Association between Remnant Cholesterol and Metabolic Syndrome among Chinese Adults: Chinese Nutrition and Health Surveillance (2015–2017)
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Surveillance Contents
2.2.1. Questionnaire Survey
2.2.2. Physical Examination
2.2.3. Laboratory Tests
2.2.4. Dietary Survey
2.3. Quality Control
2.4. Definition of Variables
2.4.1. Remnant Cholesterol
2.4.2. Metabolic Syndrome
- (1)
- High WC: WC ≥ 90 cm for male; WC ≥ 85 cm for female;
- (2)
- Elevated blood pressure: SBP ≥ 130 mm Hg or DBP ≥ 85 mm Hg or treatment of previously diagnosed hypertension.
- (3)
- High TG: serum TG ≥ 1.70 mmol/L or specific treatment for TG abnormality;
- (4)
- Low HDL-C: HDL-C ≤ 1.04 mmol/L or specific treatment for TG abnormality;
- (5)
- Elevated FBG: FBG ≥ 6.1 mmol/L or previously diagnosed type 2 DM.
2.4.3. Covariates
2.5. Statistical Analysis
3. Results
3.1. General Characteristics of the Participants
3.2. Weighted General Characteristics of Adults Aged 20 and Older in 2015–2017
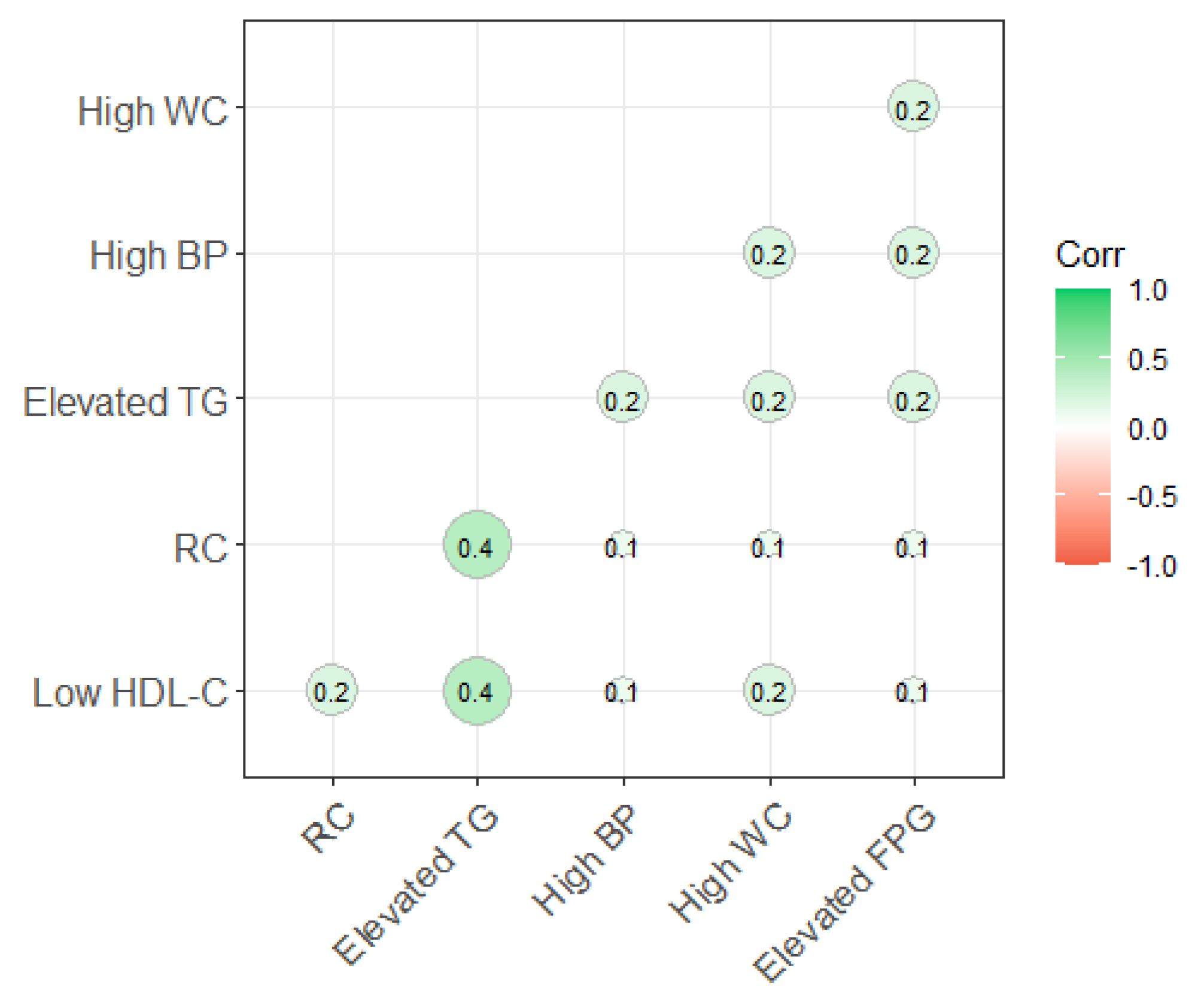
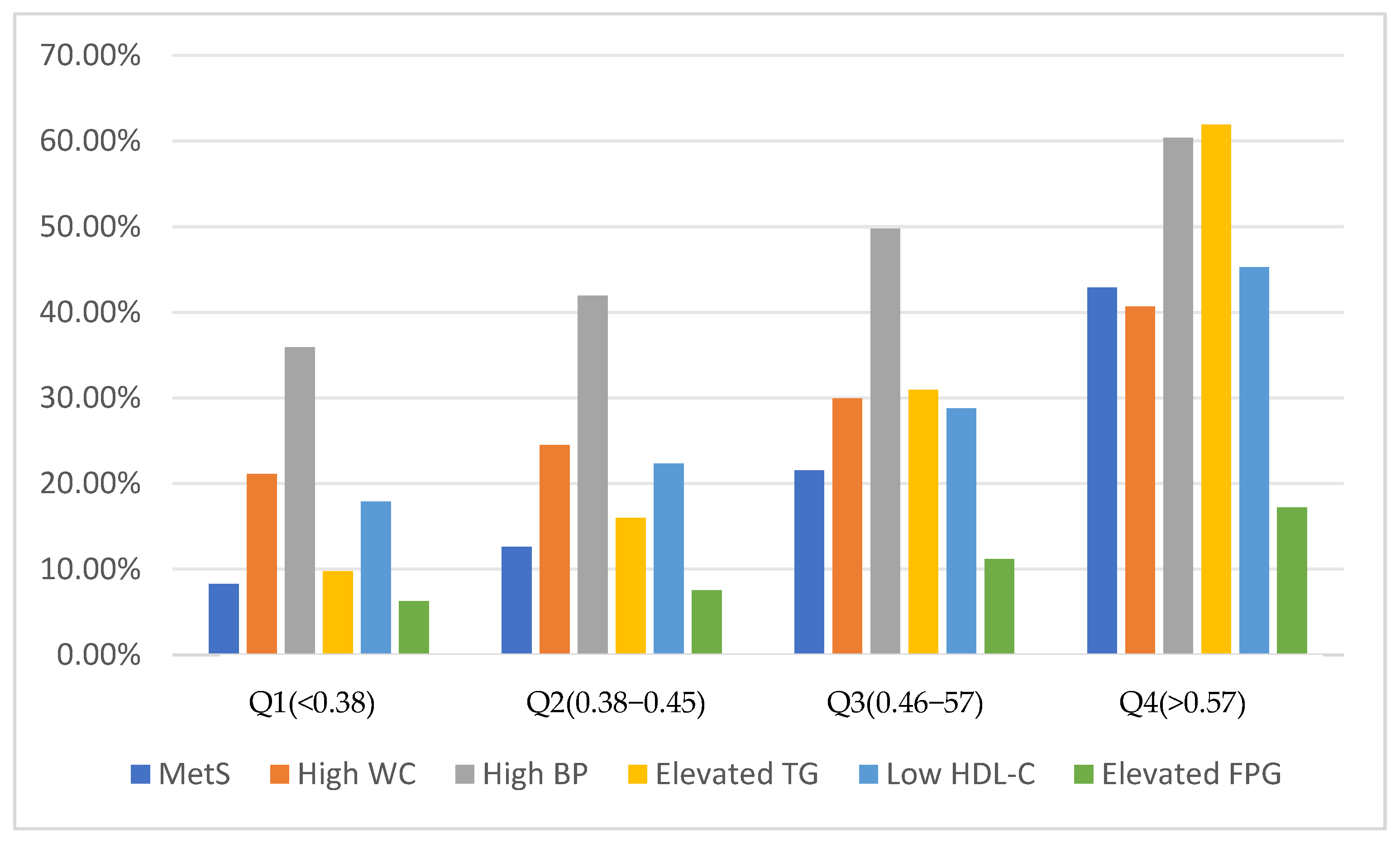
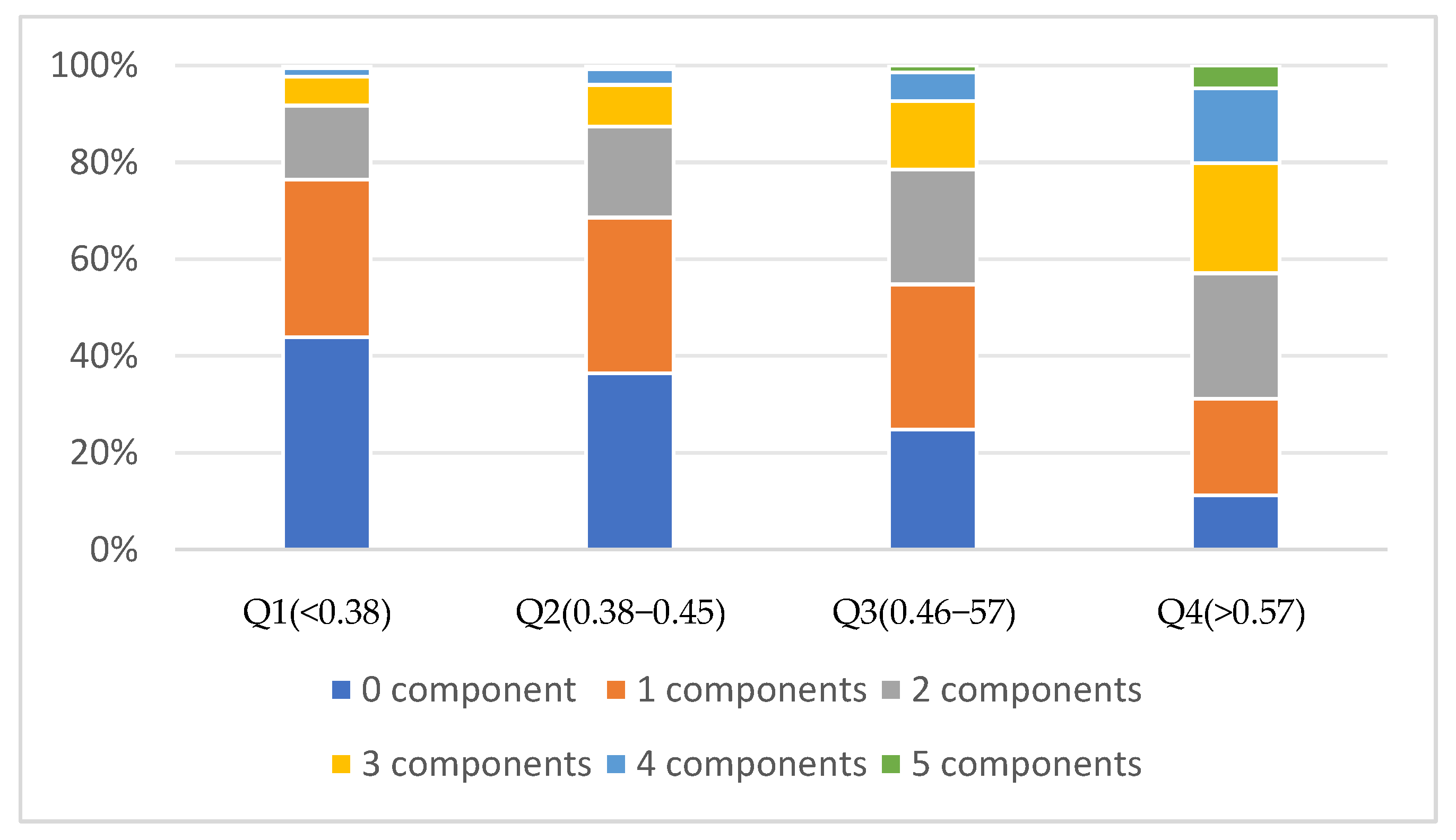
3.3. The Relationship between RC and MetS
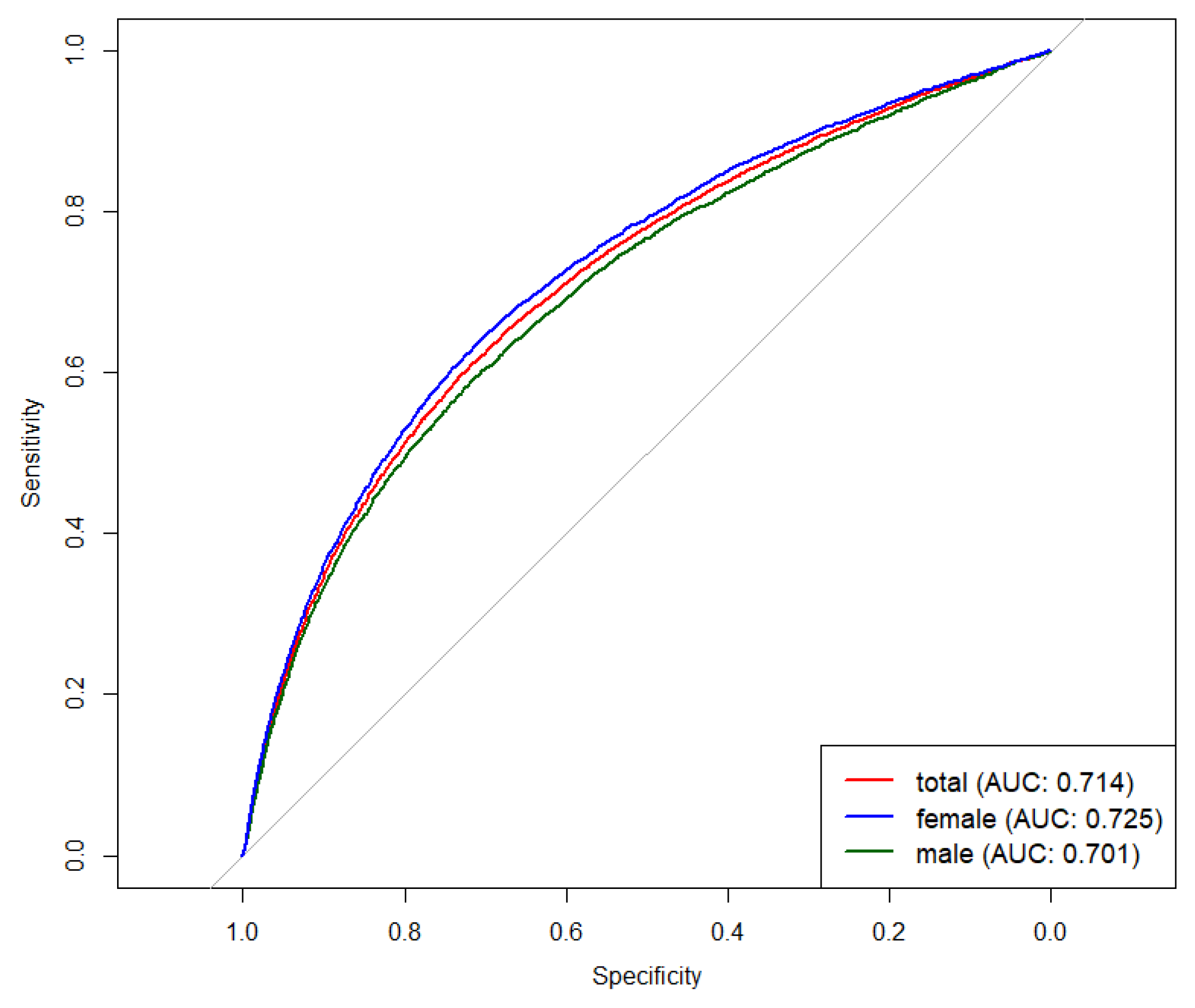
3.4. Accuracy of RC for Identifying MetS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chinese Type 2 Diabetes Prevention and Treatment Manual. Chin. J. Pract. Intern. Med. 2021, 41, 757–784. [CrossRef]
- Xi, B.; He, D.; Hu, Y.; Zhou, D. Prevalence of metabolic syndrome and its influencing factors among the Chinese adults: The China Health and Nutrition Survey in 2009. Prev. Med. 2013, 57, 867–871. [Google Scholar] [CrossRef]
- Yao, F.; Bo, Y.; Zhao, L.; Li, Y.; Ju, L.; Fang, H.; Piao, W.; Yu, D.; Lao, X. Prevalence and Influencing Factors of Metabolic Syndrome among Adults in China from 2015 to 2017. Nutrients 2021, 13, 4475. [Google Scholar] [CrossRef]
- Suh, S.; Lee, M.K. Metabolic syndrome and cardiovascular diseases in Korea. J. Atheroscler. Thromb. 2014, 21 (Suppl. S1), S31–S35. [Google Scholar] [CrossRef]
- Montemayor, S.; Mascaró, C.M.; Ugarriza, L.; Casares, M.; Llompart, I.; Abete, I.; Zulet, M.; Martínez, J.A.; Tur, J.A.; Bouzas, C. Adherence to Mediterranean Diet and NAFLD in Patients with Metabolic Syndrome: The FLIPAN Study. Nutrients 2022, 14, 3186. [Google Scholar] [CrossRef]
- Raikou, V.D.; Gavriil, S. Metabolic Syndrome and Chronic Renal Disease. Diseases 2018, 6, 12. [Google Scholar] [CrossRef]
- Kazamel, M.; Stino, A.M.; Smith, A.G. Metabolic syndrome and peripheral neuropathy. Muscle Nerve 2021, 63, 285–293. [Google Scholar] [CrossRef]
