–1542. [Google Scholar] [CrossRef]
- Braschi, A.; Naismith, D.J. The effect of a dietary supplement of potassium chloride or potassium citrate on blood pressure in predominantly normotensive volunteers. Br. J. Nutr. 2008, 99, 1284–1292. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kieneker, L.M.; Bakker, S.J.L.; de Boer, R.A.; Navis, G.J.; Gansevoort, R.T.; Joosten, M.M. Low potassium excretion but not high sodium excretion is associated with increased risk of developing chronic kidney disease. Kidney Int. 2016, 90, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Araki, S.-I.; Haneda, M.; Koya, D.; Kondo, K.; Tanaka, S.; Arima, H.; Kume, S.; Nakazawa, J.; Chin-Kanasaki, M.; Ugi, S.; et al. Urinary Potassium Excretion and Renal and Cardiovascular Complications in Patients with Type 2 Diabetes and Normal Renal Function. Clin. J. Am. Soc. Nephrol. 2015, 10, 2152–2158. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Naska, A.; Kasdagli, M.; Torres, D.; Lopes, C.; Carvalho, C.; Moreira, P.; Malavolti, M.; Orsini, N.; Whelton, P.K.; et al. Potassium Intake and Blood Pressure: A Dose-Response Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2020, 9, e015719. [Google Scholar] [CrossRef]
- Aleman, R.S.; Moncada, M.; Aryana, K.J. Leaky Gut and the Ingredients That Help Treat It: A Review. Molecules 2023, 28, 619. [Google Scholar] [CrossRef]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef]
- Snelson, M.; Tan, S.M.; Clarke, R.E.; de Pasquale, C.; Thallas-Bonke, V.; Nguyen, T.-V.; Penfold, S.A.; Harcourt, B.E.; Sourris, K.C.; Lindblom, R.S.; et al. Processed foods drive intestinal barrier permeability and microvascular diseases. Sci. Adv. 2021, 7, eabe4841. [Google Scholar] [CrossRef]
- Linh, H.T.; Iwata, Y.; Senda, Y.; Sakai-Takemori, Y.; Nakade, Y.; Oshima, M.; Nakagawa-Yoneda, S.; Ogura, H.; Sato, K.; Minami, T.; et al. Intestinal Bacterial Translocation Contributes to Diabetic Kidney Disease. J. Am. Soc. Nephrol. 2022, 33, 1105–1119. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2023, 19, 275–293. [Google Scholar] [CrossRef]
- Armstrong, H.K.; Bording-Jorgensen, M.; Santer, D.M.; Zhang, Z.; Valcheva, R.; Rieger, A.M.; Kim, J.S.-H.; Dijk, S.I.; Mahmood, R.; Ogungbola, O.; et al. Unfermented β-fructan Fibers Fuel Inflammation in Select Inflammatory Bowel Disease Patients. Gastroenterology 2023, 164, 228–240. [Google Scholar] [CrossRef]
- Miller-Hjelle, M. Polycystic Kidney Disease: An Unrecognized Emerging Infectious Disease? Emerg. Infect. Dis. 1997, 3, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Werder, A.; Amos, M.; Nielsen, A.; Wolfe, G. Comparative Effects of Germfree and Ambient Environments on the Development of Cystic Kidney-Disease in Cfwwd Mice. J. Lab. Clin. Med. 1984, 103, 399–407. [Google Scholar] [PubMed]
- Gardner, K.D.; Reed, W.P.; Evan, A.P.; Zedalis, J.; Hylarides, M.D.; Leon, A.A. Endotoxin provocation of experimental renal cystic disease. Kidney Int. 1987, 32, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.-H.; Andersen, G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, R.; Nadkarni, G.N.; McSkimming, D.I.; Chaves, L.D.; Abyad, S.; Bryniarski, M.A.; Honan, A.M.; Thomas, S.A.; Gowda, M.; He, J.C.; et al. Fecal microbiota analysis of polycystic kidney disease patients according to renal function: A pilot study. Exp. Biol. Med. 2018, 244, 505–513. [Google Scholar] [CrossRef]
- Strubl, S.; Woestmann, F.; Todorova, P.; Arjune, S.; Farowski, F.; Baar, T.; Brodesser, S.; Grundmann, F.; Vehreschild, M.J.; Mueller, R.-U. #395 Gut dysbiosis in ADPKD patients: A controlled pilot study. Nephrol. Dial. Transplant. 2024, 39, gfae069-0255-395. [Google Scholar] [CrossRef]
