Filbertone Reduces Senescence in C2C12 Myotubes Treated with Doxorubicin or H2O2 through MuRF1 and Myogenin
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. RNA Isolation and Quantitative RT-PCR (qRT-PCR)
2.3. Protein Sample Preparation and Western Blotting
2.4. Cell Viability Measurement
2.5. Senescence-Associated β-Galactosidase (SA-β-gal) Staining
2.6. Statistical Analysis
3. Results
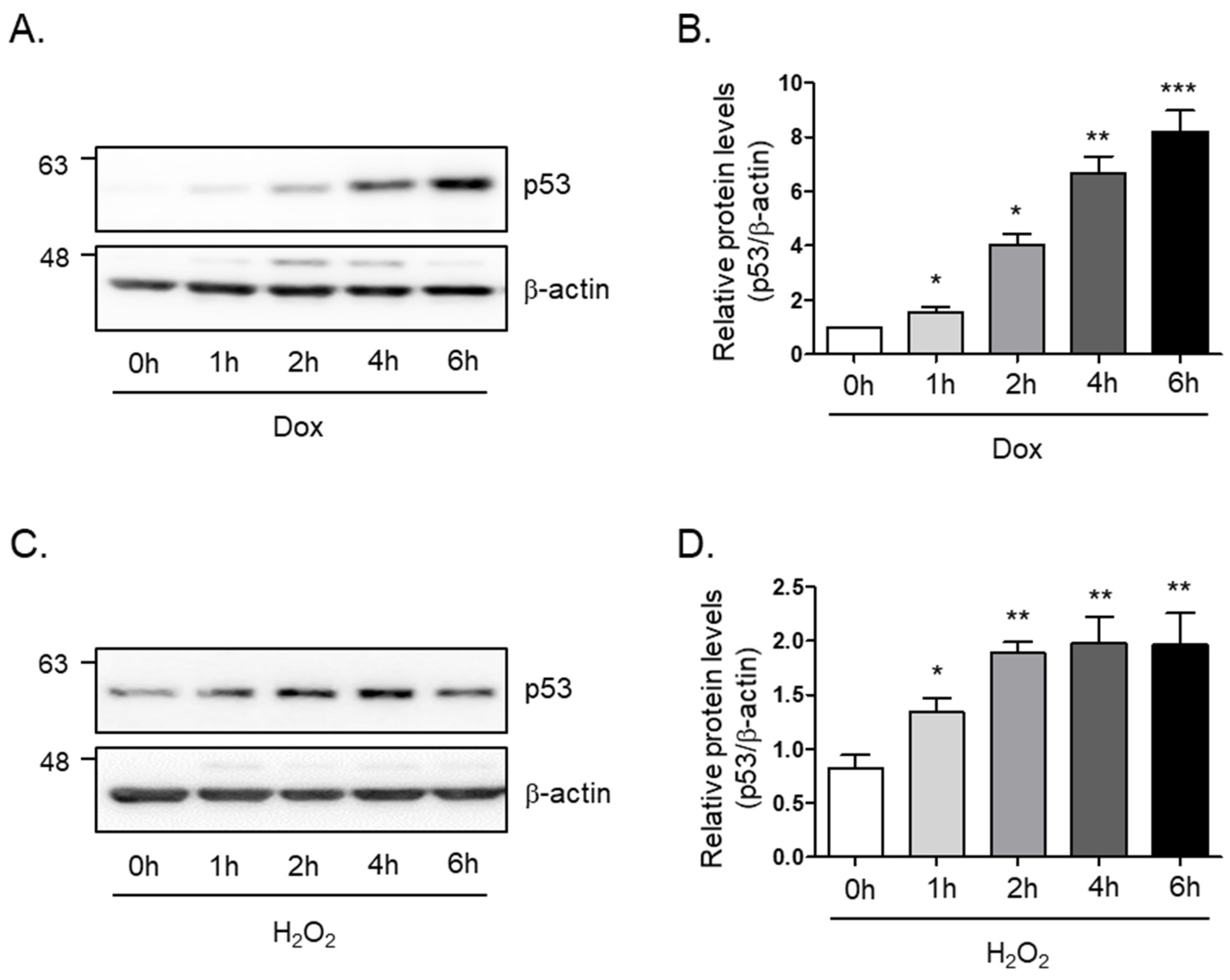
3.1. Establishment of Cellular Senescence Model in C2C12 Myotubes
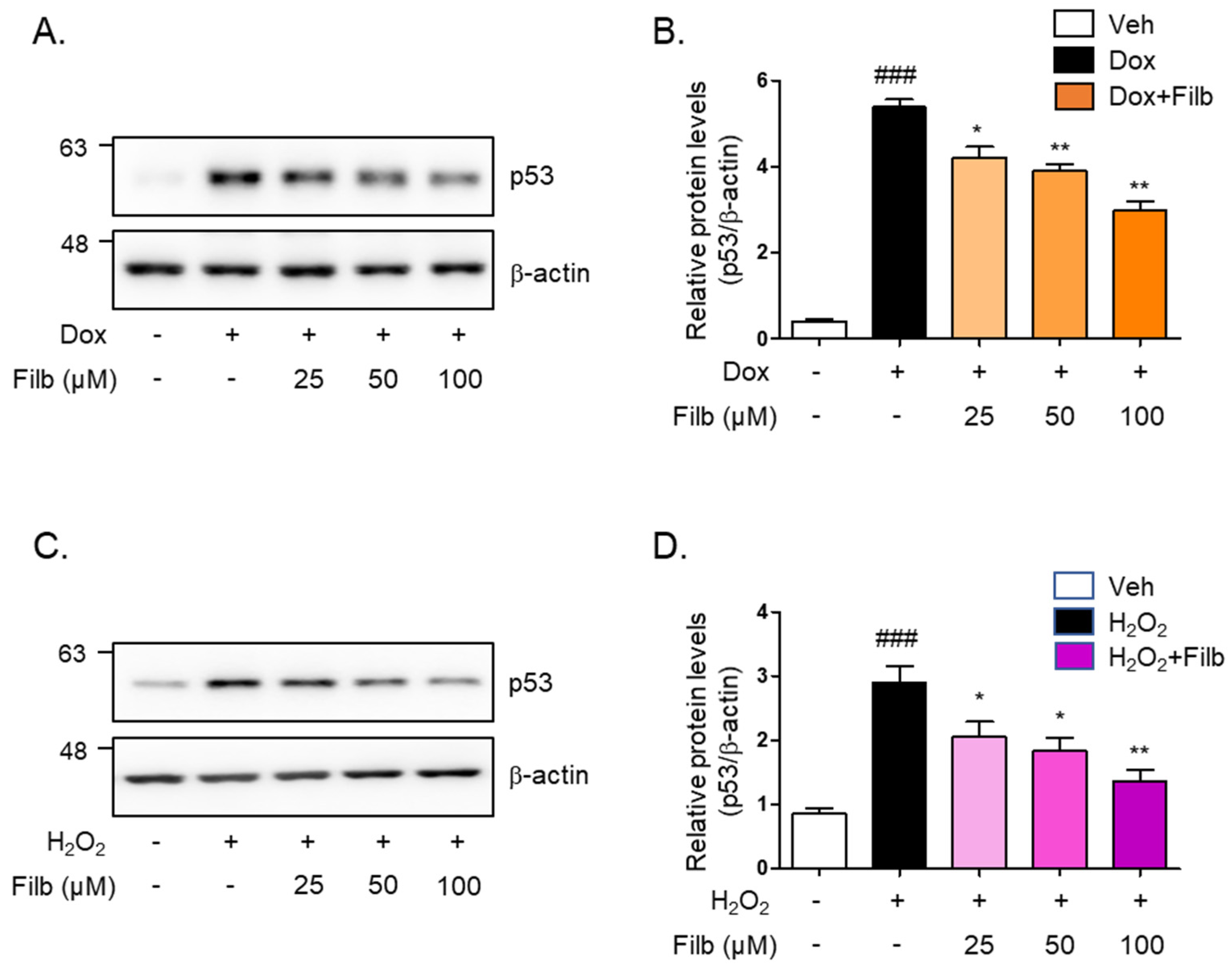
3.2. Effect of Filbertone in Doxorubicin or Hydrogen Peroxide Treated C2C12 Myotubes
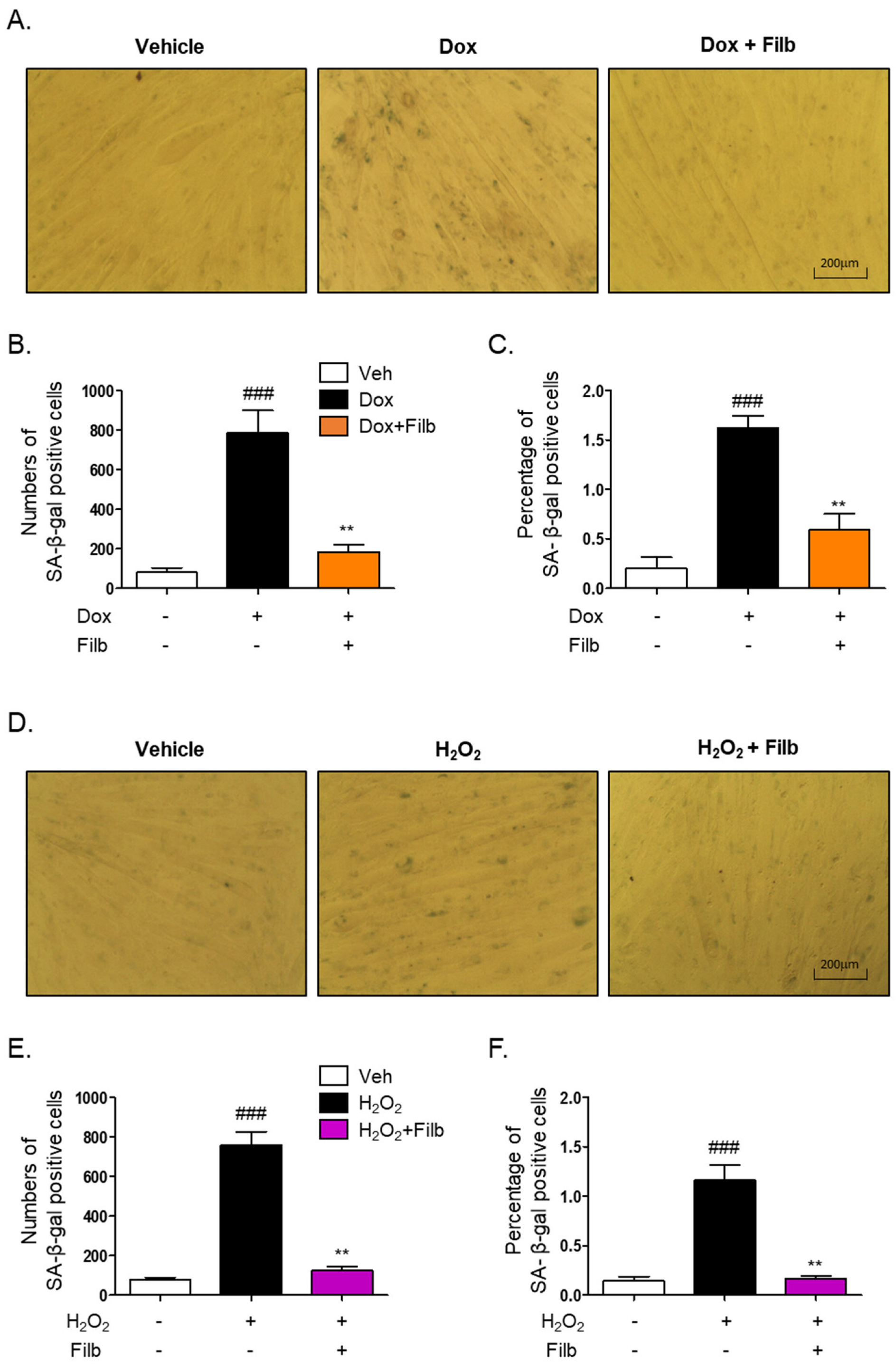
3.3. The Activity of Senescence-Associated β-Galactosidase (SA-β-gal) Is Modulated by Filbertone
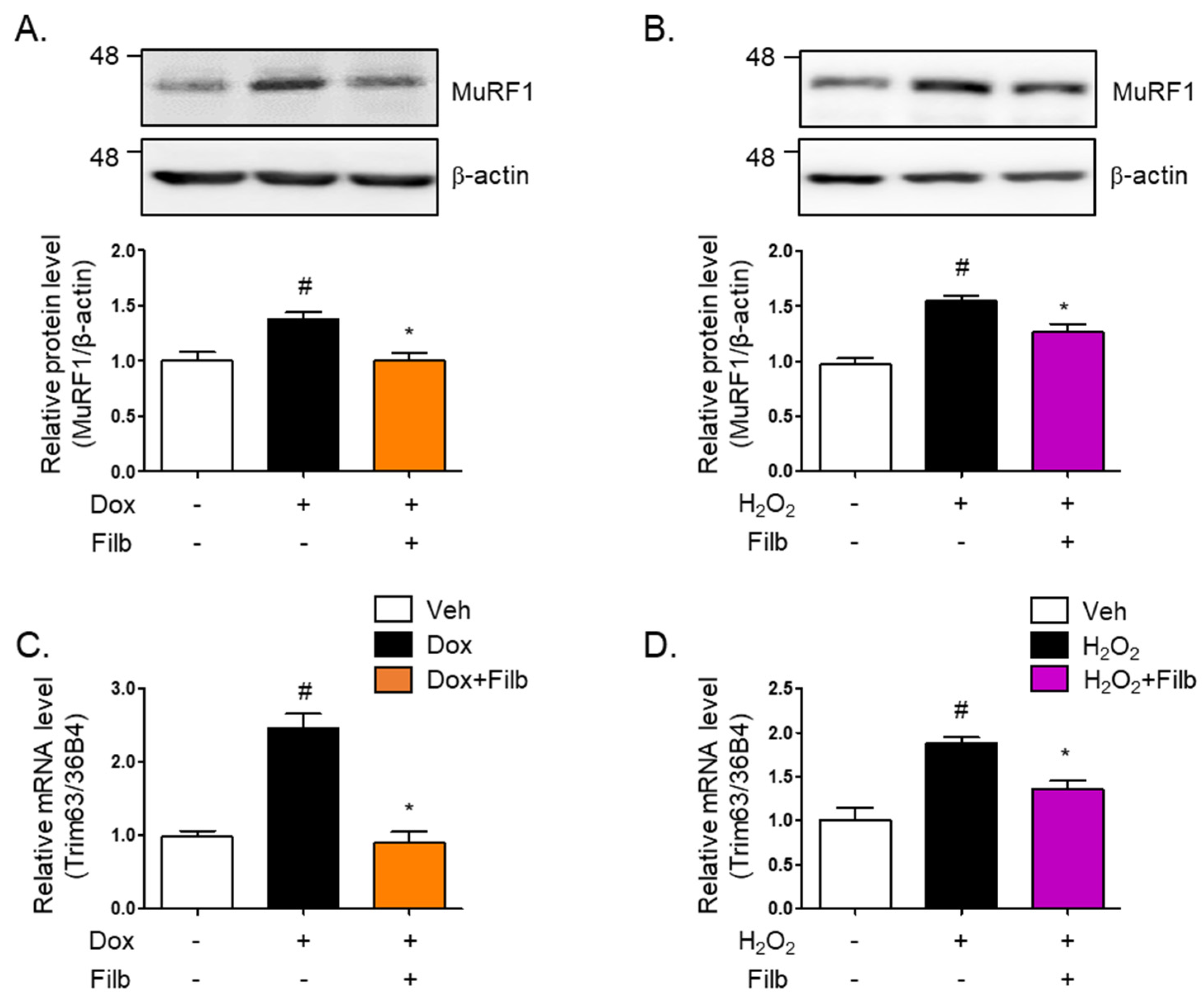
3.4. Filbertone Regulates an Associated Risk Factor with Muscle Atrophy
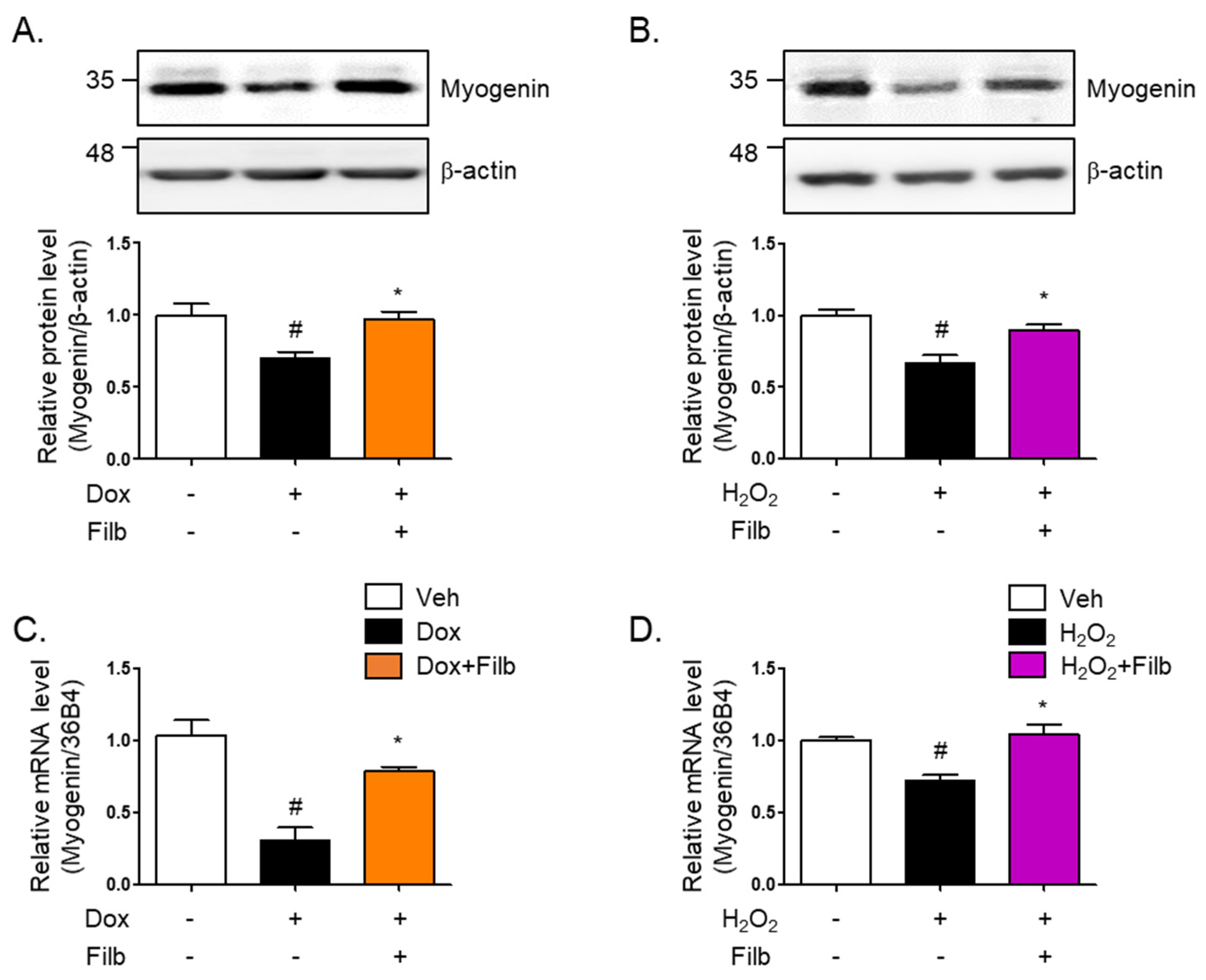
3.5. Filbertone Controls a Myogenic Regulatory Factor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brook, M.S.; Wilkinson, D.J.; Phillips, B.E.; Perez-Schindler, J.; Philp, A.; Smith, K.; Atherton, P.J. Skeletal muscle homeostasis and plasticity in youth and ageing: Impact of nutrition and exercise. Acta Physiol. 2016, 216, 15–41. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Drew, L. Fighting the inevitability of ageing. Nature 2018, 555, S15–S17. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2018, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Fyhrquist, F.; Saijonmaa, O.; Strandberg, T. The roles of senescence and telomere shortening in cardiovascular disease. Nat. Rev. Cardiol. 2013, 10, 274–283. [Google Scholar] [CrossRef]
- Izquierdo, J.M. Cellular senescence: Is the problem a solution for muscle repair? Cell. Mol. Immunol. 2023, 20, 429–431. [Google Scholar] [CrossRef]
- Takahashi, A.; Ohtani, N.; Yamakoshi, K.; Iida, S.; Tahara, H.; Nakayama, K.; Nakayama, K.I.; Ide, T.; Saya, H.; Hara, E. Mitogenic signalling and the p16INK4a-Rb pathway cooperate to enforce irreversible cellular senescence. Nat. Cell Biol. 2006, 8, 1291–1297. [Google Scholar] [CrossRef]
- Qian, Y.; Chen, X. Senescence regulation by the p53 protein family. Methods Mol. Biol. 2013, 965, 37–61. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, D.Y.; O’Connell, J.F.; Egan, J.M.; Kim, Y. Black Ginseng Ameliorates Cellular Senescence via p53-p21/p16 Pathway in Aged Mice. Biology 2022, 11, 1108. [Google Scholar] [CrossRef]
