Functional Role of Extracellular Vesicles in Skeletal Muscle Physiology and Sarcopenia: The Importance of Physical Exercise and Nutrition
Abstract
1. Introduction
2. Skeletal Muscle as an Endocrine Tissue
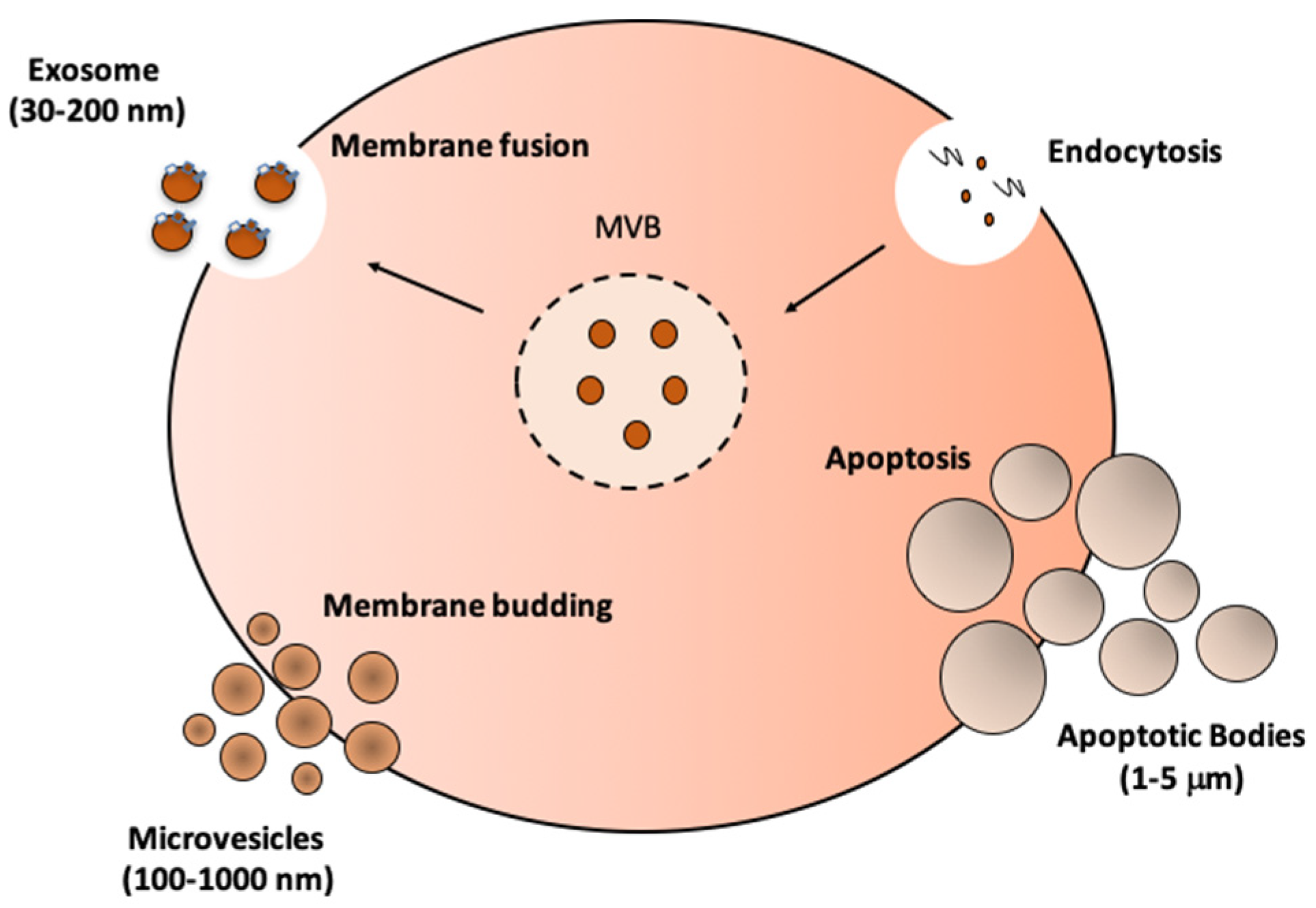
3. Biogenesis, Isolation, and Characterization of EVs
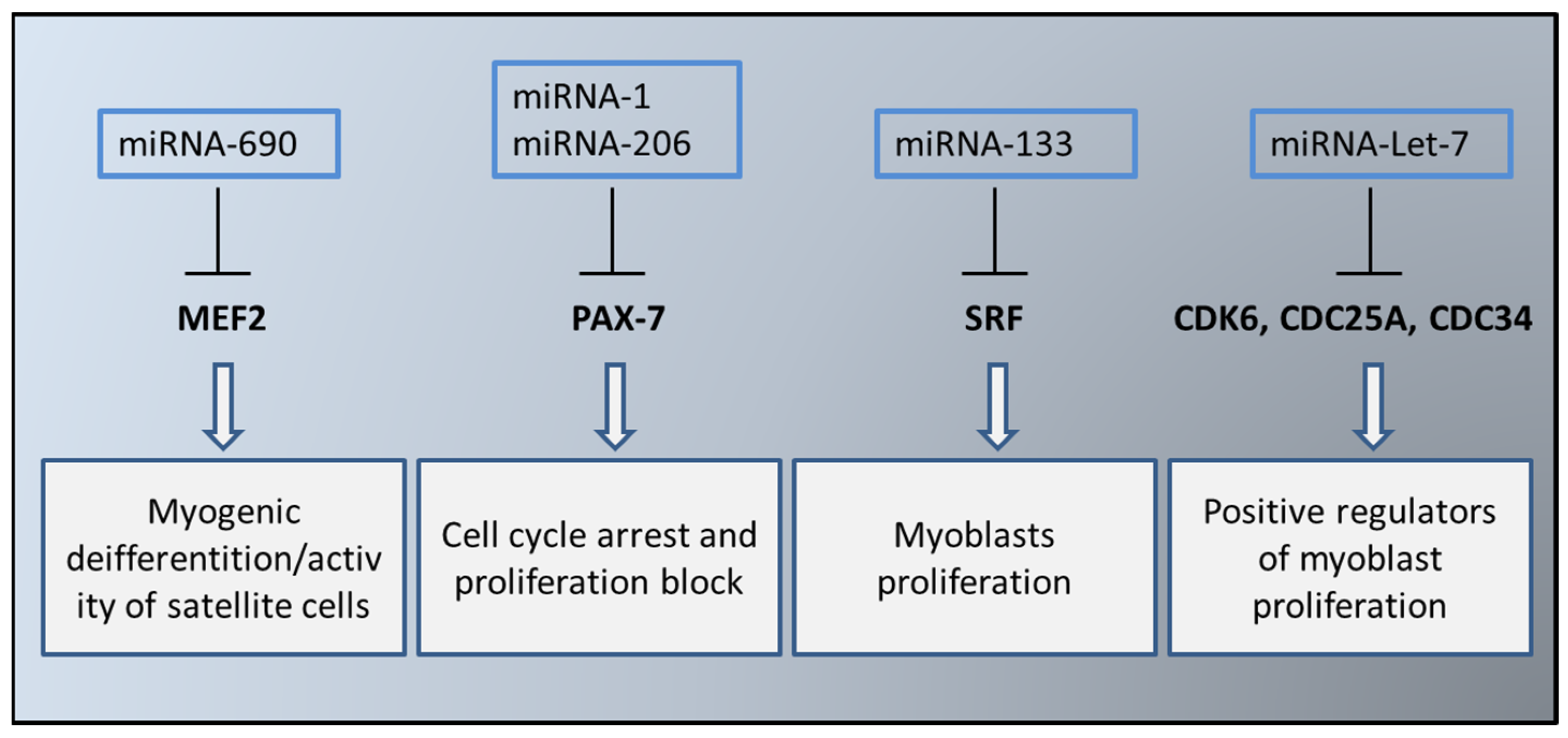
4. EVs in the Myogenesis Process
5. Sarcopenia and Muscle EVs
6. The Impact of Diet, Food, and Exercise on EVs
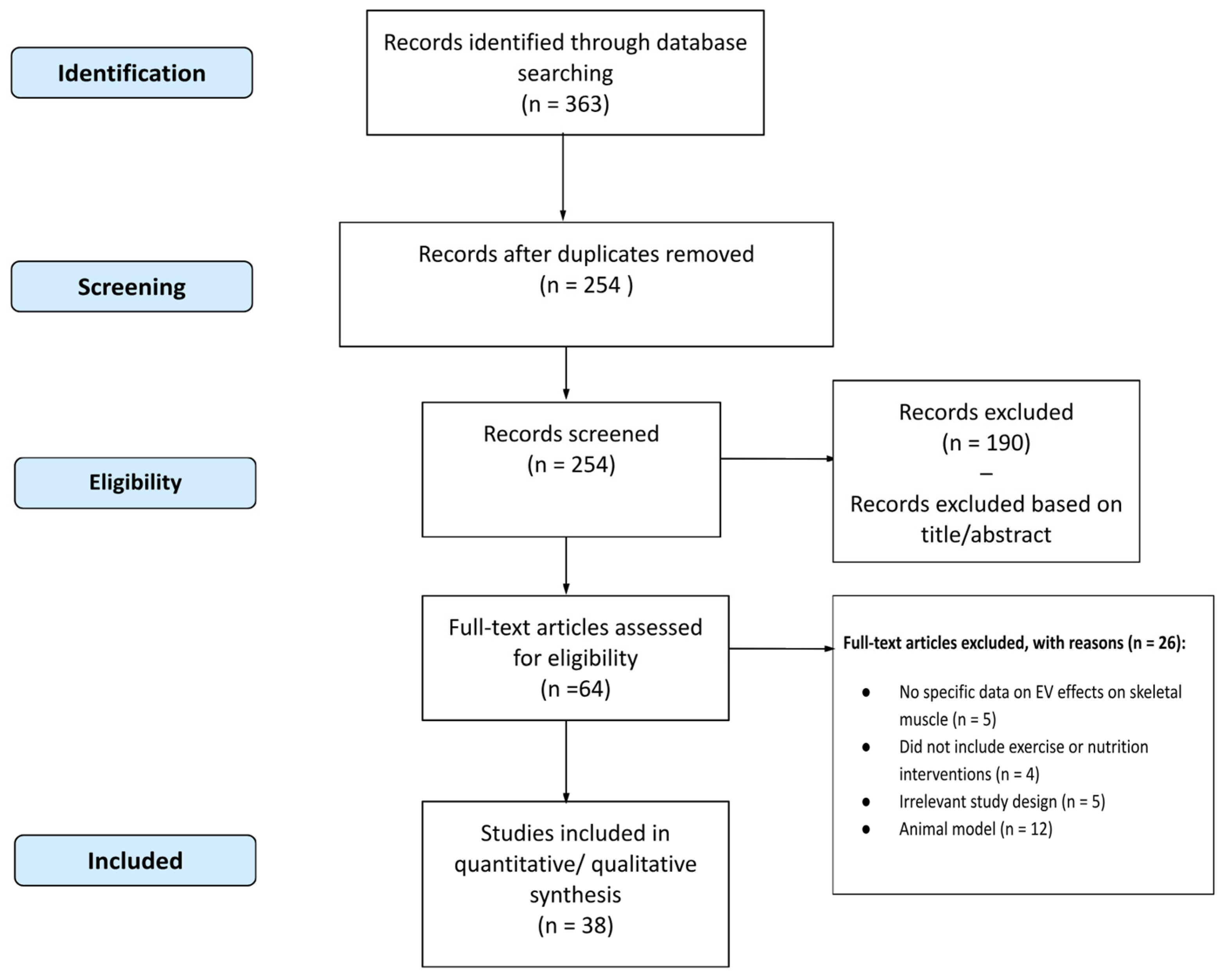
6.1. Methodology
6.2. Impact of Diet on EVs
6.3. Influence of Specific Foods on EVs
6.4. Effects of Nutritional Supplements on EVs
| First Author | Year | Sample Size (n) | Study Design | Population | Duration | Intervention Type | Effects on EV | Other Outcomes | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Phang, M. | 2012 | 30 | Comparative Study | Healthy subjects | 24 h | EPA | ↓ platelet microparticle activity, DHA did not | Both EPA and DHA ↓ platelet aggregation, gender-dependent effects observed | [36] |
| Weisshaar, S. | 2013 | 14 | RCT | Healthy male subjects | 6 h | Vitamin C | =MP formation post-LPS exposure | ↑ MP levels during acute systemic inflammation | [85] |
| Horn, P. | 2014 | 16 | Clinical Trial | Patients with coronary artery disease | 1 month | Flavanol | ↓ levels of CD31+/41− and CD144+ endothelial EVs | Improved endothelial function | [77] |
| Wu, S-Y. | 2014 | 84 | RCT | Subjects at moderate risk of cardiovascular disease | 8 weeks | Fish-oil | ↑ numbers of EPCs, ↓ numbers of EMPs | No significant effects on blood pressure, plasma lipids, or plasma glucose | [80] |
| Zhang, X. | 2014 | 22 | RCT | Patients with type 2 diabetes | 8 weeks | Oat-enriched diet | ↓ concentrations and proportions of fibrinogen- and tissue factor-related platelet and monocyte microparticles | Improved inflammatory status assessed by microparticle concentrations | [73] |
| Yang, M. | 2015 | 14 | Controlled Clinical Trial | Healthy male divers | 6 days | Ascorbic acid | diminished microparticle elevations post-SCUBA diving | ↓ neutrophil activation, no effect on intravascular bubble production | [86] |
| Chiva-Blanch, G. | 2016 | 50 | RCT | High cardiovascular-risk individuals | 1 year | MD + nuts | ↓ levels of CD142+/CD61+/AV+, CD146+/AV+, and CD45+/AV+ microparticles in patients with no-CVE compared to CVE | Predictive model for future cardiovascular events with high accuracy | [68] |
| Phang, M. | 2016 | 94 | RCT | Healthy men and women | 4 weeks | DHA, EPA | =CD36+ MPs | Cardioprotective effects of DHA and EPA do not act through CD36+ MP mechanism | [82] |
| Pirro, M. | 2016 | 100 | RCT | Patients with subclinical inflammation | 3 months | Nutraceutical combination | ↓ endothelial microparticles (−16%) and hsCRP (−41%) | Improved cholesterol profile (total and LDL cholesterol) | [84] |
| Ammollo, C. T. | 2017 | 30 | Comparative Study | Healthy volunteers | 3 weeks | Grapes | ↓ procoagulant microparticles | ↓ thrombin generation and enhanced plasma fibrinolysis, sustained anticoagulant, and profibrinolytic effects | [75] |
| Eitan, E. | 2017 | 38 | RCT | Patients with prostate cancer awaiting prostatectomy | 1 month | Dietary protein restriction | ↑ levels of leptin receptor in total plasma EVs and L1CAM+ EVs, altered phosphorylation status of IRS1 in L1CAM+ EVs | Improved insulin and leptin sensitivity | [71] |
| Burnley-Hall, N. | 2018 | 20 | RCT | Patients with coronary artery disease on clopidogrel therapy | 16 days | Nitrate supplementation | ↓ circulating platelet-derived extracellular vesicles (CD41+ EVs) | ↑ plasma RSNO levels, ↓ thrombin-receptor mediated platelet aggregation | [81] |
| Weech, M. | 2018 | 190 | RCT | Adults with moderate CVD risk | 16 weeks | Replacement of dietary saturated fat with unsaturated fats | ↓ endothelial microparticles (−47.3% for MUFA, −44.9% for n-6 PUFA) and platelet microparticles (−36.8% for MUFA, −39.1% for n-6 PUFA) | ↑ endothelial progenitor cell numbers (+28.4% for MUFA) | [72] |
| Yang, J. | 2019 | 21 | Clinical Trial | Healthy Korean adults | 4 weeks | Leuconostoc holzapfelii-enriched synbiotic beverage | Significant ↑ in species diversity of circulating urinary EVs | Lowered AST serum levels, particularly in subjects with starting levels > 40 UI/L | [79] |
| Bryl-Górecka, P. | 2020 | 50 | Open-Label Study | Patients with myocardial infarction | 8 weeks | Bilberry | ↓ platelet-derived microvesicles (PMVs) and endothelial-derived microvesicles (EMVs) | ↓ endothelial EV release, Akt phosphorylation, and vesiculation-related gene transcription | [78] |
| Chiva-Blanch, G. | 2020 | 155 | RCT | High CVD individuals | 1 year | MD | ↓ prothrombotic microvesicle release compared to low-fat diet | Lower cell activation towards a pro-atherothrombotic phenotype, suggesting delayed CV complications | [69] |
| Gröne, M. | 2020 | 39 | Clinical Trial | Healthy young and elderly subjects | 2 weeks | Cocoa flavanols | ↓ concentrations of CD31+/41−, CD144+, and CD62e+ EMPs | Improved FMD and vascular function | [74] |
| Kwon, Y-J. | 2020 | 16 | Clinical | Breast cancer survivors | 8 weeks | MD | 42 EV miRNAs significantly differentially regulated (36 up-regulated, 6 down-regulated) | Improved BMI, waist circumference, fasting glucose, insulin, and HOMA-IR | [70] |
