Carotenoids from Different Pumpkin Varieties Exert a Cytotoxic Effect on Human Neuroblastoma SH-SY5Y Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Reagents
2.3. Moisture Content and Color of Pumpkin Flesh
2.4. Isolation of Carotenoids from Pumpkin Pulp and Evaluation of Total Carotenoid Content
2.5. Cell Culture Conditions
Cell Count and Viability: AO/DAPI Double Staining
2.6. In Vitro Antioxidant Activities by Acellular Assays
2.6.1. Free Radical-Scavenging Activity Using ABTS (ABTS Assay)
2.6.2. Oxygen Radical Absorbance Capacity Assay (ORAC Assay)
2.7. HPLC-DAD Analysis of Carotenoids
2.8. LC-HRMS Analysis for Carotenoids Structural Confirmation
2.9. Statistical Analysis
3. Results and Discussion
3.1. General Characteristics of Pumpkin Cultivars
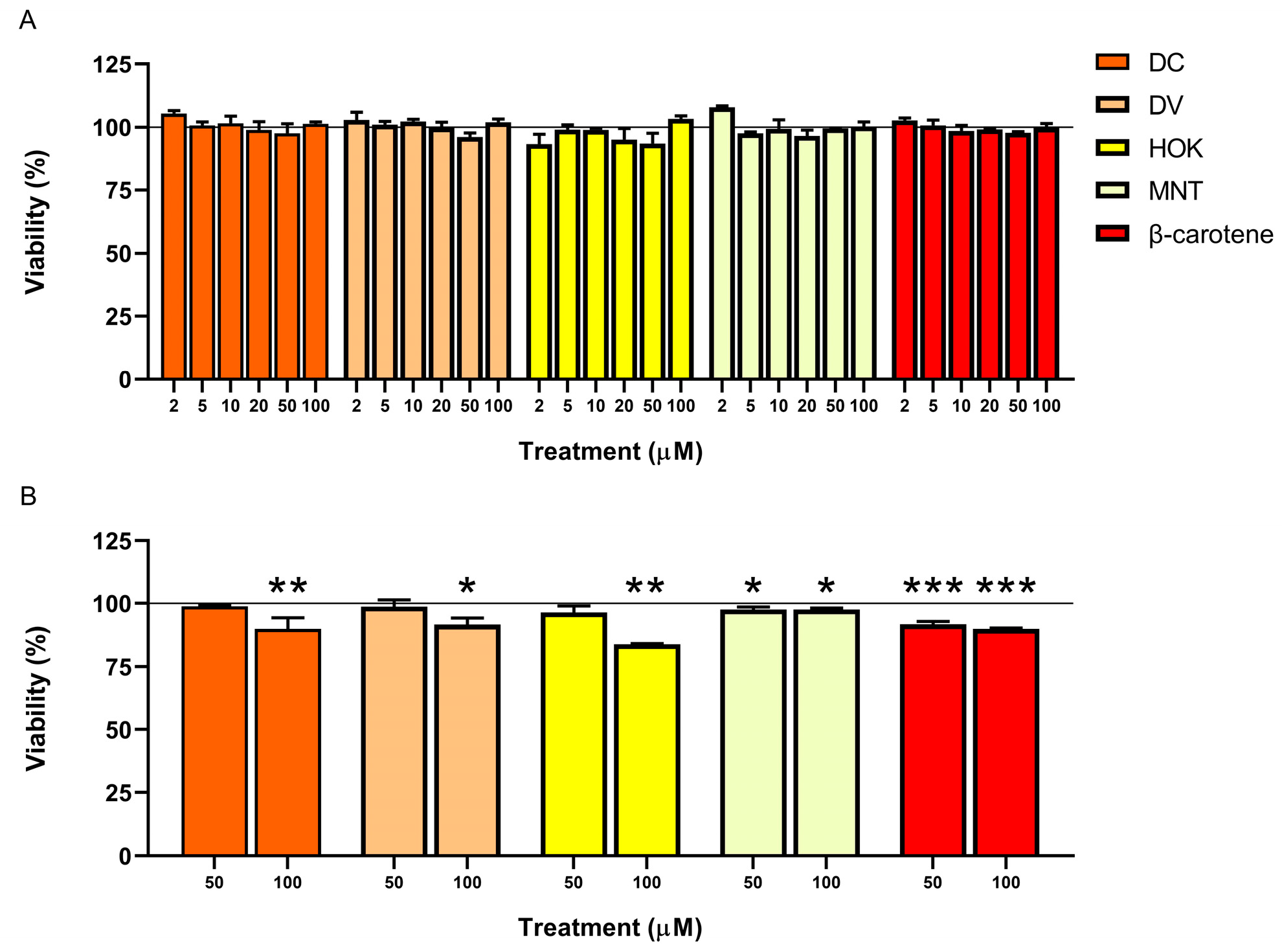
3.2. Cell Count and Viability: AO/DAPI Double Staining
3.3. TCC and Antioxidant Activity of Pumpkin Pulp
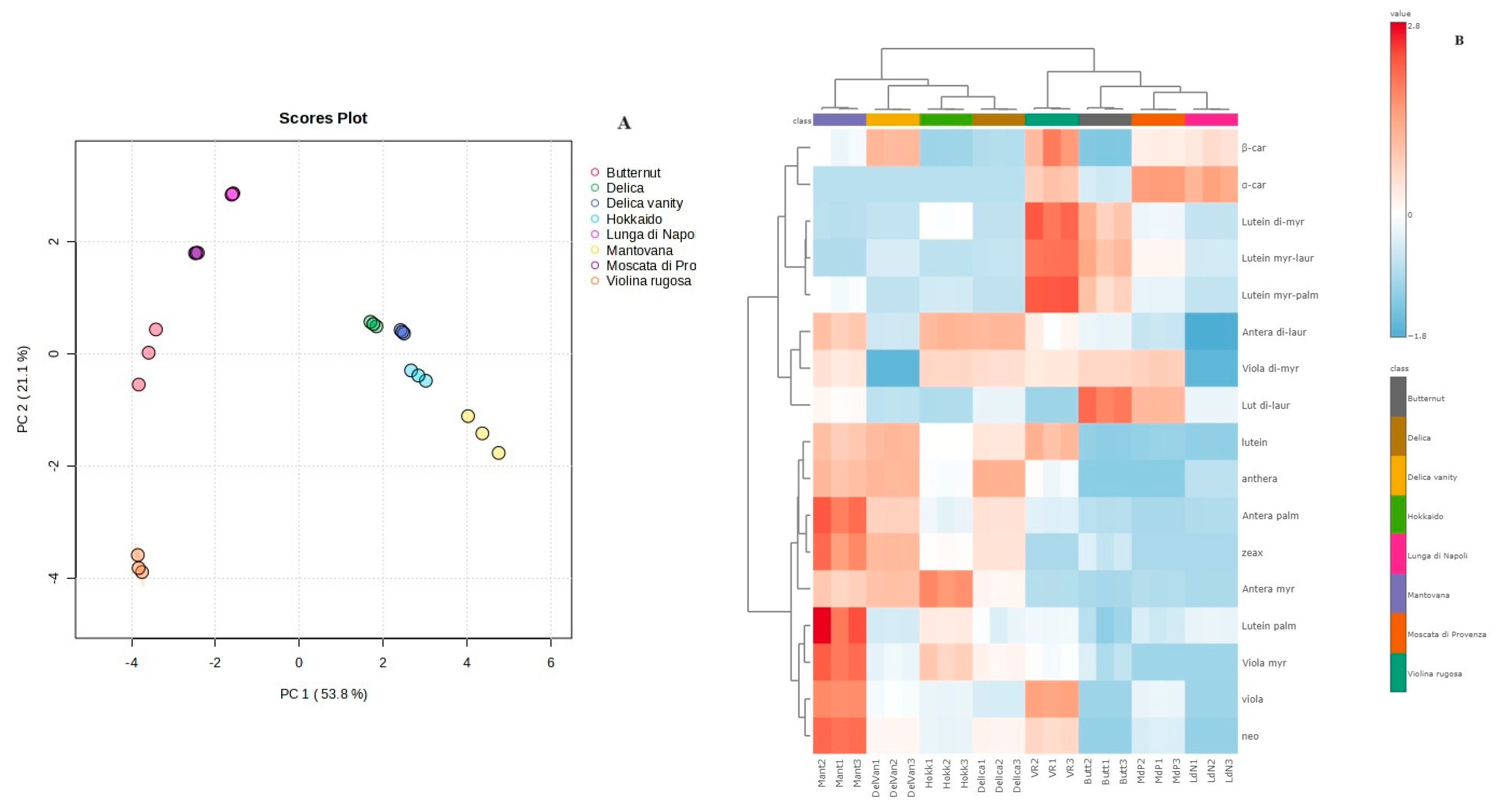
3.4. Carotenoid Composition of Pulp and Multivariate Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization 2013 WHO. Health 2020. A European Policy Framework and Strategy for the 21st Century. Available online: https://pns.dgs.pt/files/2022/02/Health2020-Long.pdf (accessed on 29 July 2024).