- Zheng, X.; Han, L.; Shen, S. Hypertension, remnant cholesterol and cardiovascular disease: Evidence from the China health and retirement longitudinal study. J. Hypertens. 2022, 40, 2292–2298. [Google Scholar] [CrossRef]
- Zou, Y.; Lan, J.; Zhong, Y.; Yang, S.; Zhang, H.; Xie, G. Association of remnant cholesterol with nonalcoholic fatty liver disease: A general population-based study. Lipids Health Dis. 2021, 20, 139. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.R.; Hooper, A.J.; Hegele, R.A. Remnant Cholesterol and Atherosclerotic Cardiovascular Disease Risk. J. Am. Coll. Cardiol. 2020, 76, 2736–2739. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N.; Packard, C.J.; Chapman, M.J.; Borén, J.; Aguilar-Salinas, C.A.; Averna, M.; Ference, B.A.; Gaudet, D.; Hegele, R.A.; Kersten, S.; et al. Triglyceride-rich lipoproteins and their remnants: Metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies—A consensus statement from the European Atherosclerosis Society. Eur. Heart J. 2021, 42, 4791–4806. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhang, D.; Ju, J.; Wang, A.; Du, T.; Chen, X.; Song, Y.; Gao, Z.; Xu, H. Remnant cholesterol associates with hypertension beyond low-density lipoprotein cholesterol among the general US adult population. Front. Endocrinol. 2023, 14, 1260764. [Google Scholar] [CrossRef]
- Gupta, R.; Guptha, S.; Agrawal, A.; Kaul, V.; Gaur, K.; Gupta, V.P. Secular trends in cholesterol lipoproteins and triglycerides and prevalence of dyslipidemias in an urban Indian population. Lipids Health Dis. 2008, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, J.; Li, Z.; Liu, J.; Lian, S.; Le, J. Association between remnant cholesterol and heart failure: A prospective cohort study. Front. Cardiovasc. Med. 2022, 9, 938647. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, Q.; Guo, X.; Wang, W.; Yu, B.; Liang, B.; Zhou, Y.; Dong, H.; Lin, J. The role of remnant cholesterol beyond low-density lipoprotein cholesterol in diabetes mellitus. Cardiovasc. Diabetol. 2022, 21, 117. [Google Scholar] [CrossRef]
- Yu, D.; Zhao, L.; Zhang, J.; Yang, Z.; Yang, L.; Huang, J.; Fang, H.; Guo, Q.; Xu, X.; Ju, L.; et al. China Nutrition and Health Surveys (1982–2017). China CDC Wkly. 2021, 3, 193–195. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, Y.; Qi, M.; Song, L.; Chen, B.; Wang, C.; Lu, J.; Yang, Y.; Zhang, X.; Cui, J.; et al. Associations of remnant cholesterol with cardiovascular and cancer mortality in a nationwide cohort. Sci. Bull. 2024, 69, 526–534. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X. The Chinese dietary Guidelines|Principle 1: Diversify food and mix it reasonably. Food Nutr. China 2022, 28, 2. [Google Scholar] [CrossRef]