- Naimi, S.; Viennois, E.; Gewirtz, A.T.; Chassaing, B. Direct impact of commonly used dietary emulsifiers on human gut microbiota. Microbiome 2021, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Dogan, S.; Celikbilek, M.; Guven, K. High Fructose Consumption Can Induce Endotoxemia. Gastroenterology 2012, 143, e29. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, J.; Xuan, R.; Chen, J.; Han, H.; Liu, J.; Niu, T.; Chen, H.; Wang, F. Dietary κ-carrageenan facilitates gut microbiota-mediated intestinal inflammation. Carbohydr. Polym. 2022, 277, 118830. [Google Scholar] [CrossRef]
- Zhu, H.; Cao, C.; Wu, Z.; Zhang, H.; Sun, Z.; Wang, M.; Xu, H.; Zhao, Z.; Wang, Y.; Pei, G.; et al. The probiotic L. casei Zhang slows the progression of acute and chronic kidney disease. Cell Metab. 2021, 33, 1926–1942.e8. [Google Scholar] [CrossRef]
- Cosola, C.; Rocchetti, M.M.; di Bari, I.; Acquaviva, P.M.; Maranzano, V.; Corciulo, S.; Di Ciaula, A.; Di Palo, D.M.; La Forgia, F.M.; Fontana, S.; et al. An Innovative Synbiotic Formulation Decreases Free Serum Indoxyl Sulfate, Small Intestine Permeability and Ameliorates Gastrointestinal Symptoms in a Randomized Pilot Trial in Stage IIIb-IV CKD Patients. Toxins 2021, 13, 334. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Li, L.; Ng, J.K.-C.; Li, P.K.-T. The Potential Benefits and Controversies of Probiotics Use in Patients at Different Stages of Chronic Kidney Disease. Nutrients 2022, 14, 4044. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, R.; Pechenyak, B.; Vyas, U.; Ranganathan, P.; Weinberg, A.; Liang, P.; Mallappallil, M.C.; Norin, A.J.; Friedman, E.A.; Saggi, S.J. Randomized Controlled Trial of Strain-Specific Probiotic Formulation (Renadyl) in Dialysis Patients. BioMed Res. Int. 2014, 2014, 568571. [Google Scholar] [CrossRef] [PubMed]
- Orr, S.E.; Bridges, C.C. Chronic Kidney Disease and Exposure to Nephrotoxic Metals. Int. J. Mol. Sci. 2017, 18, 1039. [Google Scholar] [CrossRef]
- Drożdżal, S.; Lechowicz, K.; Szostak, B.; Rosik, J.; Kotfis, K.; Machoy-Mokrzyńska, A.; Białecka, M.; Ciechanowski, K.; Gawrońska-Szklarz, B. Kidney damage from nonsteroidal anti-inflammatory drugs—Myth or truth? Review of selected literature. Pharmacol. Res. Perspect. 2021, 9, e00817. [Google Scholar] [CrossRef]
- Geertsema, P.; Stellema, R.; Casteleijn, N.F. The Importance of Recognizing Pain in Patients with Autosomal Dominant Polycystic Kidney Disease. Kidney Med. 2024, 6, 100821. [Google Scholar] [CrossRef]
- El-Damanawi, R.; Lee, M.; Harris, T.; Cowley, L.B.; Scholtes, I.; Bond, S.; Sandford, R.N.; Wilkinson, I.B.; Casteleijn, N.F.; Hogan, M.C.; et al. Developing a patient-centred tool for pain measurement and evaluation in autosomal dominant polycystic kidney disease. Clin. Kidney J. 2021, 14, 2338–2348. [Google Scholar] [CrossRef]
- Hoggan, J. An Objective Pain Assessment for Autosomal Dominant Polycystic Kidney Disease (ADPKD): A Patient’s Perspective. Clin. J. Am. Soc. Nephrol. 2023, 18, 147–148. [Google Scholar] [CrossRef] [PubMed]
- Natale, P.; Perrone, R.D.; Tong, A.; Harris, T.; Hannan, E.; Ju, A.; Burnette, E.; Casteleijn, N.F.; Chapman, A.; Eastty, S.; et al. Establishing a core outcome measure for pain in patients with autosomal dominant polycystic kidney disease: A consensus workshop report. Clin. Kidney J. 2021, 15, 407–416. [Google Scholar] [CrossRef]
- Zhan, M.; Doerfler, R.M.; Xie, D.; Chen, J.; Chen, H.-Y.; Diamantidis, C.J.; Rahman, M.; Ricardo, A.C.; Sondheimer, J.; Strauss, L.; et al. Association of Opioids and Nonsteroidal Anti-inflammatory Drugs with Outcomes in CKD: Findings from the CRIC (Chronic Renal Insufficiency Cohort) Study. Am. J. Kidney Dis. 2020, 76, 184–193. [Google Scholar] [CrossRef]