- Bohn, T.; Bonet, M.L.; Borel, P.; Keijer, J.; Landrier, J.F.; Milisav, I.; Ribot, J.; Riso, P.; Winklhofer-Roob, B.; Sharoni, Y.; et al. Mechanistic aspects of carotenoid health benefits—Where are we now? Nutr. Res. Rev. 2021, 34, 276–302. [Google Scholar] [CrossRef] [PubMed]
- Batool, M.; Ranjha, M.M.A.N.; Roobab, U.; Manzoor, M.F.; Farooq, U.; Nadeem, H.R.; Nadeem, M.; Kanwal, R.; AbdElgawad, H.; Al Jaouni, S.K.; et al. Nutritional value, phytochemical potential, and therapeutic benefits of pumpkin (Cucurbita sp.). Plants 2022, 11, 1394. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Irfan, S.; Lorenzo, J.M.; Shafique, B.; Kanwal, R.; Pateiro, M.; Arshad, R.N.; Wang, L.; Nayik, G.A.; Roobab, U.; et al. Sonication, a potential technique for extraction of phytoconstituents: A systematic review. Processes 2021, 9, 1406. [Google Scholar] [CrossRef]
- Sharma, M.; Bhat, R. Extraction of carotenoids from pumpkin peel and pulp: Comparison between innovative green extraction technologies (ultrasonic and microwave-assisted extractions using corn oil). Foods 2021, 10, 787. [Google Scholar] [CrossRef]
- Stevenson, D.G.; Eller, F.J.; Wang, L.; Jane, J.L.; Wang, T.; Inglett, G.E. Oil and tocopherol content and composition of pumpkin seed oil in 12 cultivars. J. Agric. Food Chem. 2007, 55, 4005–4013. [Google Scholar] [CrossRef]
- Nawirska-Olszańska, A.; Kita, A.; Biesiada, A.; Sokół-Łętowska, A.; Kucharska, A.Z. Characteristics of antioxidant activity and composition of pumpkin seed oils in 12 cultivars. Food Chem. 2013, 139, 155–161. [Google Scholar] [CrossRef]
- Kulczyński, B.; Gramza-Michałowska, A. The profile of secondary metabolites and other bioactive compounds in Cucurbita pepo L. and Cucurbita moschata pumpkin cultivars. Molecules 2019, 24, 2945. [Google Scholar] [CrossRef] [PubMed]
- Kostecka-Gugała, A.; Kruczek, M.; Ledwożyw-Smoleń, I.; Kaszycki, P. Antioxidants and health-beneficial nutrients in fruits of eighteen Cucurbita cultivars: Analysis of diversity and dietary implications. Molecules 2020, 25, 1792. [Google Scholar] [CrossRef]
- Kulczyński, B.; Sidor, A.; Gramza-Michałowska, A. Antioxidant potential of phytochemicals in pumpkin varieties belonging to Cucurbita moschata and Cucurbita pepo species. CyTA–J. Food 2020, 18, 472–484. [Google Scholar] [CrossRef]
- Armesto, J.; Rocchetti, G.; Biancamaria, S.; Pateiro, M.; Barba, F.J.; Domínguez, R.; Lucini, L.; Lorenzo, J.M. Nutritional characterization of Butternut squash (Cucurbita moschata D.): Effect of variety (Ariel vs. Pluto) and farming type (conventional vs. organic). Food Res. Int. 2020, 132, 109052. [Google Scholar] [CrossRef]