- Baker, D.J.; Childs, B.G.; Durik, M.; Wijers, M.E.; Sieben, C.J.; Zhong, J.; Saltness, R.A.; Jeganathan, K.B.; Verzosa, G.C.; Pezeshki, A.; et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 2016, 530, 184–189. [Google Scholar] [CrossRef] [PubMed]
- van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Tey, S.L.; Brown, R.; Chisholm, A.; Gray, A.; Williams, S.; Delahunty, C. Current guidelines for nut consumption are achievable and sustainable: A hazelnut intervention. Br. J. Nutr. 2011, 105, 1503–1511. [Google Scholar] [CrossRef]
- Alasalvar, C.; Karamac, M.; Kosinska, A.; Rybarczyk, A.; Shahidi, F.; Amarowicz, R. Antioxidant activity of hazelnut skin phenolics. J. Agric. Food Chem. 2009, 57, 4645–4650. [Google Scholar] [CrossRef]
- Puchl’ová, E.; Szolcsányi, P. Filbertone: A Review. J. Agric. Food Chem. 2018, 66, 11221–11226. [Google Scholar] [CrossRef] [PubMed]
- Burdock, G.A.; Wagner, B.M.; Smith, R.L.; Munro, I.C.; Newberne, P.M. Recent progress in the consideration of flavor ingredients under the Food Additives Amendment. 15. GRAS Substances. Food Technol. 1990, 44, 78–86. [Google Scholar]
- Moon, Y.; Tong, T.; Kang, W.; Park, T. Filbertone ameliorates adiposity in mice fed a high-fat diet via activation of cAMP signaling. Nutrients 2019, 11, 1749. [Google Scholar] [CrossRef]