- Xie, J.; Li, Y.; Zhang, Y.; Vgontzas, A.N.; Basta, M.; Chen, B.; Xu, C.; Tang, X. Sleep duration and metabolic syndrome: An updated systematic review and meta-analysis. Sleep. Med. Rev. 2021, 59, 101451. [Google Scholar] [CrossRef]
- WHO. Guidelines Approved by the Guidelines Review Committee. In WHO Guidelines on Physical Activity and Sedentary Behaviour; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Wang, Y.; Shen, R. Association of remnant cholesterol with depression among US adults. BMC Psychiatry 2023, 23, 259. [Google Scholar] [CrossRef]
- Varbo, A.; Freiberg, J.J.; Nordestgaard, B.G. Remnant Cholesterol and Myocardial Infarction in Normal Weight, Overweight, and Obese Individuals from the Copenhagen General Population Study. Clin. Chem. 2018, 64, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Farjam, M.; Kheirandish, M.; Ghanbarnejad, A.; Nikpoor, A.R.; Nejatizadeh, A.; Aghamolaei, T.; Shahmoradi, M.; Alizade, H.; Homayounfar, R.; Zarei, H.; et al. Reference Interval for Non-HDL-Cholesterol, Remnant Cholesterol and Other Lipid Parameters in the Southern Iranian Population; Findings from Bandare Kong and Fasa Cohort Studies. Arch. Iran. Med. 2024, 27, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.H.; Roh, E.; Lee, S.J.; Ihm, S.H.; Han, K.D.; Kang, J.G. Remnant Cholesterol Is an Independent Predictor of Type 2 Diabetes: A Nationwide Population-Based Cohort Study. Diabetes Care 2023, 46, 305–312. [Google Scholar] [CrossRef]
- Jin, J.; Hu, X.; Francois, M.; Zeng, P.; Wang, W.; Yu, B.; Zhou, Y.; Dong, H. Association between remnant cholesterol, metabolic syndrome, and cardiovascular disease: Post hoc analysis of a prospective national cohort study. Eur. J. Med. Res. 2023, 28, 420. [Google Scholar] [CrossRef]
- Sandesara, P.B.; Virani, S.S.; Fazio, S.; Shapiro, M.D. The Forgotten Lipids: Triglycerides, Remnant Cholesterol, and Atherosclerotic Cardiovascular Disease Risk. Endocr. Rev. 2019, 40, 537–557. [Google Scholar] [CrossRef]
- Liu, J.; Fan, F.; Liu, B.; Li, K.; Jiang, Y.; Jia, J.; Chen, C.; Zheng, B.; Zhang, Y. Association between remnant cholesterol and arterial stiffness in a Chinese community-based population: A cross-sectional study. Front. Cardiovasc. Med. 2022, 9, 993097. [Google Scholar] [CrossRef] [PubMed]
- Kosmas, C.E.; Rodriguez Polanco, S.; Bousvarou, M.D.; Papakonstantinou, E.J.; Peña Genao, E.; Guzman, E.; Kostara, C.E. The Triglyceride/High-Density Lipoprotein Cholesterol (TG/HDL-C) Ratio as a Risk Marker for Metabolic Syndrome and Cardiovascular Disease. Diagnostics 2023, 13, 929. [Google Scholar] [CrossRef]