| Chiva-Blanch, G. | 2021 | 156 | RCT | Elderly subjects post-myocardial infarction | 1 year | ω-3 PUFA | No significant modulation of prothrombotic microvesicle release from blood and vascular cells | ↑ levels of various microvesicle subtypes in both ω 3 and placebo groups | [83] |
| Kim, M-J. | 2021 | 28 | RCT | Patients with acne vulgaris | 12 weeks | Lactobacillus plantarum CJLP55 | ↓ prevalence of Proteobacteria and ↑ Firmicutes in urine bacterial EVs | Improved acne lesion count and grade, ↓ sebum triglycerides, ↑ skin hydration, and ceramide 2 | [87] |
| López de Las Hazas, M.-C. | 2021 | 211 | RCT | Elderly subjects | 1 year | Walnuts | Induced exosomal miRNAs (hsa-miR-32-5p and hsa-miR-29b-3p) | No major changes in exosomal lipids, nanoparticle concentration, or size | [76] |
| Shin, C. M. | 2021 | 112 | RCT | Patients with lower gastrointestinal symptoms | 8 weeks | ID-JPL934 | Significant ↑ in Lactobacillus johnsonii and Bifidobacterium lactis in feces post-treatment | Higher relief of overall gastrointestinal symptoms, ↓ abdominal pain, and bloating scores | [88] |
| Nederveen, J. P. | 2023 | 55 | RCT | Overweight and obese individuals | 12 weeks | Multi-ingredient supplement | Significant ↓ in EVs-associated miRNA species miR-122 and miR-34a | Improved weight, fat mass, liver health, and metabolism | [89] |
| Bozbas, E. | 2024 | 40 | RCT | Individuals with moderate CVD risk | 12 weeks | ω-3 PUFA | ↓ numbers of circulating EVs, doubled n-3 PUFA content in EVs, ↓ EV capacity to support thrombin generation by >20% | =thrombus formation in ex vivo assay | [29] |
6.5. Exercise-Induced Changes in EVs
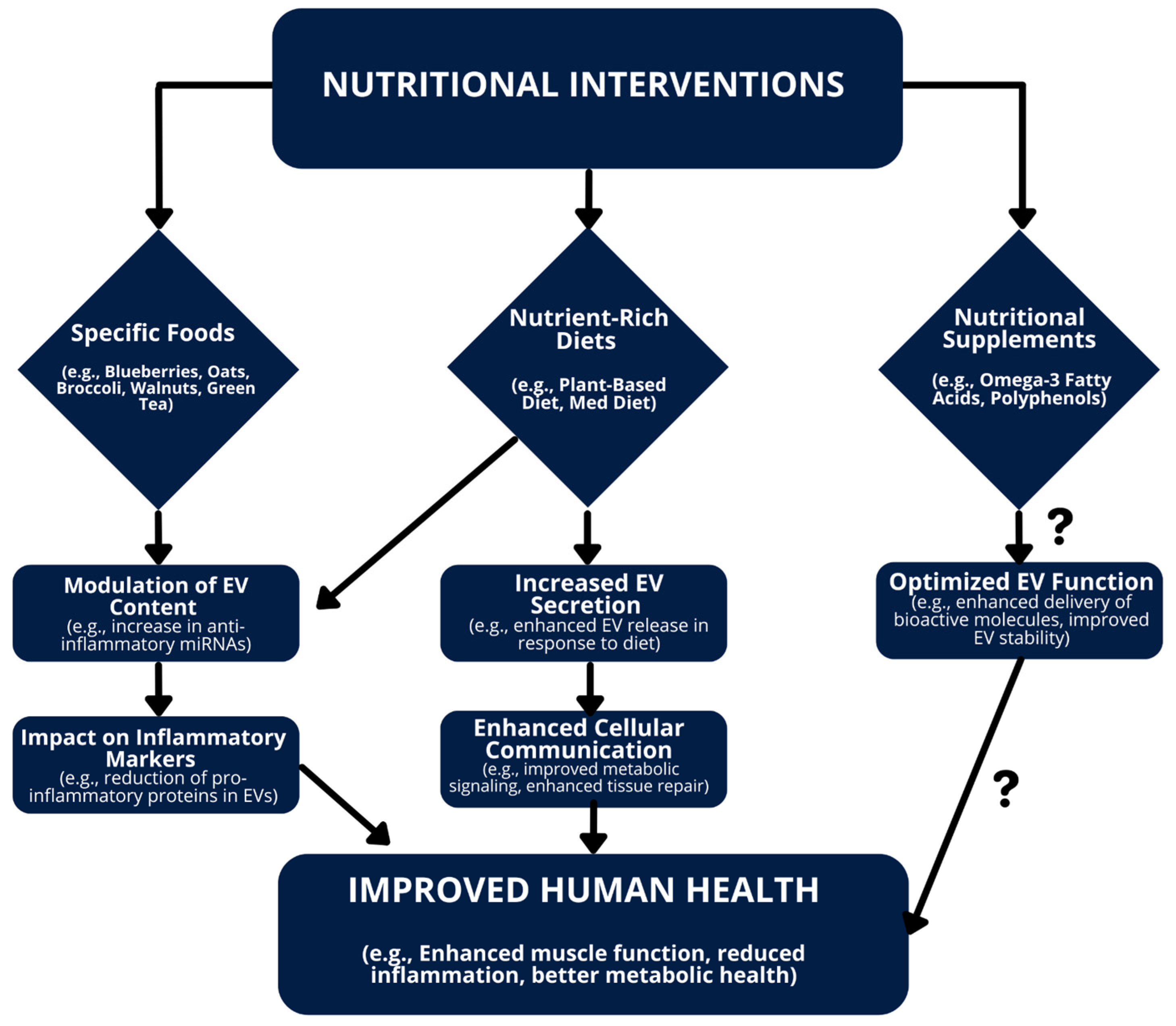
6.6. Modulating EVs for Health Benefits
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Neven, K.Y.; Nawrot, T.S.; Bollati, V. Extracellular Vesicles: How the External and Internal Environment Can Shape Cell-To-Cell Communication. Curr. Environ. Health Rep. 2017, 4, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Olejarz, W.; Sadowski, K.; Radoszkiewicz, K. Extracellular Vesicles in Atherosclerosis: State of the Art. Int. J. Mol. Sci. 2023, 25, 388. [Google Scholar] [CrossRef]
- He, Y.; Wu, Q. The Effect of Extracellular Vesicles on Thrombosis. J. Cardiovasc. Transl. Res. 2023, 16, 682–697. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Liu, S.; Guo, J.; Lu, Y.; Cheng, J.; Liu, J. Macrophage-derived extracellular vesicles: Diverse mediators of pathology and therapeutics in multiple diseases. Cell Death Dis. 2020, 11, 924. [Google Scholar] [CrossRef]
- de Freitas, R.C.; Hirata, R.D.; Hirata, M.H.; Aikawa, E. Circulating Extracellular Vesicles As Biomarkers and Drug Delivery Vehicles in Cardiovascular Diseases. Biomolecules 2021, 11, 388. [Google Scholar] [CrossRef]
- Sheehan, C.; D’Souza-Schorey, C. Tumor-derived extracellular vesicles: Molecular parcels that enable regulation of the immune response in cancer. J. Cell Sci. 2019, 132, jcs235085. [Google Scholar] [CrossRef]
- Mathew, M.; Zade, M.; Mezghani, N.; Patel, R.; Wang, Y.; Momen-Heravi, F. Extracellular Vesicles as Biomarkers in Cancer Immunotherapy. Cancers 2020, 12, 2825. [Google Scholar] [CrossRef]
- Qiu, L.; Liu, X.; Zhu, L.; Luo, L.; Sun, N.; Pei, R. Current Advances in Technologies for Single Extracellular Vesicle Analysis and Its Clinical Applications in Cancer Diagnosis. Biosensors 2023, 13, 129. [Google Scholar] [CrossRef]
- Grieco, G.E.; Fignani, D.; Formichi, C.; Nigi, L.; Licata, G.; Maccora, C.; Brusco, N.; Sebastiani, G.; Dotta, F. Extracellular Vesicles in Immune System Regulation and Type 1 Diabetes: Cell-to-Cell Communication Mediators, Disease Biomarkers, and Promising Therapeutic Tools. Front. Immunol. 2021, 12, 682948. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, X.; Zhang, F.-L.; Jin, H.; Yan, X.-L.; Huang, S.; Guo, Z.-N.; Yang, Y. Clinical Potential of Extracellular Vesicles in Type 2 Diabetes. Front. Endocrinol. 2020, 11, 596811. [Google Scholar] [CrossRef] [PubMed]
- Aloi, N.; Drago, G.; Ruggieri, S.; Cibella, F.; Colombo, P.; Longo, V. Extracellular Vesicles and Immunity: At the Crossroads of Cell Communication. Int. J. Mol. Sci. 2024, 25, 1205. [Google Scholar] [CrossRef]
- Buzas, E.I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 2023, 23, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular vesicles in cancer: Exosomes, microvesicles and the emerging role of large oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Chiaradia, E.; Tancini, B.; Emiliani, C.; Delo, F.; Pellegrino, R.M.; Tognoloni, A.; Urbanelli, L.; Buratta, S. Extracellular Vesicles under Oxidative Stress Conditions: Biological Properties and Physiological Roles. Cells 2021, 10, 1763. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, S.; Wu, X.; Chandra, S.; Lyon, C.; Ning, B.; Jiang, L.; Fan, J.; Hu, T.Y. Extracellular vesicles: Emerging tools as therapeutic agent carriers. Acta Pharm. Sin. B 2022, 12, 3822–3842. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Plomgaard, P.; Halban, P.A.; Bouzakri, K. Bimodal impact of skeletal muscle on pancreatic beta-cell function in health and disease. Diabetes Obes. Metab. 2012, 14 (Suppl. 3), 78–84. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Zhang, Z.-K.; Liang, C.; Li, J.; Liu, J.; Lu, A.; Zhang, B.-T.; Zhang, G. Molecular Communication from Skeletal Muscle to Bone: A Review for Muscle-Derived Myokines Regulating Bone Metabolism. Calcif. Tissue Int. 2017, 100, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jun, H.-S. Role of Myokines in Regulating Skeletal Muscle Mass and Function. Front. Physiol. 2019, 10, 42. [Google Scholar] [CrossRef]