- Mutsnaini, L.; Yang, J.; Kim, J.; Kim, C.-S.; Lee, C.-H.; Kim, M.-S.; Park, T.; Goto, T.; Yu, R. Filbertone protects obesity-induced hypothalamic inflammation by reduction of microglia-mediated inflammatory responses. Biotechnol. Bioprocess Eng. 2021, 26, 86–92. [Google Scholar] [CrossRef]
- Park, J.; Gong, J.H.; Chen, Y.; Nghiem, T.-H.T.; Chandrawanshi, S.; Hwang, E.; Yang, C.H.; Kim, B.-S.; Park, J.W.; Ryter, S.W. Activation of ROS-PERK-TFEB by filbertone ameliorates neurodegenerative diseases via enhancing the autophagy-lysosomal pathway. J. Nutr. Biochem. 2023, 118, 109325. [Google Scholar] [CrossRef]
- Kim, H.; Ahn, B. Filbertone,(2E)-5-methyl-2-hepten-4-one, regulates thermogenesis and lipid metabolism in skeletal muscle of a high-fat diet fed mice. Appl. Biol. Chem. 2023, 66, 24. [Google Scholar] [CrossRef]
- Kim, E.M.; Jung, C.-H.; Kim, J.; Hwang, S.-G.; Park, J.K.; Um, H.-D. The p53/p21 Complex Regulates Cancer Cell Invasion and Apoptosis by Targeting Bcl-2 Family Proteins. Cancer Res. 2017, 77, 3092–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Arndt, J.; O’Connell, J.F.; Egan, J.M.; Kim, Y. Red Ginseng Attenuates the Hepatic Cellular Senescence in Aged Mice. Biology 2024, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Gartel, A.L.; Radhakrishnan, S.K. Lost in Transcription: p21 Repression, Mechanisms, and Consequences. Cancer Res. 2005, 65, 3980–3985. [Google Scholar] [CrossRef]