- Connelly, P.J.; Currie, G.; Delles, C. Sex Differences in the Prevalence, Outcomes and Management of Hypertension. Curr. Hypertens. Rep. 2022, 24, 185–192. [Google Scholar] [CrossRef]
- Guo, D.C.; Gao, J.W.; Wang, X.; Chen, Z.T.; Gao, Q.Y.; Chen, Y.X.; Wang, J.F.; Liu, P.M.; Zhang, H.F. Remnant cholesterol and risk of incident hypertension: A population-based prospective cohort study. Hypertens. Res. 2024, 47, 1157–1166. [Google Scholar] [CrossRef]
- Xie, G.; Zhong, Y.; Yang, S.; Zou, Y. Remnant Cholesterol is an Independent Predictor of New-Onset Diabetes: A Single-Center Cohort Study. Diabetes Metab. Syndr. Obes. 2021, 14, 4735–4745. [Google Scholar] [CrossRef]
- Søndergaard, E.; Nielsen, S. VLDL triglyceride accumulation in skeletal muscle and adipose tissue in type 2 diabetes. Curr. Opin. Lipidol. 2018, 29, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Ramdas Nayak, V.K.; Satheesh, P.; Shenoy, M.T.; Kalra, S. Triglyceride Glucose (TyG) Index: A surrogate biomarker of insulin resistance. J. Pak. Med. Assoc. 2022, 72, 986–988. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, X.; Adams, H.; Kubena, K.; Guo, S. Etiology of Metabolic Syndrome and Dietary Intervention. Int. J. Mol. Sci. 2018, 20, 128. [Google Scholar] [CrossRef] [PubMed]
- Son, D.H.; Lee, H.S.; Lee, Y.J.; Lee, J.H.; Han, J.H. Comparison of triglyceride-glucose index and HOMA-IR for predicting prevalence and incidence of metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 596–604. [Google Scholar] [CrossRef]
- Zou, Y.; Kuang, M.; Zhong, Y.; Jiang, C. Remnant cholesterol can identify individuals at higher risk of metabolic syndrome in the general population. Sci. Rep. 2023, 13, 5957. [Google Scholar] [CrossRef]
| Total | Male | Female | |
|---|---|---|---|
| N | 65,618 | 30,363 | 35,255 |
| Remnant Cholesterol * | 0.50 ± 0.21 | 0.50 ± 0.21 | 0.49 ± 0.22 |
| MetS, N (%) * | 15,644 (23.8) | 7778 (11.9) | 7866 (12.0) |
| Age, year * | 52.52 ± 14.31 | 53.56 ± 14.40 | 51.63 ± 14.16 |
| BMI * | |||
| Normal | 33,752 (51.4) | 15,741 (24.0) | 18,011 (27.5) |
| Overweight | 22,859 (34.8) | 10,777 (16.4) | 12,082 (18.4) |
| Obese | 9007 (13.7) | 3845 (5.9) | 5162 (7.9) |
| Education status, N (%) * | |||
| Primary school graduate or below | 31,984 (48.7) | 12,485 (19.0) | 19,499 (29.7) |
| Middle/high school | 28,681 (43.7) | 15,493 (23.6) | 13,188 (20.1) |
| College graduate or above | 4953 (7.6) | 2385 (3.6) | 2568 (3.9) |
| Household yearly income per capita, N (%) | |||
| No given | 10,682 (16.3) | 4861 (7.4) | 5821 (8.9) |
| <10,000 CNY | 24,473 (37.3) | 11,477 (17.5) | 12,996 (19.8) |
| 10,000–20,000 CNY | 16,195 (24.7) | 7431 (11.3) | 8764 (13.4) |
| >20,000 CNY | 14,268 (21.7) | 6594 (10.1) | 7674 (11.7) |
| Marital status, N (%) * | |||