- Guescini, M.; Maggio, S.; Ceccaroli, P.; Battistelli, M.; Annibalini, G.; Piccoli, G.; Sestili, P.; Stocchi, V. Extracellular Vesicles Released by Oxidatively Injured or Intact C2C12 Myotubes Promote Distinct Responses Converging toward Myogenesis. Int. J. Mol. Sci. 2017, 18, 2488. [Google Scholar] [CrossRef]
- Annibalini, G.; Contarelli, S.; Lucertini, F.; Guescini, M.; Maggio, S.; Ceccaroli, P.; Gervasi, M.; Ferri Marini, C.; Fardetti, F.; Grassi, E.; et al. Muscle and Systemic Molecular Responses to a Single Flywheel Based Iso-Inertial Training Session in Resistance-Trained Men. Front. Physiol. 2019, 10, 554. [Google Scholar] [CrossRef]
- Whitham, M.; Parker, B.L.; Friedrichsen, M.; Hingst, J.R.; Hjorth, M.; Hughes, W.E.; Egan, C.L.; Cron, L.; Watt, K.I.; Kuchel, R.P.; et al. Extracellular Vesicles Provide a Means for Tissue Crosstalk during Exercise. Cell Metab. 2018, 27, 237–251.e4. [Google Scholar] [CrossRef]
- Choi, H.; Lee, D.S. Illuminating the physiology of extracellular vesicles. Stem Cell Res. Ther. 2016, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Takafuji, Y.; Kawao, N.; Ohira, T.; Mizukami, Y.; Okada, K.; Jo, J.I.; Tabata, Y.; Kaji, H. Extracellular vesicles secreted from mouse muscle cells improve delayed bone repair in diabetic mice. Endocr. J. 2023, 70, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Takafuji, Y.; Tatsumi, K.; Ishida, M.; Kawao, N.; Okada, K.; Kaji, H. Extracellular vesicles secreted from mouse muscle cells suppress osteoclast formation: Roles of mitochondrial energy metabolism. Bone 2020, 134, 115298. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Jin, X.; Chen, S.; Yang, N.; Feng, G. Plant-derived extracellular vesicles -a novel clinical anti-inflammatory drug carrier worthy of investigation. Biomed. Pharmacother. 2023, 169, 115904. [Google Scholar] [CrossRef] [PubMed]
- Mukund, K.; Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1462. [Google Scholar] [CrossRef]
- Schnyder, S.; Handschin, C. Skeletal muscle as an endocrine organ: PGC-1 alpha, myokines and exercise. Bone 2015, 80, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.L.; Steinberg, G.R.; Macaulay, S.L.; Thomas, W.G.; Holmes, A.G.; Ramm, G.; Prelovsek, O.; Hohnen-Behrens, C.; Watt, M.J.; James, D.E.; et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 2006, 55, 2688–2697. [Google Scholar] [CrossRef]
- Bozbas, E.; Zhou, R.; Soyama, S.; Allen-Redpath, K.; Mitchell, J.L.; Fisk, H.L.; Calder, P.C.; Jones, C.; Gibbins, J.M.; Fischer, R.; et al. Dietary n-3 polyunsaturated fatty acids alter the number, fatty acid profile and coagulatory activity of circulating and platelet-derived extracellular vesicles: A randomized, controlled crossover trial. Am. J. Clin. Nutr. 2024, 119, 1175–1186. [Google Scholar] [CrossRef]
- Liu, D.S.K.; Yang, Q.Z.C.; Asim, M.; Krell, J.; Frampton, A.E. The Clinical Significance of Transfer RNAs Present in Extracellular Vesicles. Int. J. Mol. Sci. 2022, 23, 3692. [Google Scholar] [CrossRef]
- Vechetti, I.J.; Peck, B.D.; Wen, Y.; Walton, R.G.; Valentino, T.R.; Alimov, A.P.; Dungan, C.M.; Van Pelt, D.W.; von Walden, F.; Alkner, B.; et al. Mechanical overload-induced muscle-derived extracellular vesicles promote adipose tissue lipolysis. FASEB J. 2021, 35, e21644. [Google Scholar] [CrossRef]
- Aswad, H.; Forterre, A.; Wiklander, O.P.B.; Vial, G.; Danty-Berger, E.; Jalabert, A.; Lamazière, A.; Meugnier, E.; Pesenti, S.; Ott, C.; et al. Exosomes participate in the alteration of muscle homeostasis during lipid-induced insulin resistance in mice. Diabetologia 2014, 57, 2155–2164. [Google Scholar] [CrossRef]
- Lischnig, A.; Bergqvist, M.; Ochiya, T.; Lässer, C. Quantitative Proteomics Identifies Proteins Enriched in Large and Small Extracellular Vesicles. Mol. Cell Proteom. 2022, 21, 100273. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Sudo, Y.; Makino, T.; Kimura, S.; Tomita, K.; Noguchi, M.; Sakurai, H.; Shimizu, M.; Takahashi, Y.; Sato, R.; et al. Skeletal muscle releases extracellular vesicles with distinct protein and microRNA signatures that function in the muscle microenvironment. Proc. Natl. Acad. Sci. USA Nexus 2022, 1, pgac173. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Kim, D.; Kim, Y.; Gho, Y.S. Proteomics of extracellular vesicles: Exosomes and ectosomes. Mass. Spectrom. Rev. 2015, 34, 474–490. [Google Scholar] [CrossRef] [PubMed]