- Rufini, A.; Tucci, P.; Celardo, I.; Melino, G. Senescence and aging: The critical roles of p53. Oncogene 2013, 32, 5129–5143. [Google Scholar] [CrossRef]
- Walter, D.; Hoffmann, S.; Komseli, E.S.; Rappsilber, J.; Gorgoulis, V.; Sørensen, C.S. SCF(Cyclin F)-dependent degradation of CDC6 suppresses DNA re-replication. Nat. Commun. 2016, 7, 10530. [Google Scholar] [CrossRef]
- Soriano-Arroquia, A.; McCormick, R.; Molloy, A.P.; McArdle, A.; Goljanek-Whysall, K. Age-related changes in miR-143-3p:Igfbp5 interactions affect muscle regeneration. Aging Cell 2016, 15, 361–369. [Google Scholar] [CrossRef]
- Glass, D.; Roubenoff, R. Recent advances in the biology and therapy of muscle wasting. Ann. N. Y. Acad. Sci. 2010, 1211, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef]
- Heras, G.; Namuduri, A.V.; Traini, L.; Shevchenko, G.; Falk, A.; Bergström Lind, S.; Jia, M.; Tian, G.; Gastaldello, S. Muscle RING-finger protein-1 (MuRF1) functions and cellular localization are regulated by SUMO1 post-translational modification. J. Mol. Cell Biol. 2018, 11, 356–370. [Google Scholar] [CrossRef]
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of muscle atrophy and hypertrophy: Implications in health and disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef]