| Never married | 2302 (3.5) | 1460 (2.2) | 842 (1.3) |
| Married | 60,425 (92.1) | 28,027 (42.7) | 32,398 (49.4) |
| Other | 2891 (4.4) | 876 (1.3) | 2015 (3.1) |
| Area of the country, N (%) | |||
| North | 29,603 (45.1) | 13,799 (21.0) | 15,804 (24.1) |
| South | 36,015 (54.9) | 16,564 (25.2) | 19,451 (29.6) |
| Residence location, N (%) * | |||
| Urban | 26,894 (41.0) | 12,112 (18.5) | 14,782 (22.5) |
| Rural | 38,724 (59.0) | 18,251 (27.8) | 20,473 (31.2) |
| Smoking status, N (%) * | |||
| Never smoke | 44,182 (67.3) | 10,215 (15.6) | 33,967 (51.8) |
| Former smoke | 4511 (6.9) | 4242 (6.5) | 269 (0.4) |
| Current smoking | 16,925 (25.8) | 15,906 (24.2) | 1019 (1.6) |
| Alcohol consumption, N (%) * | |||
| Never | 40,939 (62.4) | 12,696 (19.4) | 28,243 (43.0) |
| Moderate | 10,369 (15.8) | 5728 (8.7) | 4641 (7.1) |
| Excessive | 14,310 (21.8) | 11,939 (18.2) | 2371 (3.6) |
| Red meat intake * | |||
| Normal | 52,093 (79.4) | 22,683 (34.6) | 29,410 (44.8) |
| Excessive | 13,525 (20.61) | 7680 (11.7) | 5845 (8.9) |
| Vegetable intake * | |||
| Normal | 20,672 (31.5) | 9989 (15.2) | 10,683 (16.3) |
| Insufficient | 44,946 (68.5) | 20,374 (31.1) | 24,572 (37.5) |
| Fruit intake * | |||
| Normal | 4452 (6.8) | 1643 (2.5) | 2809 (4.3) |
| Insufficient | 61,166 (93.2) | 28,720 (43.8) | 32,446 (49.5) |
| Sleep time, N (%) * | |||
| Low | 13,455 (20.5) | 6098 (9.3) | 7357 (11.2) |
| Normal | 44,762 (68.2) | 20,838 (31.8) | 23,924 (36.5) |
| High | 7401 (11.3) | 3427 (5.2) | 3974 (6.1) |
| Physically active, N (%) * | |||
| active | 54,182 (82.6) | 24,462 (37.3) | 29,720 (45.3) |
| inactive | 11,436 (17.4) | 5901 (9.0) | 5535 (8.4) |
| Laboratory results | |||
| TC, mmol/L * | 4.75 ± 0.92 | 4.71 ± 0.90 | 4.80 ± 0.93 |
| TG, mmol/L * | 1.39 ± 0.83 | 1.44 ± 0.87 | 1.35 ± 0.79 |
| LDL-C, mmol/L * | 2.96 ± 0.82 | 2.94 ± 0.81 | 2.97 ± 0.82 |
| HDL-C, mmol/L * | 1.30 ± 0.32 | 1.26 ± 0.33 | 1.33 ± 0.31 |
| FBG, mmol/L * | 5.28 ± 0.89 | 5.31 ± 0.91 | 5.26 ± 0.88 |
| hba1c, % | 4.97 ± 0.64 | 4.96 ± 0.63 | 4.97 ± 0.65 |
| Characteristics | Total | Quartiles of Remnant Cholesterol Index | ||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | p | ||
| Remnant Cholesterol | 0.48 ± 0.00 | 0.31 ± 0.00 | 0.42 ± 0.00 | 0.51 ± 0.00 | 0.75 ± 0.00 | <0.01 |
| MetS | 20.3 (19.7, 20.9) | 2.4 (2.1, 2.7) | 3.1 (2.9, 3.3) | 5.1 (4.8, 5.5) | 9.7 (9.3, 10.1) | <0.01 |
| Age, year | 44.44 ± 0.13 | 40.53 ± 0.24 | 42.83 ± 0.26 | 46.66 ± 0.28 | 48.89 ± 0.27 | <0.01 |
| Sex | <0.01 | |||||
| Male | 49.9 (49.1, 50.6) | 13.4 (12.8, 14.0) | 12.3 (11.7, 12.9) | 12.1 (11.6, 12.7) | 12.06 (11.6, 12.5) | |