- Phang, M.; Lincz, L.; Seldon, M.; Garg, M.L. Acute supplementation with eicosapentaenoic acid reduces platelet microparticle activity in healthy subjects. J. Nutr. Biochem. 2012, 23, 1128–1133. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaka, Y.; Tanihata, J.; Komaki, H.; Ishiyama, A.; Oya, Y.; Rüegg, U.; Takeda, S.-I.; Hashido, K. Characterization and Functional Analysis of Extracellular Vesicles and Muscle-Abundant miRNAs (miR-1, miR-133a, and miR-206) in C2C12 Myocytes and mdx Mice. PLoS ONE 2016, 11, e0167811. [Google Scholar] [CrossRef]
- Chal, J.; Pourquie, O. Making muscle: Skeletal myogenesis in vivo and in vitro. Development 2017, 144, 2104–2122. [Google Scholar] [CrossRef]
- Mytidou, C.; Koutsoulidou, A.; Katsioloudi, A.; Prokopi, M.; Kapnisis, K.; Michailidou, K.; Anayiotos, A.; Phylactou, L.A. Muscle-derived exosomes encapsulate myomiRs and are involved in local skeletal muscle tissue communication. FASEB J. 2021, 35, e21279. [Google Scholar] [CrossRef]
- Chen, J.-F.; Mandel, E.M.; Thomson, J.M.; Wu, Q.; E Callis, T.; Hammond, S.M.; Conlon, F.L.; Wang, D.-Z. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 2006, 38, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wang, Y.M.; Zhang, W.R.; Liu, X.F.; Li, X.; Bin Ding, X.; Guo, H. The role of microRNA-1 and microRNA-206 in the proliferation and differentiation of bovine skeletal muscle satellite cells. Vitr. Cell. Dev. Biol.-Anim. 2016, 52, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Goljanek-Whysall, K.; Pais, H.; Rathjen, T.; Sweetman, D.; Dalmay, T.; Münsterberg, A. Regulation of multiple target genes by miR-1 and miR-206 is pivotal for C2C12 myoblast differentiation. J. Cell Sci. 2012, 125, 3590–3600. [Google Scholar] [CrossRef]
- Feng, Y.; Niu, L.L.; Wei, W.; Zhang, W.Y.; Li, X.Y.; Cao, J.H.; Zhao, S.H. A feedback circuit between miR-133 and the ERK1/2 pathway involving an exquisite mechanism for regulating myoblast proliferation and differentiation. Cell Death Dis. 2013, 4, e934. [Google Scholar] [CrossRef]
- Guescini, M.; Guidolin, D.; Vallorani, L.; Casadei, L.; Gioacchini, A.M.; Tibollo, P.; Battistelli, M.; Falcieri, E.; Battistin, L.; Agnati, L.F.; et al. C2C12 myoblasts release micro-vesicles containing mtDNA and proteins involved in signal transduction. Exp. Cell Res. 2010, 316, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.-R.; Lee, S.; Song, S.-K. A Review of Sarcopenia Pathophysiology, Diagnosis, Treatment and Future Direction. J. Korean Med. Sci. 2022, 37, e146. [Google Scholar] [CrossRef]
- Bian, A.-L.; Hu, H.-Y.; Rong, Y.-D.; Wang, J.; Wang, J.-X.; Zhou, X.-Z. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-alpha. Eur. J. Med. Res. 2017, 22, 25. [Google Scholar] [CrossRef]
- Kapetanovic, R.; Bokil, N.J.; Sweet, M.J. Innate immune perturbations, accumulating DAMPs and inflammasome dysregulation: A ticking time bomb in ageing. Ageing Res. Rev. 2015, 24, 40–53. [Google Scholar] [CrossRef]
- Shao, X.; Gong, W.; Wang, Q.; Wang, P.; Shi, T.; Mahmut, A.; Qin, J.; Yao, Y.; Yan, W.; Chen, D.; et al. Atrophic skeletal muscle fibre-derived small extracellular vesicle miR-690 inhibits satellite cell differentiation during ageing. J. Cachexia Sarcopenia Muscle 2022, 13, 3163–3180. [Google Scholar] [CrossRef]
- Mokalled, M.H.; Johnson, A.N.; Creemers, E.E.; Olson, E.N. MASTR directs MyoD-dependent satellite cell differentiation during skeletal muscle regeneration. Genes Dev. 2012, 26, 190–202. [Google Scholar] [CrossRef]
- Chen, J.-F.; Tao, Y.; Li, J.; Deng, Z.; Yan, Z.; Xiao, X.; Wang, D.-Z. microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7. J. Cell Biol. 2010, 190, 867–879. [Google Scholar] [CrossRef]
- Nakasa, T.; Ishikawa, M.; Shi, M.; Shibuya, H.; Adachi, N.; Ochi, M. Acceleration of muscle regeneration by local injection of muscle-specific microRNAs in rat skeletal muscle injury model. J. Cell. Mol. Med. 2010, 14, 2495–2505. [Google Scholar] [CrossRef]
- Drummond, M.J.; McCarthy, J.J.; Sinha, M.; Spratt, H.M.; Volpi, E.; Esser, K.A.; Rasmussen, B.B. Aging and microRNA expression in human skeletal muscle: A microarray and bioinformatics analysis. Physiol. Genom. 2011, 43, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Lezza, A.M.S.; Leeuwenburgh, C.; Pesce, V.; Calvani, R.; Bossola, M.; Manes-Gravina, E.; Landi, F.; Bernabei, R.; Marzetti, E. Circulating Mitochondrial DNA at the Crossroads of Mitochondrial Dysfunction and Inflammation during Aging and Muscle Wasting Disorders. Rejuvenation Res. 2018, 21, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Byappanahalli, A.M.; Noren Hooten, N.; Vannoy, M.; Mode, N.A.; Ezike, N.; Zonderman, A.B.; Evans, M.K. Mitochondrial DNA and inflammatory proteins are higher in extracellular vesicles from frail individuals. Immun. Ageing 2023, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, A.L.; Lessard, S.J.; Ouchida, A.T.; Araujo, H.N.; Gonçalves, D.A.; Guimarães, D.S.; Teodoro, B.G.; So, K.; Espreafico, E.M.; Hirshman, M.F.; et al. The MicroRNA miR-696 is regulated by SNARK and reduces mitochondrial activity in mouse skeletal muscle through Pgc1 alpha inhibition. Mol. Metab. 2021, 51, 101226. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Naito, Y.; Mizushima, K.; Takanami, Y.; Kawai, Y.; Ichikawa, H.; Yoshikawa, T. The microRNA miR-696 regulates PGC-1 alpha in mouse skeletal muscle in response to physical activity. Am. J. Physiol.-Endocrinol. Metab. 2010, 298, E799–E806. [Google Scholar] [CrossRef]