- Nabeshima, Y.; Hanaoka, K.; Hayasaka, M.; Esumi, E.; Li, S.; Nonaka, I.; Nabeshima, Y. Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature 1993, 364, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Gautel, M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat. Rev. Mol. Cell Biol. 2011, 12, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hernández, J.M.; García-González, E.G.; Brun, C.E.; Rudnicki, M.A. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 2017, 72, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef] [PubMed]
- Batsis, J.A.; Mackenzie, T.A.; Barre, L.K.; Lopez-Jimenez, F.; Bartels, S.J. Sarcopenia, sarcopenic obesity and mortality in older adults: Results from the National Health and Nutrition Examination Survey III. Eur. J. Clin. Nutr. 2014, 68, 1001–1007. [Google Scholar] [CrossRef]
- Villareal, D.T.; Banks, M.; Siener, C.; Sinacore, D.R.; Klein, S. Physical frailty and body composition in obese elderly men and women. Obes. Res. 2004, 12, 913–920. [Google Scholar] [CrossRef]
- Prado, C.M.; Wells, J.C.; Smith, S.R.; Stephan, B.C.; Siervo, M. Sarcopenic obesity: A Critical appraisal of the current evidence. Clin. Nutr. 2012, 31, 583–601. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, R.; Parrillo, L.; Longo, M.; Florese, P.; Desiderio, A.; Zatterale, F.; Miele, C.; Raciti, G.A.; Beguinot, F. Molecular basis of ageing in chronic metabolic diseases. J. Endocrinol. Investig. 2020, 43, 1373–1389. [Google Scholar] [CrossRef]
- Halter, J.B.; Musi, N.; McFarland Horne, F.; Crandall, J.P.; Goldberg, A.; Harkless, L.; Hazzard, W.R.; Huang, E.S.; Kirkman, M.S.; Plutzky, J.; et al. Diabetes and Cardiovascular Disease in Older Adults: Current Status and Future Directions. Diabetes 2014, 63, 2578–2589. [Google Scholar] [CrossRef]
- Shimizu, H.; Langenbacher, A.D.; Huang, J.; Wang, K.; Otto, G.; Geisler, R.; Wang, Y.; Chen, J.N. The Calcineurin-FoxO-MuRF1 signaling pathway regulates myofibril integrity in cardiomyocytes. eLife 2017, 6, e27955. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Cornwell, E.W.; Jackman, R.W.; Kandarian, S.C. NF-κB but not FoxO sites in the MuRF1 promoter are required for transcriptional activation in disuse muscle atrophy. Am. J. Physiol. Cell Physiol. 2014, 306, C762–C767. [Google Scholar] [CrossRef] [PubMed]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Suetta, C.; Frandsen, U.; Mackey, A.L.; Jensen, L.; Hvid, L.G.; Bayer, M.L.; Petersson, S.J.; Schrøder, H.D.; Andersen, J.L.; Aagaard, P.; et al. Ageing is associated with diminished muscle re-growth and myogenic precursor cell expansion early after immobility-induced atrophy in human skeletal muscle. J. Physiol. 