| Female | 50.1 (49.4, 50.9) | 15.6 (15.0, 16.2) | 12.3 (11.8, 12.8) | 11.7 (11.3, 12.5) | 10.55 (10.1, 11.0) | |
| BMI | <0.01 | |||||
| Normal | 53.8 (53.0, 54.6) | 18.3 (17.6, 19.0) | 14.4 (13.8, 15.0) | 12.1 (11.6, 12.6) | 9.1 (8.7, 9.5) | |
| Overweight | 32.6 (31.9, 33.3) | 8.0 (7.6, 8.4) | 7.4 (7.0, 7.8) | 8.4 (8.0, 8.8) | 8.9 (8.5, 9.3) | |
| Obese | 13.6 (13.0, 14.1) | 2.8 (2.5, 3.0) | 2.8 (2.6, 3.1) | 3.4 (3.0, 3.6) | 4.7 (4.3, 5.0) | |
| Education status | <0.01 | |||||
| Primary school graduate or below | 36.8 (36.1, 37.5) | 9.3 (8.9, 9.7) | 8.9 (8.5, 9.2) | 9.4 (9.1, 9.8) | 9.2 (8.8, 9.5) | |
| Middle/high school | 48.1 (47.3, 48.9) | 14.2 (13.7, 14.8) | 11.7 (11.1, 12.2) | 11.4 (10.9, 11.8) | 10.9 (10.4, 11.3) | |
| College graduate or above | 15.1 (14.4, 15.9) | 5.5 (5.0, 6.0) | 4.0 (3.6, 4.5) | 3.0 (2.7, 3.4) | 2.6 (2.3, 2.9) | |
| Household yearly income per capita | <0.01 | |||||
| No given | 15.9 (15.3, 16.4) | 4.8 (4.4, 5.2) | 3.9 (3.6, 4.2) | 3.7 (3.4, 3.9) | 3.5 (3.2, 3.7) | |
| <10,000 | 35.4 (34.7, 36.1) | 9.4 (9.0, 9.8) | 8.7 (8.3, 9.1) | 8.8 (8.4, 9.2) | 8.4 (8.0, 8.8) | |
| 10,000–20,000 | 24.4 (23.7, 25.1) | 7.2 (6.8, 7.6) | 6.1 (5.6, 6.5) | 5.7 (5.4, 6.11) | 5.4 (5.1, 5.8) | |
| >20,000 | 24.3 (23.7, 25.0) | 7.6 (7.1, 8.1) | 5.8 (5.5, 6.2) | 5.6 (5.2, 6.00) | 5.3 (5.0, 5.6) | |
| Marital status | <0.01 | |||||
| Never married | 10.5 (9.8, 11.3) | 4.3 (3.8, 4.8) | 2.8 (2.5, 3.2) | 2.0 (1.6, 2.4) | 1.3 (1.1, 1.6) | |
| Married | 85.9 (85.2, 86.7) | 23.9 (23.3, 24.6) | 21.0 (20.4, 21.6) | 20.8 (20.2, 21.4) | 20.2 (19.7, 20.8) | |
| Other | 3.6 (3.4, 3.8) | 0.8 (0.7, 0.9) | 0.8 (0.7, 0.9) | 1.0 (0.9, 1.1) | 1.1 (0.9, 1.2) | |
| Area of the country | <0.01 | |||||
| North | 47.3 (46.5, 48.1) | 14.5 (13.9, 15.1) | 11.5 (11.0, 12.0) | 10.6 (10.1, 11.1) | 10.7 (10.3, 11.2) | |
| South | 52.7 (51.9, 53.5) | 14.5 (13.9, 15.0) | 13.1 (12.6, 13.6) | 13.3 (12.8, 13.8) | 11.9 (11.4, 12.3) | |
| Residence location | <0.01 | |||||
| Urban | 48.5 (47.7, 49.3) | 15.6 (15.0, 16.3) | 11.7 (11.2, 12.3) | 10.8 (10.2, 11.3) | 10.4 (9.9, 10.9) | |
| Rural | 51.6 (50.7, 52.3) | 13.4 (12.9, 13.8) | 12.9 (12.4, 13.3) | 13.1 (12.6, 13.5) | 12.2 (11.8, 12.7) | |
| Smoking status | <0.01 | |||||
| Never smoke | 67.8 (67.0, 68.5) | 21.2 (20.6, 21.9) | 16.7 (16.1, 17.3) | 15.8 (15.3, 16.4) | 14.0 (13.5, 14.5) | |
| Former smoke | 5.4 (5.1, 5.7) | 1.3 (1.2, 1.5) | 1.2 (1.0, 1.4) | 1.4 (1.2, 1.5) | 1.5 (1.3, 1.7) | |
| Current smoking | 26.9 (26.2, 27.6) | 6.4 (6.1, 6.8) | 6.7 (6.3, 7.1) | 6.6 (6.3, 7.0) | 7.1 (6.7, 7.5) | |
| Alcohol consumption | 0.06 | |||||
| Never | 59.2 (58.4, 59.9) | 17.0 (16.4, 17.6) | 14.6 (14.0, 15.1) | 14.4 (13.9, 15.0) | 13.2 (12.7, 13.7) | |