- Qi, X.; Jha, S.K.; Jha, N.K.; Dewanjee, S.; Dey, A.; Deka, R.; Pritam, P.; Ramgopal, K.; Liu, W.; Houet, K. Antioxidants in brain tumors: Current therapeutic significance and future prospects. Mol. Cancer 2022, 21, 204. [Google Scholar] [CrossRef] [PubMed]
- Koklesova, L.; Liskova, A.; Samec, M.; Buhrmann, C.; Samuel, S.M.; Varghese, E.; Ashrafizadeh, M.; Najafi, M.; Shakibaei, M.; Büsselberg, D.; et al. Carotenoids in cancer apoptosis-the road from bench to bedside and back. Cancers 2020, 12, 2425. [Google Scholar] [CrossRef] [PubMed]
- Caili, F.; Huan, S.; Quanhong, L. A review on pharmacological activities and utilization technologies of pumpkin. Plant Foods Hum. Nutr. 2006, 61, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Moccia, S.; Russo, M.; Durante, M.; Lenucci, M.S.; Mita, G.; Russo, G.L. A carotenoid-enriched extract from pumpkin delays cell proliferation in a human chronic lymphocytic leukemia cell line through the modulation of autophagic flux. Curr. Res. Biotech. 2020, 2, 74–82. [Google Scholar] [CrossRef]
- Gandla, K.; Babu, A.K.; Unnisa, A.; Sharma, I.; Singh, L.P.; Haque, M.A.; Dashputre, N.L.; Baig, S.; Siddiqui, F.A.; Khandaker, M.U.; et al. Carotenoids: Role in neurodegenerative diseases remediation. Brain Sci. 2023, 13, 457. [Google Scholar] [CrossRef]
- Murakoshi, M.; Takayasu, J.; Kimura, O.; Kohmura, E.; Nishino, H.; Iwashima, A.; Okuzumi, J.; Sakai, T.; Sugimoto, T.; Imanishi, J.; et al. Inhibitory effects of alpha-carotene on proliferation of the human neuroblastoma cell line GOTO. J. Natl. Cancer Inst. 1989, 81, 1649–1652. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, H.A.; Lim, J.K.; Kim, Y.; Jung, C.H.; Yoo, S.H.; Kim, Y. β-Carotene inhibits neuroblastoma cell invasion and metastasis in vitro and in vivo by decreasing level of hypoxia-inducible factor-1α. J. Nutr. Biochem. 2014, 25, 655–664. [Google Scholar] [CrossRef]
- Pinna, N.; Ianni, F.; Blasi, F.; Stefani, A.; Codini, M.; Sabatini, S.; Schoubben, A.; Cossignani, L. Unconventional extraction of total non-polar carotenoids from pumpkin pulp and their nanoencapsulation. Molecules 2022, 27, 8240. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- EasyRGB. 2024. Convert Color Data into Different Standards and Color Spaces. Available online: https://www.easyrgb.com/en/convert.php (accessed on 29 July 2024).