- Nie, Y.; Sato, Y.; Wang, C.; Yue, F.; Kuang, S.; Gavin, T.P. Impaired exercise tolerance, mitochondrial biogenesis, and muscle fiber maintenance in miR-133a-deficient mice. FASEB J. 2016, 30, 3745–3758. [Google Scholar] [CrossRef]
- Sligar, J.; DeBruin, D.A.; Saner, N.J.; Philp, A.M.; Philp, A. The importance of mitochondrial quality control for maintaining skeletal muscle function across health span. Am. J. Physiol.-Cell Physiol. 2022, 322, C461–C467. [Google Scholar] [CrossRef]
- Liu, D.; Fan, Y.-B.; Tao, X.-H.; Pan, W.-L.; Wu, Y.-X.; Wang, X.-H.; He, Y.-Q.; Xiao, W.-F.; Li, Y.-S. Mitochondrial Quality Control in Sarcopenia: Updated Overview of Mechanisms and Interventions. Aging Dis. 2021, 12, 2016–2030. [Google Scholar] [CrossRef]
- Soriano-Arroquia, A.; House, L.; Tregilgas, L.; Canty-Laird, E.; Goljanek-Whysall, K. The functional consequences of age-related changes in microRNA expression in skeletal muscle. Biogerontology 2016, 17, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Lee, K.-P.; Milholland, B.; Shin, Y.J.; Kang, J.S.; Kwon, K.-S.; Suh, Y. Comprehensive miRNA Profiling of Skeletal Muscle and Serum in Induced and Normal Mouse Muscle Atrophy during Aging. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2017, 72, 1483–1491. [Google Scholar] [CrossRef]
- Silva, W.J.; Graça, F.A.; Cruz, A.; Silvestre, J.G.; Labeit, S.; Miyabara, E.H.; Yan, C.Y.I.; Wang, D.Z.; Moriscot, A.S. miR-29c improves skeletal muscle mass and function throughout myocyte proliferation and differentiation and by repressing atrophy-related genes. Acta Physiol. 2019, 226, e13278. [Google Scholar] [CrossRef]
- Xu, J.; Li, R.; Workeneh, B.; Dong, Y.; Wang, X.; Hu, Z. Transcription factor FoxO1, the dominant mediator of muscle wasting in chronic kidney disease, is inhibited by microRNA-486. Kidney Int. 2012, 82, 401–411. [Google Scholar] [CrossRef]
- Ge, Y.; Sun, Y.; Chen, J. IGF-II is regulated by microRNA-125b in skeletal myogenesis. J. Cell Biol. 2011, 192, 69–81. [Google Scholar] [CrossRef]
- Li, G.; Luo, W.; A Abdalla, B.; Ouyang, H.; Yu, J.; Hu, F.; Nie, Q.; Zhang, X. miRNA-223 upregulated by MYOD inhibits myoblast proliferation by repressing IGF2 and facilitates myoblast differentiation by inhibiting ZEB1. Cell Death Dis. 2017, 8, e3094. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Liang, R.; Yang, Y.; Hou, X.; Wang, Z.; Zhu, S.; Wang, C.; Tang, Z.; Li, K. MicroRNA-21 Regulates PI3K/Akt/mTOR Signaling by Targeting TGF beta I during Muscle Development in Pigs. PLoS ONE 2015, 10, e0119396. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Crespo, J.; Suades, R.; Arderiu, G.; Padro, T.; Vilahur, G.; Cubedo, J.; Corella, D.; Salas-Salvadó, J.; Arós, F.; et al. CD142+/CD61+, CD146+ and CD45+ microparticles predict cardiovascular events in high risk patients following a Mediterranean diet supplemented with nuts. Thromb. Haemost. 2016, 116, 103–114. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Sala-Vila, A.; Crespo, J.; Ros, E.; Estruch, R.; Badimon, L. The Mediterranean diet decreases prothrombotic microvesicle release in asymptomatic individuals at high cardiovascular risk. Clin. Nutr. 2020, 39, 3377–3384. [Google Scholar] [CrossRef]
- Kwon, Y.-J.; Cho, Y.-E.; Cho, A.-R.; Choi, W.J.; Yun, S.; Park, H.; Kim, H.-S.; Cashion, A.K.; Gill, J.; Lee, H.; et al. The Possible Influence of Mediterranean Diet on Extracellular Vesicle miRNA Expression in Breast Cancer Survivors. Cancers 2020, 12, 1355. [Google Scholar] [CrossRef]
- Eitan, E.; Tosti, V.; Suire, C.N.; Cava, E.; Berkowitz, S.; Bertozzi, B.; Raefsky, S.M.; Veronese, N.; Spangler, R.; Spelta, F.; et al. In a randomized trial in prostate cancer patients, dietary protein restriction modifies markers of leptin and insulin signaling in plasma extracellular vesicles. Aging Cell 2017, 16, 1430–1433. [Google Scholar] [CrossRef]
- Weech, M.; Altowaijri, H.; Mayneris-Perxachs, J.; Vafeiadou, K.; Madden, J.; Todd, S.; Jackson, K.G.; Lovegrove, J.A.; Yaqoob, P. Replacement of dietary saturated fat with unsaturated fats increases numbers of circulating endothelial progenitor cells and decreases numbers of microparticles: Findings from the randomized, controlled Dietary Intervention and VAScular function (DIVAS) study. Am. J. Clin. Nutr. 2018, 107, 876–882. [Google Scholar] [PubMed]
- Zhang, X.; McGeoch, S.C.; Megson, I.L.; MacRury, S.M.; Johnstone, A.M.; Abraham, P.; Pearson, D.W.M.; Roos, B.; Holtrop, G.; O’Kennedy, N.; et al. Oat-enriched diet reduces inflammatory status assessed by circulating cell-derived microparticle concentrations in type 2 diabetes. Mol. Nutr. Food Res. 2014, 58, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Gröne, M.; Sansone, R.; Höffken, P.; Horn, P.; Rodriguez-Mateos, A.; Schroeter, H.; Kelm, M.; Heiss, C. Cocoa Flavanols Improve Endothelial Functional Integrity in Healthy Young and Elderly Subjects. J. Agric. Food Chem. 2020, 68, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Ammollo, C.T.; Semeraro, F.; Milella, R.A.; Antonacci, D.; Semeraro, N.; Colucci, M. Grape intake reduces thrombin generation and enhances plasma fibrinolysis. Potential role of circulating procoagulant microparticles. J. Nutr. Biochem. 2017, 50, 66–73. [Google Scholar]