| Moderate | 17.0 (16.5, 17.6) | 5.3 (4.9, 5.7) | 4.2 (3.8, 4.5) | 3.9 (3.6, 4.2) | 3.7 (3.5, 4.0) | |
| Excessive | 23.8 (23.1, 24.5) | 6.7 (6.3, 7.2) | 5.8 (5.5, 6.2) | 5.6 (5.2, 5.9) | 5.7 (5.3, 6.1) | |
| Red meat intake | 0.98 | |||||
| Normal | 77.3 (76.6, 77.9) | 22.5 (21.8, 23.1) | 18.9 (18.3, 19.6) | 18.4 (17.8, 19.0) | 17.5 (17.0, 18.0) | |
| Excessive | 22.8 (22.1, 23.4) | 6.6 (6.1, 7.0) | 5.6 (5.3, 6.0) | 5.4 (5.1, 5.8) | 5.1 (4.8, 5.5) | |
| Vegetable intake | 0.71 | |||||
| Normal | 30.6 (29.9, 31.3) | 9.0 (8.6, 9.5) | 7.6 (7.2, 8.0) | 7.2 (6.8, 7.7) | 6.8 (6.4, 7.1) | |
| Insufficient | 69.4 (68.7, 70.1) | 20.0 (19.3, 20.7) | 17.0 (16.4, 17.6) | 16.6 (16.0, 17.1) | 15.8 (15.3, 16.4) | |
| Fruit intake | <0.05 | |||||
| Normal | 8.2 (7.7, 8.6) | 2.6 (2.3, 2.8) | 2.1 (1.8, 2.4) | 1.9 (1.7, 2.1) | 1.6 (1.5, 1.8) | |
| Insufficient | 91.8 (91.4, 92.3) | 26.4 (25.7, 27.1) | 22.5 (21.8, 23.2) | 21.9 (21.3, 22.6) | 21.0 (20.4, 21.6) | |
| Sleep time | <0.01 | |||||
| Low | 16.5 (16.0, 17.0) | 4.0 (3.8, 4.3) | 4.0 (3.7, 4.3) | 4.3 (4.1, 4.6) | 4.1 (3.9, 4.3) | |
| Normal | 72.2 (71.5, 72.8) | 21.9 (21.2, 22.6) | 17.8 (17.2, 18.4) | 16.8 (16.2, 17.4) | 15.7 (15.2, 16.3) | |
| High | 11.4 (10.9, 11.9) | 3.1 (2.8, 3.4) | 2.8 (2.5, 3.0) | 2.7 (2.9, 3.0) | 2.8 (2.5, 3.0) | |
| Physically active | 0.92 | |||||
| active | 80.7 (80.1, 81.4) | 23.4 (22.7, 24.1) | 19.9 (19.3, 20.5) | 19.2 (18.6, 19.8) | 18.2 (17.6, 18.7) | |
| inactive | 19.3 (18.6, 19.9) | 5.6 (5.2, 6.0) | 4.7 (4.3, 5.0) | 4.6 (4.2, 5.0) | 4.4 (4.1, 4.8) | |
| Laboratory results | ||||||
| TC, mmol/L | 4.60 ± 0.01 | 4.24 ± 0.01 | 4.44 ± 0.01 | 4.73 ± 0.01 | 5.12 ± 0.01 | <0.01 |
| TG, mmol/L | 1.37 ± 0.01 | 0.96 ± 0.01 | 1.12 ± 0.01 | 1.38 ± 0.01 | 2.14 ± 0.02 | <0.01 |
| LDL-C, mmol/L | 2.86 ± 0.01 | 2.58 ± 0.01 | 2.72 ± 0.01 | 2.97 ± 0.01 | 3.23 ± 0.01 | <0.01 |
| HDL-C, mmol/L | 1.26 ± 0.00 | 1.35 ± 0.00 | 1.30 ± 0.00 | 1.25 ± 0.00 | 1.14 ± 0.00 | <0.01 |
| FBG, mmol/L | 5.17 ± 0.01 | 5.01 ± 0.01 | 5.08 ± 0.01 | 5.24 ± 0.01 | 5.42 ± 0.01 | <0.01 |
| hba1c,% | 4.88 ± 0.00 | 4.82 ± 0.01 | 4.85 ± 0.01 | 4.91 ± 0.01 | 4.95 ± 0.01 | <0.01 |
| Indicators | Group of Quartile | MetS OR (95% CI) | ||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||
| RC | Q1 | reference | reference | reference |
| Q2 | 1.60 (1.38, 1.85) | 1.50 (1.29, 1.73) | 1.47 (1.26, 1.72) | |
| Q3 | 3.03 (2.65, 3.48) | 2.64 (2.30, 3.03) | 2.54 (2.19, 2.94) | |
| Q4 | 8.32 (7.30, 9.47) | 7.06 (6.20, 8.03) | 6.66 (5.82, 7.62) | |
| Indicators | Group of Quartile | MetS OR (95% CI) | ||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||
| High WC | Q1 | reference | reference | reference |