- Villarini, M.; Acito, M.; Di Vito, R.; Vannini, S.; Dominici, L.; Fatigoni, C.; Pagiotti, R.; Moretti, M. Pro-apoptotic activity of artichoke leaf extracts in human HT-29 and RKO colon cancer cells. Int. J. Environ. Res. Public Health 2021, 18, 4166. [Google Scholar] [CrossRef]
- Shao, Y.; Ni, Y.; Yang, J.; Lin, X.; Li, J.; Zhang, L. Astaxanthin inhibits proliferation and induces apoptosis and cell cycle arrest of mice H22 hepatoma cells. Med. Sci. Monit. 2016, 22, 2152–2160. [Google Scholar] [CrossRef]
- Ramamoorthy, K.; Raghunandhakumar, S.; Anand, R.S.; Paramasivam, A.; Kamaraj, S.; Nagaraj, S.; Ezhilarasan, D.; Lakshmi, T.; Dua, K.; Chellappan, D.K.; et al. Anticancer effects and lysosomal acidification in A549 cells by Astaxanthin from Haematococcus lacustris. Bioinformation 2020, 16, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Pollini, L.; Rocchi, R.; Cossignani, L.; Mañes, J.; Compagnone, D.; Blasi, F. Phenol profiling and nutraceutical potential of Lycium spp. leaf extracts obtained with ultrasound and microwave assisted techniques. Antioxidants 2019, 8, 260. [Google Scholar] [CrossRef]
- Persichetti, L.E.; De Michele, A.; Codini, M.; Traina, G. Antioxidative capacity of Lactobacillus fermentum LF31 evaluated in vitro by oxygen radical absorbance capacity assay. Nutrition 2014, 30, 936–938. [Google Scholar] [CrossRef] [PubMed]
- Montesano, D.; Rocchetti, G.; Cossignani, L.; Senizza, B.; Pollini, L.; Lucini, L.; Blasi, F. Untargeted metabolomics to evaluate the stability of extra-virgin olive oil with added Lycium barbarum carotenoids during storage. Foods 2019, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Pinna, N.; Ianni, F.; Selvaggini, R.; Urbani, S.; Codini, M.; Grispoldi, L.; Cenci-Goga, B.T.; Cossignani, L.; Blasi, F. Valorization of pumpkin byproducts: Antioxidant activity and carotenoid characterization of extracts from peel and filaments. Foods 2023, 12, 4035. [Google Scholar] [CrossRef]
- Kurz, C.; Carle, R.; Schieber, A. HPLC-DAD-MSn characterisation of carotenoids from apricots and pumpkins for the evaluation of fruit product authenticity. Food Chem. 2008, 110, 522–530. [Google Scholar] [CrossRef]
- Petry, F.C.; Mercadante, A.Z. Composition by LC-MS/MS of new carotenoid esters in mango and citrus. J. Agric. Food Chem. 2016, 64, 8207–8224. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef]
- Hosen, M.; Rafii, M.Y.; Mazlan, N.; Jusoh, M.; Oladosu, Y.; Chowdhury, M.F.N.; Muhammad, I.; Khan, M.M.H. Pumpkin (Cucurbita spp.): A crop to mitigate food and nutritional challenges. Horticulturae 2021, 7, 352. [Google Scholar] [CrossRef]
- Zhou, C.L.; Mi, L.; Hu, X.Y.; Zhu, B.H. Evaluation of three pumpkin species: Correlation with physicochemical, antioxidant properties and classification using SPME-GC–MS and E-nose methods. J. Food Sci. Technol. 2017, 54, 3118–3131. [Google Scholar] [CrossRef]
- Paciulli, M.; Rinaldi, M.; Rodolfi, M.; Ganino, T.; Morbarigazzi, M.; Chiavaro, E. Effects of high hydrostatic pressure on physico-chemical and structural properties of two pumpkin species. J. Food Chem. 2019, 274, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Norfezah, M.N.; Hardacre, A.; Brennan, C.S. Comparison of waste pumpkin material and its potential use in extruded snack foods. Food Sci. Technol. Int. 2011, 17, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Itle, R.A.; Kabelk, E.A. Correlation between L*a*b* color space values and carotenoid content in pumpkins and squash (Cucurbita spp.). HortScience 2009, 44, 633–637. [Google Scholar] [CrossRef]