- López de Las Hazas, M.-C.; Gil-Zamorano, J.; Cofán, M.; Mantilla-Escalante, D.C.; Garcia-Ruiz, A.; del Pozo-Acebo, L.; Pastor, O.; Yañez-Mo, M.; Mazzeo, C.; Serra-Mir, M.; et al. One-year dietary supplementation with walnuts modifies exosomal miRNA in elderly subjects. Eur. J. Nutr. 2021, 60, 1999–2011. [Google Scholar] [CrossRef]
- Horn, P.; Amabile, N.; Angeli, F.S.; Sansone, R.; Stegemann, B.; Kelm, M.; Springer, M.L.; Yeghiazarians, Y.; Schroeter, H.; Heiss, C. Dietary flavanol intervention lowers the levels of endothelial microparticles in coronary artery disease patients. Br. J. Nutr. 2014, 111, 1245–1252. [Google Scholar] [CrossRef]
- Bryl-Górecka, P.; Sathanoori, R.; Arevström, L.; Landberg, R.; Bergh, C.; Evander, M.; Olde, B.; Laurell, T.; Fröbert, O.; Erlinge, D. Bilberry Supplementation after Myocardial Infarction Decreases Microvesicles in Blood and Affects Endothelial Vesiculation. Mol. Nutr. Food Res. 2020, 64, e2000108. [Google Scholar] [CrossRef]
- Yang, J.; McDowell, A.; Kim, E.K.; Seo, H.; Yum, K.; Lee, W.H.; Jee, Y.-K.; Kim, Y.-K. Consumption of a Leuconostoc holzapfelii-enriched synbiotic beverage alters the composition of the microbiota and microbial extracellular vesicles. Exp. Mol. Med. 2019, 51, 1–11. [Google Scholar] [CrossRef]
- Wu, S.Y.; Mayneris-Perxachs, J.; Lovegrove, J.A.; Todd, S.; Yaqoob, P. Fish-oil supplementation alters numbers of circulating endothelial progenitor cells and microparticles independently of eNOS genotype. Am. J. Clin. Nutr. 2014, 100, 1232–1243. [Google Scholar] [CrossRef]
- Burnley-Hall, N.; Abdul, F.; Androshchuk, V.; Morris, K.; Ossei-Gerning, N.; Anderson, R.; Rees, D.A.; James, P.E. Dietary Nitrate Supplementation Reduces Circulating Platelet-Derived Extracellular Vesicles in Coronary Artery Disease Patients on Clopidogrel Therapy: A Randomised, Double-Blind, Placebo-Controlled Study. Thromb. Haemost. 2018, 118, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Phang, M.; Thorne, R.F.; Alkhatatbeh, M.J.; Garg, M.L.; Lincz, L.F. Circulating CD36+ microparticles are not altered by docosahexaenoic or eicosapentaenoic acid supplementation. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Bratseth, V.; Laake, K.; Arnesen, H.; Solheim, S.; Schmidt, E.B.; Badimon, L.; Seljeflot, I. One year of omega 3 polyunsaturated fatty acid supplementation does not reduce circulating prothrombotic microvesicles in elderly subjects after suffering a myocardial infarction. Clin. Nutr. 2021, 40, 5674–5677. [Google Scholar] [CrossRef]
- Pirro, M.; Mannarino, M.R.; Ministrini, S.; Fallarino, F.; Lupattelli, G.; Bianconi, V.; Bagaglia, F.; Mannarino, E. Effects of a nutraceutical combination on lipids, inflammation and endothelial integrity in patients with subclinical inflammation: A randomized clinical trial. Sci. Rep. 2016, 6, 23587. [Google Scholar] [CrossRef]
- Weisshaar, S.; Gouya, G.; Nguyen, D.; Kapiotis, S.; Wolzt, M. The LPS-induced increase in circulating microparticles is not affected by vitamin C in humans. Eur. J. Clin. Investig. 2013, 43, 708–715. [Google Scholar] [CrossRef]
- Yang, M.; Barak, O.F.; Dujic, Z.; Madden, D.; Bhopale, V.M.; Bhullar, J.; Thom, S.R.; Brugniaux, J.V.; Coombs, G.B.; Sekhon, M.S.; et al. Ascorbic acid supplementation diminishes microparticle elevations and neutrophil activation following SCUBA diving. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R338–R344. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, K.P.; Choi, E.; Yim, J.H.; Choi, C.; Yun, H.S.; Ahn, H.Y.; Oh, J.Y.; Cho, Y. Effects of Lactobacillus plantarum CJLP55 on Clinical Improvement, Skin Condition and Urine Bacterial Extracellular Vesicles in Patients with Acne Vulgaris: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2021, 13, 1368. [Google Scholar] [CrossRef]
- Shin, C.M.; Choi, Y.J.; Lee, D.H.; Moon, J.S.; Kim, T.-Y.; Kim, Y.-K.; Lee, W.-H.; Yoon, H.; Park, Y.S.; Kim, N. Validity and safety of ID-JPL934 in lower gastrointestinal symptom improvement. Sci. Rep. 2021, 11, 13046. [Google Scholar] [CrossRef] [PubMed]
- Nederveen, J.P.; Mastrolonardo, A.J.; Xhuti, D.; Di Carlo, A.; Manta, K.; Fuda, M.R.; Tarnopolsky, M.A. Novel Multi-Ingredient Supplement Facilitates Weight Loss and Improves Body Composition in Overweight and Obese Individuals: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2023, 15, 3693. [Google Scholar] [CrossRef]
- Anderson, E.; Durstine, J.L. Physical activity, exercise, and chronic diseases: A brief review. Sports Med. Health Sci. 2019, 1, 3–10. [Google Scholar] [CrossRef]
- Graham, Z.A.; Lavin, K.M.; O’bryan, S.M.; Thalacker-Mercer, A.E.; Buford, T.W.; Ford, K.M.; Broderick, T.J.; Bamman, M.M. Mechanisms of exercise as a preventative measure to muscle wasting. Am. J. Physiol. Cell Physiol. 2021, 321, C40–C57. [Google Scholar] [CrossRef] [PubMed]