| Q2 | 1.21 (1.09, 1.34) | 1.17 (1.06, 1.30) | 1.06 (0.93, 1.21) | |
| Q3 | 1.60 (1.45, 1.76) | 1.47 (1.33, 1.62) | 1.16 (1.03, 1.32) | |
| Q4 | 2.56 (2.33, 2.81) | 2.29 (2.08, 2.52) | 1.47 (1.31, 1.66) | |
| p-values | <0.01 | <0.01 | <0.01 | |
| High BP | Q1 | reference | reference | reference |
| Q2 | 1.29 (1.18, 1.41) | 1.15 (1.04, 1.27) | 1.10 (1.00, 1.22) | |
| Q3 | 1.77 (1.62, 1.93) | 1.31 (1.19, 1.44) | 1.18 (1.07, 1.31) | |
| Q4 | 2.72 (2.49, 2.97) | 1.86 (1.69, 2.05) | 1.51 (1.37, 1.68) | |
| p-values | <0.01 | <0.01 | <0.01 | |
| Elevated TG | Q1 | reference | reference | reference |
| Q2 | 1.76 (1.52, 2.03) | 1.71 (1.48, 1.98) | 1.72 (1.49, 1.98) | |
| Q3 | 4.14 (3.62, 4.73) | 3.98 (3.49, 4.54) | 3.96 (3.47, 4.53) | |
| Q4 | 14.99 (13.18, 17.06) | 14.31 (12.61, 16.26) | 13.94 (12.31, 15.79) | |
| p-values | <0.01 | <0.01 | <0.01 | |
| Low HDL-C | Q1 | reference | reference | reference |
| Q2 | 1.32 (1.17, 1.48) | 1.31 (1.16, 1.47) | 1.31 (1.16, 1.48) | |
| Q3 | 1.86 (1.66, 2.08) | 1.87 (1.67, 2.10) | 1.84 (1.63, 2.06) | |
| Q4 | 3.80 (3.43, 4.22) | 3.86 (3.48, 4.29) | 3.57 (3.21, 3.98) | |
| p-values | <0.01 | <0.01 | <0.01 | |
| Elevated FPG | Q1 | reference | reference | reference |
| Q2 | 1.23 (1.07, 1.41) | 1.12 (0.97, 1.28) | 1.10 (0.96, 1.26) | |
| Q3 | 1.89 (1.65, 2.18) | 1.51 (1.31, 1.76) | 1.43 (1.23, 1.65) | |
| Q4 | 3.12 (2.74, 3.55) | 2.36 (2.06, 2.70) | 2.05 (1.79, 2.35) | |
| p-values | <0.01 | <0.01 | <0.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Yuan, H.; Cai, S.; Piao, W.; Nan, J.; Yang, Y.; Zhao, L.; Yu, D. Association between Remnant Cholesterol and Metabolic Syndrome among Chinese Adults: Chinese Nutrition and Health Surveillance (2015–2017). Nutrients 2024, 16, 3275. https://doi.org/10.3390/nu16193275
Li F, Yuan H, Cai S, Piao W, Nan J, Yang Y, Zhao L, Yu D. Association between Remnant Cholesterol and Metabolic Syndrome among Chinese Adults: Chinese Nutrition and Health Surveillance (2015–2017). Nutrients. 2024; 16(19):3275. https://doi.org/10.3390/nu16193275
Chicago/Turabian StyleLi, Fusheng, Hongtao Yuan, Shuya Cai, Wei Piao, Jing Nan, Yuxiang Yang, Liyun Zhao, and Dongmei Yu. 2024. "Association between Remnant Cholesterol and Metabolic Syndrome among Chinese Adults: Chinese Nutrition and Health Surveillance (2015–2017)" Nutrients 16, no. 19: 3275. https://doi.org/10.3390/nu16193275
APA StyleLi, F., Yuan, H., Cai, S., Piao, W., Nan, J., Yang, Y., Zhao, L., & Yu, D. (2024). Association between Remnant Cholesterol and Metabolic Syndrome among Chinese Adults: Chinese Nutrition and Health Surveillance (2015–2017). Nutrients, 16(19), 3275. https://doi.org/10.3390/nu16193275