- Karanja, J.K.; Mugendi, B.J.; Khamis, F.M.; Muchugi, A.N. Nutritional Evaluation of Some Kenyan Pumpkins (Cucurbita spp.). Int. J. Agric. 2014, 4, 195–200. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; ILSI Press: Washington, DC, USA, 2001; ISBN 978-1-57881-072-7. [Google Scholar]
- Feng, C.; Luo, T.; Zhang, S.; Liu, K.; Zhang, Y.; Luo, Y.; Ge, P. Lycopene protects human SH-SY5Y neuroblastoma cells against hydrogen peroxide-induced death via inhibition of oxidative stress and mitochondria-associated apoptotic pathways. Mol. Med. Rep. 2016, 13, 4205–4214. [Google Scholar] [CrossRef]
- Pruccoli, L.; Balducci, M.; Pagliarani, B.; Tarozzi, A. Antioxidant and neuroprotective effects of fucoxanthin and its metabolite fucoxanthinol: A comparative in vitro study. Curr. Issues Mol. Biol. 2024, 46, 5984–5998. [Google Scholar] [CrossRef]
- Lakey-Beitia, J.; Kumar, D.J.; Hegde, M.L.; Rao, K.S. Carotenoids as novel therapeutic molecules against neurodegenerative disorders: Chemistry and molecular docking analysis. Int. J. Mol. Sci. 2019, 7, 5553. [Google Scholar] [CrossRef]
- Huang, L.; Luo, Y.; Pu, Z.; Kong, X.; Fu, X.; Xing, H.; Wei, S.; Chen, W.; Tang, H. Oxoisoaporphine alkaloid derivative 8-1 reduces Aβ1-42 secretion and toxicity in human cell and Caenorhabditis elegans models of Alzheimer’s disease. Neurochem. Int. 2017, 108, 157–168. [Google Scholar] [CrossRef]
- Darendelioğlu, E. Studies of anticancer activity of beta-carotene, alpha-tocopherol and ascorbic acid in SH-SY5Y neuroblastoma cells. J. Inst. Sci. Technol. 2019, 9, 1657–1665. [Google Scholar] [CrossRef]
- de Carvalho, L.M.J.; Gomes, P.B.; de Oliveira Godoy, R.L.; Pacheco, S.; do Monte, P.H.F.; de Carvalho, J.L.V. Total carotenoid content, α-carotene and β-carotene, of landrace pumpkins (Cucurbita moschata Duch): A preliminary study. Food Res. Int. 2012, 47, 337–340. [Google Scholar] [CrossRef]
- Hussain, A.; Kausar, T.; Sehar, S.; Sarwar, A.; Ashraf, A.H.; Jamil, M.A.; Noreen, S.; Rafique, A.; Iftikhar, K.; Quddoos, M.Y.; et al. A Comprehensive review of functional ingredients, especially bioactive compounds present in pumpkin peel, flesh and seeds, and their health benefits. Food Chem. Adv. 2022, 1, 100067. [Google Scholar] [CrossRef]
- Biesiada, A.; Nawirska, A.; Kucharska, A.; Sokół-Łętowska, A. Chemical composition of pumpkin fruit depending on cultivar and storage. Ecol. Chem. Eng. A 2011, 18, 9–18. [Google Scholar]
- Azizah, A.H.; Wee, K.C.; Azizah, O.; Azizah, M. Effect of boiling and stir frying on total phenolics, carotenoids and radical scavenging activity of pumpkin (Cucurbita moschata). Int. Food Res. 2009, 16, 45–51. [Google Scholar]
- Kreck, M.; Kurbel, P.; Ludwig, M.; Paschold, P.J.; Dietrich, H. Identification and quantification of carotenoids in pumpkin cultivars (Cucurbita maxima L.) and their juices by liquid chromatography with ultraviolet-diode array detection. J. Appl. Bot. Food Qual. 2006, 80, 93–99. [Google Scholar]