- Guescini, M.; Canonico, B.; Lucertini, F.; Maggio, S.; Annibalini, G.; Barbieri, E.; Luchetti, F.; Papa, S.; Stocchi, V. Muscle Releases Alpha-Sarcoglycan Positive Extracellular Vesicles Carrying miRNAs in the Bloodstream. PLoS ONE 2015, 10, e0125094. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.D.; Ge, Y.; Li, S.; Pincas, H.; Jain, N.; Seenarine, N.; Amper, M.A.S.; Goodpaster, B.H.; Walsh, M.J.; Coen, P.M.; et al. Sedentary and Trained Older Men Have Distinct Circulating Exosomal microRNA Profiles at Baseline and in Response to Acute Exercise. Front. Physiol. 2020, 11, 605. [Google Scholar] [CrossRef] [PubMed]
- Uhlemann, M.; Möbius-Winkler, S.; Fikenzer, S.; Adam, J.; Redlich, M.; Möhlenkamp, S.; Hilberg, T.; Schuler, G.C.; Adams, V. Circulating microRNA-126 increases after different forms of endurance exercise in healthy adults. Eur. J. Prev. Cardiol. 2014, 21, 484–491. [Google Scholar] [CrossRef]
- Kargl, C.K.; Sterczala, A.J.; Santucci, D.; Conkright, W.R.; Krajewski, K.T.; Martin, B.J.; Greeves, J.P.; O’Leary, T.J.; Wardle, S.L.; Sahu, A.; et al. Circulating extracellular vesicle characteristics differ between men and women following 12 weeks of concurrent exercise training. Physiol. Rep. 2024, 12, e16016. [Google Scholar] [CrossRef]
- Frühbeis, C.; Helmig, S.; Tug, S.; Simon, P.; Krämer-Albers, E. Physical exercise induces rapid release of small extracellular vesicles into the circulation. J. Extracell. Vesicles 2015, 4, 28239. [Google Scholar] [CrossRef]
- McIlvenna, L.C.; Whitham, M. Exercise, healthy ageing, and the potential role of small extracellular vesicles. J. Physiol. 2023, 601, 4937–4951. [Google Scholar] [CrossRef]
- Nie, Y.; Sato, Y.; Garner, R.T.; Kargl, C.; Wang, C.; Kuang, S.; Gilpin, C.J.; Gavin, T.P. Skeletal muscle-derived exosomes regulate endothelial cell functions via reactive oxygen species-activated nuclear factor-kappaB signalling. Exp. Physiol. 2019, 104, 1262–1273. [Google Scholar] [CrossRef]
- Catitti, G.; De Bellis, D.; Vespa, S.; Simeone, P.; Canonico, B.; Lanuti, P. Extracellular Vesicles as Players in the Anti-Inflammatory Inter-Cellular Crosstalk Induced by Exercise Training. Int. J. Mol. Sci. 2022, 23, 14098. [Google Scholar] [CrossRef]
- Sullivan, B.P.; Nie, Y.; Evans, S.; Kargl, C.K.; Hettinger, Z.R.; Garner, R.T.; Hubal, M.J.; Kuang, S.; Stout, J.; Gavin, T.P. Obesity and exercise training alter inflammatory pathway skeletal muscle small extracellular vesicle microRNAs. Exp. Physiol. 2022, 107, 462–475. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Jenkins, N.T.; Landers, R.Q.; Thakkar, S.R.; Fan, X.; Brown, M.D.; Prior, S.J.; Spangenburg, E.E.; Hagberg, J.M. Prior endurance exercise prevents postprandial lipaemia-induced increases in reactive oxygen species in circulating CD31+ cells. J. Physiol. 2011, 589 Pt 22, 5539–5553. [Google Scholar] [CrossRef] [PubMed]
- Strohacker, K.; Breslin, W.L.; Carpenter, K.C.; Davidson, T.R.; Agha, N.H.; McFarlin, B.K. Moderate-intensity, premeal cycling blunts postprandial increases in monocyte cell surface CD18 and CD11a and endothelial microparticles following a high-fat meal in young adults. Appl. Physiol. Nutr. Metab. 2012, 37, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Highton, P.J.; Goltz, F.R.; Martin, N.; Stensel, D.J.; Thackray, A.E.; Bishop, N.C. Microparticle Responses to Aerobic Exercise and Meal Consumption in Healthy Men. Med. Sci. Sports Exerc. 2019, 51, 1935–1943. [Google Scholar] [CrossRef] [PubMed]
- Kirk, R.J.; Peart, D.J.; Madden, L.A.; Vince, R.V. Repeated supra-maximal sprint cycling with and without sodium bicarbonate supplementation induces endothelial microparticle release. Eur. J. Sport Sci. 2014, 14, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.D.; Arem, H.; Hubal, M.J.; Cartmel, B.; Li, F.; Harrigan, M.; Sanft, T.; Cheng, C.J.; Pusztai, L.; Irwin, M.L. Exercise and weight loss interventions and miRNA expression in women with breast cancer. Breast Cancer Res. Treat. 2018, 170, 55–67. [Google Scholar] [CrossRef]
- Harrison, M.; Murphy, R.P.; O’connor, P.L.; O’gorman, D.J.; McCaffrey, N.; Cummins, P.M.; Moyna, N.M. The endothelial microparticle response to a high fat meal is not attenuated by prior exercise. Eur. J. Appl. Physiol. 2009, 106, 555–562. [Google Scholar] [CrossRef]
- Nielsen, S.; Åkerström, T.; Rinnov, A.; Yfanti, C.; Scheele, C.; Pedersen, B.K.; Laye, M.J. The miRNA plasma signature in response to acute aerobic exercise and endurance training. PLoS ONE 2014, 9, e87308. [Google Scholar] [CrossRef]
| First Author | Year | Sample Size (n) | Study Design | Population | Duration | Intervention Type | Effects on EV | Other Outcomes | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Harrison, M. | 2009 | 8 | Clinical Trial | Recreationally active young men | Single session | High-fat meals, exercise | EMP ↑ postprandially, no attenuation by prior exercise | ↓ postprandial triglycerides, ↑ HDL-C, no changes in sICAM-1, sVCAM-1, IL-6, or leukocytes | [107] |
| Jenkins, N. T. | 2011 | 10 | Controlled Clinical Trial | Healthy men | Single session | Endurance exercise | Prevented postprandial lipaemia-induced ↑ in ROS in CD31+ cells | ↑ antioxidant gene expression, ↓ intracellular lipid uptake, lower serum triglyceride and oxidized LDL-cholesterol, lower plasma endothelial microparticle concentrations | [102] |
| Strohacker, K. | 2012 | 12 | RCT | Young adults | Single session | Moderate-intensity premeal cycling | ↑ in EMP and CD11a/CD18 monocyte cell surface receptors | Exercise ↓ postprandial monocyte and endothelial cell activation | [103] |