- Zhang, M.; Srichai, M.B.; Zhao, M.; Chen, J.; Davis, L.S.; Wu, G.; Breyer, M.D.; Hao, C.-M. Nonselective Cyclooxygenase Inhibition Retards Cyst Progression in a Murine Model of Autosomal Dominant Polycystic Kidney Disease. Int. J. Med. Sci. 2019, 16, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.H.; Gregoire, M.; Devassy, J.G.; Wu, Y.; Yoshihara, D.; Yamaguchi, T.; Nagao, S.; Aukema, H.M. Cyclooxygenase product inhibition with acetylsalicylic acid slows disease progression in the Han:SPRD-Cy rat model of polycystic kidney disease. Prostaglandins Other Lipid Mediat. 2015, 116–117, 19–25. [Google Scholar] [CrossRef]
- Aukema, H.M. Prostaglandins as potential targets for the treatment of polycystic kidney disease. Prostaglandins Leukot. Essent. Fat. Acids 2020, 164, 102220. [Google Scholar] [CrossRef] [PubMed]
- Crellin, E.; Mansfield, K.E.; Leyrat, C.; Nitsch, D.; Douglas, I.J.; Root, A.; Williamson, E.; Smeeth, L.; Tomlinson, L.A. Trimethoprim use for urinary tract infection and risk of adverse outcomes in older patients: Cohort study. BMJ 2018, 360, k341. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.M.-W.; Juurlink, D.N. Considerations when prescribing trimethoprim-sulfamethoxazole. Can. Med. Assoc. J. 2011, 183, 1851–1858. [Google Scholar] [CrossRef]
- Anderson, B.; Berdzuli, N.; Ilbawi, A.; Kestel, D.; Kluge, H.P.; Krech, R.; Mikkelsen, B.; Neufeld, M.; Poznyak, V.; Rekve, D.; et al. Health and cancer risks associated with low levels of alcohol consumption. Lancet Public Health 2023, 8, e6–e7. [Google Scholar] [CrossRef]
- Yuan, H.C.; Yu, Q.T.; Bai, H.; Xu, H.Z.; Gu, P.; Chen, L.Y. Alcohol intake and the risk of chronic kidney disease: Results from a systematic review and dose–response meta-analysis. Eur. J. Clin. Nutr. 2021, 75, 1555–1567. [Google Scholar] [CrossRef]
- Orth, S.R.; Hallan, S.I. Smoking: A risk factor for progression of chronic kidney disease and for cardiovascular morbidity and mortality in renal patients—Absence of evidence or evidence of absence? Clin. J. Am. Soc. Nephrol. 2008, 3, 226–236. [Google Scholar] [CrossRef]
- Gul, C.B.; Yildiz, A.; Sag, S.; Oruc, A.; Ersoy, A.; Gullulu, S. The Effect of Smoking on Endothelial Dysfunction in Autosomal Dominant Polycystic Kidney Disease Patients with Preserved Renal Function. Ren. Fail. 2021, 43, 1124–1129. [Google Scholar] [CrossRef]
- Sousa, M.V.; Amaral, A.G.; Freitas, J.A.; Murata, G.M.; Watanabe, E.H.; Balbo, B.E.; Tavares, M.D.; Hortegal, R.A.; Rocon, C.; Souza, L.E.; et al. Smoking accelerates renal cystic disease and worsens cardiac phenotype in Pkd1-deficient mice. Sci. Rep. 2021, 11, 14443. [Google Scholar] [CrossRef]
- Shankar, A.; Klein, R.; Klein, B.E.K. The Association among Smoking, Heavy Drinking, and Chronic Kidney Disease. Am. J. Epidemiol. 2006, 164, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.H.; Wu, Y.; Liu, M.; Attina, T.M.; Naidu, M.; Karthikraj, R.; Kannan, K.; Warady, B.A.; Furth, S.; Vento, S.; et al. Serially assessed bisphenol A and phthalate exposure and association with kidney function in children with chronic kidney disease in the US and Canada: A longitudinal cohort study. PLoS Med. 2020, 17, e1003384. [Google Scholar] [CrossRef] [PubMed]
- Robert, T.; Tang, E.; Kervadec, J.; Zaworski, J.; Daudon, M.; Letavernier, E. Kidney Injury and Hair-Straightening Products Containing Glyoxylic Acid. N. Engl. J. Med. 2024, 390, 1147–1149. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J.; Dissanayake, C.B. Factors Affecting the Environmentally Induced, Chronic Kidney Disease of Unknown Aetiology in Dry Zonal Regions in Tropical Countries—Novel Findings. Environments 2019, 7, 2. [Google Scholar] [CrossRef]