- Murkovic, M.; Mülleder, U.; Neunteufl, H. Carotenoid content in different varieties of pumpkins. J. Food Comp. Anal. 2002, 15, 633–638. [Google Scholar] [CrossRef]
- Dhenge, R.; Rinaldi, M.; Ganino, T.; Santi, S.; Ferrarese, I.; Dall’Acqua, S. Variations of polyphenols, sugars, carotenoids, and volatile constituents in pumpkin (Cucurbita moschata) during high pressure processing: A kinetic study. Inn. Food Sci. Emerg. Technol. 2022, 78, 103005. [Google Scholar] [CrossRef]
- Grassino, A.N.; Brnčić, S.R.; Sabolović, M.B.; Žlabur, J.Š.; Marović, R.; Brnčić, M. Carotenoid content and profiles of pumpkin products and by-products. Molecules 2023, 28, 858. [Google Scholar] [CrossRef]
- Khachik, F.; Beecher, G.R. Separation and identification of carotenoids and carotenol fatty acid esters in some squash products by liquid chromatography. 1. Quantification of carotenoids and related esters by HPLC. J. Agric. Food Chem. 1988, 36, 929–937. [Google Scholar] [CrossRef]
- Ouyang, M.; Huang, Y.; Wang, Y.; Luo, F.; Liao, L. Stability of carotenoids and carotenoid esters in pumpkin (Cucurbita maxima) slices during hot air drying. Food Chem. 2022, 367, 130710. [Google Scholar] [CrossRef]
- Mariutti, L.R.B.; Mercadante, A.Z. Carotenoid esters analysis and occurrence: What do we know so far? Arch. Biochem. Biophys. 2018, 648, 36–43. [Google Scholar] [CrossRef]
- Lewis, E.; Nogueira, R.M.; Enfissi, E.M.A.; Fraser, P.D. The esterification of xanthophylls in Solanum lycopersicum (tomato) chromoplasts; the role of a non-specific acyltransferase. Phytochemistry 2021, 191, 112912. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Rauf, A.; Tareq, A.M.; Jahan, S.; Bin Emran, T.; Shahriar, T.G.; Dhama, K.; Alhumaydhi, F.A.; Aljohani, A.S.M.; Rebezov, M.; et al. Potential health benefits of carotenoid lutein: An updated review. Food Chem. Toxicol. 2021, 154, 112328. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, M.; Mohammadi, M.; Pezeshki, A.; Jafari, S.M. Health benefits of beta-carotene. In Handbook of Food Bioactive Ingredients; Jafari, S.M., Rashidinejad, A., Simal-Gandara, J., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]



| Fruit Appearance |  |  |  |  |
| C. moschata species | Butternut | Lunga di Napoli | Moscata di Provenza | Violina rugosa |
| Skin color | Orange | Green | Orange with green spot | Orange |
| Fruit shape | Pear-like shape | Pear-like shape | Round shape | Pear-like shape |
| Fruit weight, kg | 3.318 | 2.537 | 5.433 | 3.120 |
| Flesh color | Orange | Orange | Orange | Orange |
| Usage | For food use | For food use | For food use | For food use |
| Rind | Smooth and regular | Smooth and regular | Smooth and regular | Smooth and regular |
| Harvest time | October | October | October | October |
| Fruit appearance |  |  |  |  |
| C. maxima species | Delica | Delica vanity | Hokkaido | Mantovana |
| Skin color | Green | Orange | Orange/Red | Brown with green spot |
| Fruit shape | Round shape | Round shape | Round shape | Round shape |
| Fruit weight, kg | 1.350 | 1.011 | 0.539 | 4.490 |
| Flesh color | Orange | Orange | Orange | Orange |
| Usage | For food use | For food use | For food use | For food use |