2013, 591, 3789–3804. [Google Scholar] [CrossRef]
- Ganassi, M.; Badodi, S.; Wanders, K.; Zammit, P.S.; Hughes, S.M. Myogenin is an essential regulator of adult myofibre growth and muscle stem cell homeostasis. eLife 2020, 9, e60445. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.; Ahn, B. Filbertone Reduces Senescence in C2C12 Myotubes Treated with Doxorubicin or H2O2 through MuRF1 and Myogenin. Nutrients 2024, 16, 3177. https://doi.org/10.3390/nu16183177
Jung S, Ahn B. Filbertone Reduces Senescence in C2C12 Myotubes Treated with Doxorubicin or H2O2 through MuRF1 and Myogenin. Nutrients. 2024; 16(18):3177. https://doi.org/10.3390/nu16183177
Chicago/Turabian StyleJung, Sumin, and Byungyong Ahn. 2024. "Filbertone Reduces Senescence in C2C12 Myotubes Treated with Doxorubicin or H2O2 through MuRF1 and Myogenin" Nutrients 16, no. 18: 3177. https://doi.org/10.3390/nu16183177
APA StyleJung, S., & Ahn, B. (2024). Filbertone Reduces Senescence in C2C12 Myotubes Treated with Doxorubicin or H2O2 through MuRF1 and Myogenin. Nutrients, 16(18), 3177. https://doi.org/10.3390/nu16183177