- Rogers, K.L.; Roncal-Jimenez, C.A.; Leiva, R.; Stem, A.; Wijkstrom, J.; Serpas, L.; González-Quiroz, M.A.; Sasai, F.; Wernerson, A.; Schaeffer, J.; et al. Silica Nanoparticles and Mesoamerican Nephropathy: A Case Series. Am. J. Kidney Dis. 2024, 83, 420–423. [Google Scholar] [CrossRef]
- Roncal-Jimenez, C.; García-Trabanino, R.; Barregard, L.; Lanaspa, M.A.; Wesseling, C.; Harra, T.; Aragón, A.; Grases, F.; Jarquin, E.R.; González, M.A.; et al. Heat Stress Nephropathy from Exercise-Induced Uric Acid Crystalluria: A Perspective on Mesoamerican Nephropathy. Am. J. Kidney Dis. 2016, 67, 20–30. [Google Scholar] [CrossRef]
- Fulgoni, V.L., 3rd. Current protein intake in America: Analysis of the National Health and Nutrition Examination Survey, 2003–2004. Am. J. Clin. Nutr. 2008, 87, 1554S–1557S. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Obeid, W.; Hiremath, S.; Topf, J.M. Protein Restriction for CKD: Time to Move On. Kidney360 2022, 3, 1611–1615. [Google Scholar] [CrossRef]
- Devries, M.C.; Sithamparapillai, A.; Brimble, K.S.; Banfield, L.; Morton, R.W.; Phillips, S.M. ‘Changes in Kidney Function Do Not Differ between Healthy Adults Consuming Higher-Compared with Lower- or Normal-Protein Diets: A Systematic Review and Meta-Analysis. J. Nutr. 2018, 148, 1760–1775. [Google Scholar] [CrossRef]
- Cheng, Y.; Zheng, G.; Song, Z.; Zhang, G.; Rao, X.; Zeng, T. Association between dietary protein intake and risk of chronic kidney disease: A systematic review and meta-analysis. Front. Nutr. 2024, 11, 1408424. [Google Scholar] [CrossRef] [PubMed]
- Tummalapalli, S.L.; Shlipak, M.G. Hyperfiltration. Hyperfiltration: Much Ado about Nothing? Clin. J. Am. Soc. Nephrol. 2019, 14, 789–791. [Google Scholar] [CrossRef] [PubMed]
- Molitch, M.E.; Gao, X.; Bebu, I.; de Boer, I.H.; Lachin, J.; Paterson, A.; Perkins, B.; Saenger, A.K.; Steffes, M.; Zinman, B. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group. Early Glomerular Hyperfiltration and Long-Term Kidney Outcomes in Type 1 Diabetes: The DCCT/EDIC Experience. Clin. J. Am. Soc. Nephrol. 2019, 14, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Carballo-Casla, A.; Avesani, C.M.; Beridze, G.; Ortolá, R.; García-Esquinas, E.; Lopez-Garcia, E.; Dai, L.; Dunk, M.M.; Stenvinkel, P.; Lindholm, B.; et al. Protein Intake and Mortality in Older Adults with Chronic Kidney Disease. JAMA Netw. Open 2024, 7, e2426577. [Google Scholar] [CrossRef] [PubMed]
- Klahr, S.; Breyer, J.A.; Beck, G.J.; Dennis, V.W.; Hartman, J.A.; Roth, D.; Steinman, T.I.; Wang, S.R.; Yamamoto, M.E. Dietary protein restriction, blood pressure control, and the progression of polycystic kidney disease. Modification of Diet in Renal Disease Study Group. J. Am. Soc. Nephrol. 1995, 5, 2037–2047. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messing, M.; Torres, J.A.; Holznecht, N.; Weimbs, T. Trigger Warning: How Modern Diet, Lifestyle, and Environment Pull the Trigger on Autosomal Dominant Polycystic Kidney Disease Progression. Nutrients 2024, 16, 3281. https://doi.org/10.3390/nu16193281
Messing M, Torres JA, Holznecht N, Weimbs T. Trigger Warning: How Modern Diet, Lifestyle, and Environment Pull the Trigger on Autosomal Dominant Polycystic Kidney Disease Progression. Nutrients. 2024; 16(19):3281. https://doi.org/10.3390/nu16193281
Chicago/Turabian StyleMessing, Melina, Jacob A. Torres, Nickolas Holznecht, and Thomas Weimbs. 2024. "Trigger Warning: How Modern Diet, Lifestyle, and Environment Pull the Trigger on Autosomal Dominant Polycystic Kidney Disease Progression" Nutrients 16, no. 19: 3281. https://doi.org/10.3390/nu16193281
APA StyleMessing, M., Torres, J. A., Holznecht, N., & Weimbs, T. (2024). Trigger Warning: How Modern Diet, Lifestyle, and Environment Pull the Trigger on Autosomal Dominant Polycystic Kidney Disease Progression. Nutrients, 16(19), 3281. https://doi.org/10.3390/nu16193281