| Rind | Rough and irregular | Rough and irregular | Smooth and regular | Rough and irregular |
| Harvest time | October | October | October | October |
| Cultivar | L* | a* | b* | C* | H* |
|---|---|---|---|---|---|
| C. moschata species | |||||
| Butternut | 51.10 ± 0.89 a,d | 7.28 ± 0.61 a,d | 44.73 ± 0.58 a,e | 45.32 ± 0.71 a,e | 80.76 ± 0.78 a |
| Lunga di Napoli | 55.58 ± 0.93 a,e | 3.97 ± 0.12 b | 40.15 ± 1.09 a | 40.35 ± 0.87 b | 84.35 ± 0.20 a,c,d |
| Moscata di Provenza | 55.17 ± 1.20 a,e | 4.87 ± 0.93 a,b | 40.98 ± 0.29 a | 41.27 ± 0.75 a,b | 83.22 ± 1.19 a,c,d |
| Violina rugosa | 34.52 ± 0.85 b | 14.98 ± 0.31 c | 33.98 ± 0.41 b | 37.13 ± 0.33 b | 66.21 ± 1.15 b |
| C. maxima species | |||||
| Delica | 52.71 ± 1.08 a,e | 4.87 ± 0.21 a,b | 49.28 ± 0.89 c,e | 49.52 ± 0.97 c,e | 84.36 ± 1.51 c,d |
| Delica vanity | 26.98 ± 1.08 c | 10.47 ± 0.42 d | 25.19 ± 1.13 d | 27.28 ± 1.22 d | 67.43 ± 1.94 b |
| Hokkaido | 45.98 ± 1.95 d | 5.18 ± 0.18 a,b | 41.58 ± 2.43 a | 41.90 ± 1.97 a,b | 82.90 ± 1.08 c |
| Mantovana | 56.87 ± 2.47 e | 2.58 ± 0.05 b | 48.62 ± 1.77 e | 48.69 ± 1.15 e | 86.96 ± 2.03 d |
| Cultivar | Yield (%) | TCC (μg β-CE/g) | ABTS (μg TE/g) | ORAC (μg TE/g) |
|---|---|---|---|---|
| C. moschata species | ||||
| Butternut | 1.63 ± 0.20 a,b | 161.08 ± 7.80 a | 280.91 ± 27.45 a | 1352.34 ± 10.34 a |
| Lunga di Napoli | 1.29 ± 0.06 a,d | 303.27 ± 2.08 b | 343.13 ± 18.82 a,b | 1802.54 ± 76.54 a,b |
| Moscata di Provenza | 2.00 ± 0.25 b,e | 365.73 ± 9.49 c | 417.62 ± 53.94 b,c,e | 1500.30 ± 53.04 a,d |
| Violina rugosa | 1.42 ± 0.15 a,d | 443.89 ± 7.58 d | 525.39 ± 32.24 c,f | 2560.11 ± 324.24 b |
| C. maxima species | ||||
| Delica | 4.15 ± 0.00 c | 379.36 ± 44.08 c | 1192.11 ± 48.44 d | 3996.18 ± 72.58 c |
| Delica vanity | 1.03 ± 0.01 d | 241.32 ± 21.55 e | 313.23 ± 16.22 a,e | 1267.86 ± 166.22 a |
| Hokkaido | 1.58 ± 0.06 a,b | 310.77 ± 23.82 b | 548.41 ± 68.45 f | 2341.24 ± 68.45 b,d |
| Mantovana | 2.20 ± 0.28 e | 247.05 ± 13.62 e | 500.90 ± 11.69 c,f | 2615.96 ± 52.10 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinna, N.; Ianni, F.; Conte, C.; Codini, M.; di Vito, R.; Urbani, S.; Selvaggini, R.; Cossignani, L.; Blasi, F. Carotenoids from Different Pumpkin Varieties Exert a Cytotoxic Effect on Human Neuroblastoma SH-SY5Y Cells. Nutrients 2024, 16, 3043. https://doi.org/10.3390/nu16173043
Pinna N, Ianni F, Conte C, Codini M, di Vito R, Urbani S, Selvaggini R, Cossignani L, Blasi F. Carotenoids from Different Pumpkin Varieties Exert a Cytotoxic Effect on Human Neuroblastoma SH-SY5Y Cells. Nutrients. 2024; 16(17):3043. https://doi.org/10.3390/nu16173043
Chicago/Turabian StylePinna, Nicola, Federica Ianni, Carmela Conte, Michela Codini, Raffaella di Vito, Stefania Urbani, Roberto Selvaggini, Lina Cossignani, and Francesca Blasi. 2024. "Carotenoids from Different Pumpkin Varieties Exert a Cytotoxic Effect on Human Neuroblastoma SH-SY5Y Cells" Nutrients 16, no. 17: 3043. https://doi.org/10.3390/nu16173043
APA StylePinna, N., Ianni, F., Conte, C., Codini, M., di Vito, R., Urbani, S., Selvaggini, R., Cossignani, L., & Blasi, F. (2024). Carotenoids from Different Pumpkin Varieties Exert a Cytotoxic Effect on Human Neuroblastoma SH-SY5Y Cells. Nutrients, 16(17), 3043. https://doi.org/10.3390/nu16173043