| Kirk, R. J. | 2013 | 7 | RCT | Healthy male volunteers | Single session | Sprint cycling, sodium bicarbonate | ↑ in endothelial CD105+ and CD106+ MPs post-exercise, no effect of supplementation | Endothelium rapidly recovers post-exercise in healthy individuals | [105] |
| Nielsen, S. | 2014 | 32 (13 acute, 7 chronic) | Clinical Trial | Healthy, trained men | 12 weeks + single session | Acute endurance exercise and chronic endurance training | Acute: ci-miRNAs downregulated immediately post-exercise, followed by upregulation at 1 and 3 h. Chronic: 7 ci-miRNAs ↓ and 2 ci-miRNAs ↑ post-training | Identified dynamic plasma miRNA changes in response to both acute exercise and chronic endurance training | [108] |
| Uhlemann, M. | 2014 | 58 (13 + 12 + 22 + 11) | Comparative Study | Healthy adults | Four different exercise protocols | Maximal symptom-limited exercise test, 4-h cycling, marathon, resistance training | ↑ miRNA-126 after endurance exercises; ↑ miRNA-133 after resistance exercise | Different exercise modalities impact endothelial and muscle cells differently; miRNA-126 linked to endothelial damage | [94] |
| Fruhbeis, C. | 2015 | 12 | Clinical Trial | Healthy, physically active men | Single session | Incremental cycling and treadmill running until exhaustion | Significant ↑ in small EVs (100–130 nm) immediately after exercise, declining within 90 min; treadmill-induced EVs sustained longer | EV release initiated early during exercise, before reaching anaerobic threshold; potential role in exercise adaptation | [96] |
| Guescini, M. | 2015 | 22 | Clinical Trial | Sedentary and fit young men | Cross-sectional | Physical exercise | SGCA+ EVs enriched for miR-206; correlation between fitness and muscle-specific miRNAs | EV miR-133b and miR-181a-5p significantly upregulated after acute exercise; role in muscle communication | [92] |
| Adams, B. D. | 2018 | 121 | Clinical Trial | Breast cancer survivors | 6 months | Exercise, weight loss | Identified eight miRNAs associated with BMI and weight loss interventions, including miR-191-5p and miR-122-5p | Correlated miRNAs with biological pathways such as “Estrogen-mediated S-phase entry” and “Molecular mechanisms of cancer” | [106] |
| Whitham, M. | 2018 | 10 | Clinical Trial | Healthy humans | Single session | 1-h cycling exercise | Increase in over 300 EV-contained proteins; localization in the liver | Identified new candidate myokines released into circulation independently of classical secretion | [21] |
| Highton, P. J. | 2019 | 15 | RCT | Healthy men | Single session (x3) | Aerobic exercise, meal consumption | ↓ tissue factor (TF) expression on platelet and neutrophil-derived microparticles (MPs) after exercise | [104] | |
| Nair, V.D. | 2020 | 10 | Clinical Trial | Sedentary and trained older men | Single session (3 points: Pre, Post, 3hPost) | Aerobic exercise (cycle ergometer) | Baseline: ↑ miR-486-5p, ↑ miR-215-5p, ↑ miR-941, ↓ miR-151b. Acute exercise: Distinct exomiRs in trained vs sedentary groups | IGF-1 signaling pathway regulation differs by training status; potential role in counteracting anabolic resistance | [93] |
| Sullivan, B.P. | 2022 | 16 | Clinical Trial | Sedentary lean and obese adults (8 lean, 8 obese) | 7 days | Concurrent aerobic and resistance exercise training | Obesity alters small EV miRNAs targeting inflammatory and growth pathways; exercise training induces anti-inflammatory changes in EVs | ↓ IL-8 and Jun mRNA after training; exercise-induced EV miRNAs target pathways related to inflammation and growth | [100] |
| Kargl, C.K. | 2024 | 18 | Clinical Trial | Healthy, recreationally active men and women (18–36 years) | 12 weeks | Concurrent resistance and endurance exercise training (CET) | AHRET ↑ EV abundance in trained men only; sex-specific differences in miRNA contents | Predicted regulation of hypertrophy and growth pathways in men more than women | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardo, M.; Aiello, G.; Fratantonio, D.; Karav, S.; Baldelli, S. Functional Role of Extracellular Vesicles in Skeletal Muscle Physiology and Sarcopenia: The Importance of Physical Exercise and Nutrition. Nutrients 2024, 16, 3097. https://doi.org/10.3390/nu16183097
Lombardo M, Aiello G, Fratantonio D, Karav S, Baldelli S. Functional Role of Extracellular Vesicles in Skeletal Muscle Physiology and Sarcopenia: The Importance of Physical Exercise and Nutrition. Nutrients. 2024; 16(18):3097. https://doi.org/10.3390/nu16183097
Chicago/Turabian StyleLombardo, Mauro, Gilda Aiello, Deborah Fratantonio, Sercan Karav, and Sara Baldelli. 2024. "Functional Role of Extracellular Vesicles in Skeletal Muscle Physiology and Sarcopenia: The Importance of Physical Exercise and Nutrition" Nutrients 16, no. 18: 3097. https://doi.org/10.3390/nu16183097
APA StyleLombardo, M., Aiello, G., Fratantonio, D., Karav, S., & Baldelli, S. (2024). Functional Role of Extracellular Vesicles in Skeletal Muscle Physiology and Sarcopenia: The Importance of Physical Exercise and Nutrition. Nutrients, 16(18), 3097. https://doi.org/10.3390/nu16183097











