Implementing a Diet Risk Score (DRS) for Spanish-Speaking Adults in a Clinical Setting: A Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. The DRS Questionnaire
2.2. Study Approval and Informed Consent
2.3. Recruitment
2.4. Training
2.5. Data Collection
2.6. Statistical Analysis
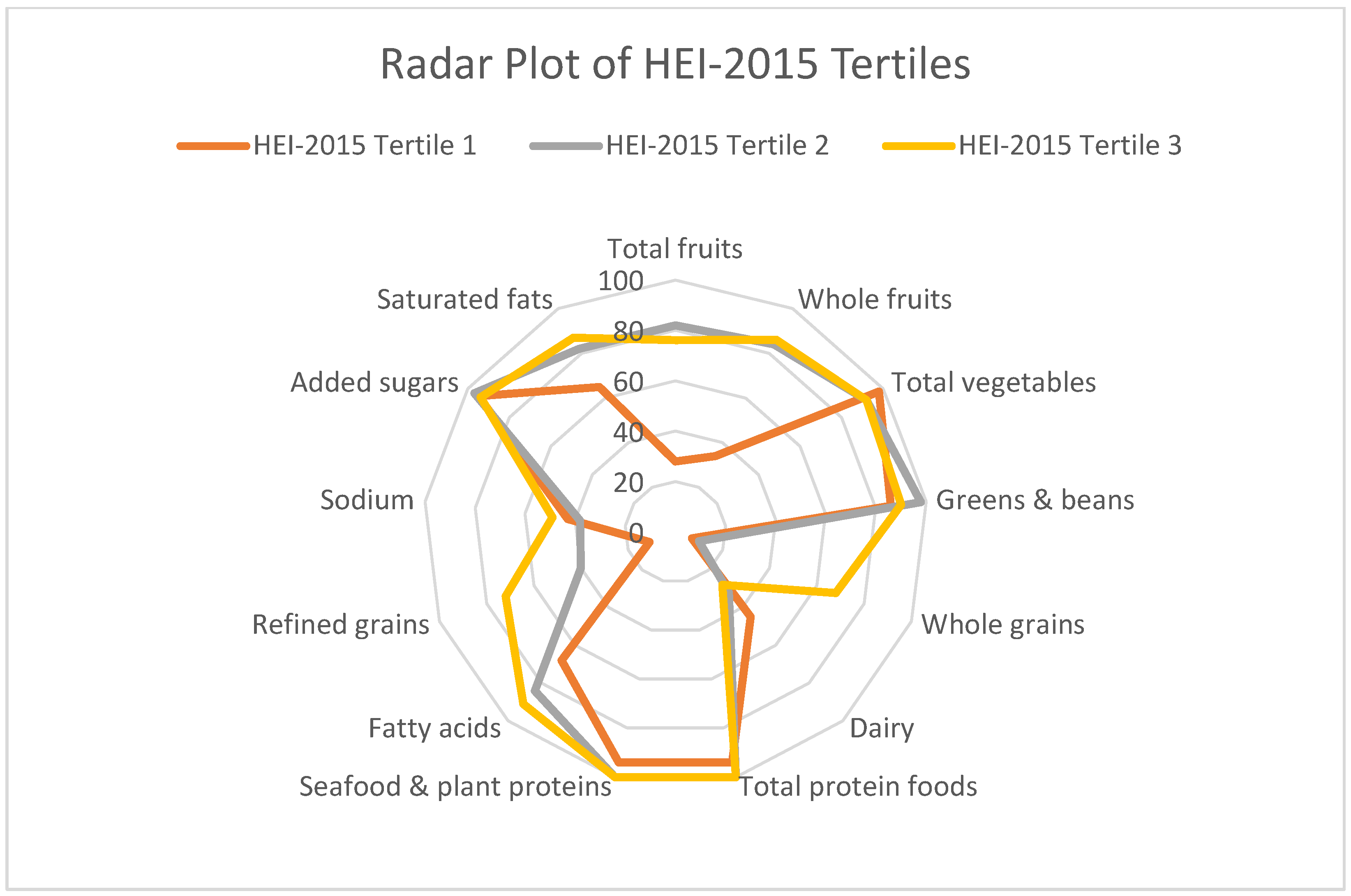
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Diet Risk Score Questionnaire with Scoring (Spanish and English) | ||||
|---|---|---|---|---|
| Para los siguientes alimentos, por favor seleccione con qué frecuencia come cada alimento o grupo de alimentos en una semana normal. For the following foods, please select the frequency that best describes how often you eat each food or group of foods in a normal day. | Diariamente Daily | 2–3 veces por semana 2–3 times/week | 1 vez por semana 1 time/week | Nunca Never |
| Comidas tipo comida rápida, comidas para sentarse o para llevar, cenas congeladas, incluyendo pizza * Fast food-type meals, sit down or takeout meals, frozen dinners, including pizza | 3 | 2 | 1 | 0 |
| Panes, bollos, sandwiches * Bread, rolls, sandwiches | 3 | 2 | 1 | 0 |
| Papas fritas, palomitas de maíz, pretzels, mezclas de bocadillos, galletas saladas * Chips, popcorn, pretzels, snack mixes, crackers | 3 | 2 | 1 | 0 |
| Salchichas, carnes curadas o carnes frias, hot dogs, embutidos † Sausage, cured or deli meats, hot dogs | 3 | 3 | 3 | 0 |
| Refresco regular, té helado endulzado, jugo, leche con sabor o bebidas de café con sabor ‡ Regular soda, sweetened iced tea, juice, flavored milk or flavored coffee drinks | 3 | 2 | 1 | 0 |
| Cacahuates, nueces de árbol, semillas, crema de cacahuate u alguna mantequilla de nuez § Peanuts, tree nuts, seeds, peanut butter or other nut butter | 0 | 0 | 2 | 3 |
| Pescado o mariscos ¶ Fish or shellfish | 0 | 0 | 1 | 3 |
| Verduras, frijoles, legumbres ** Vegetables, beans, peas | 0 | 3 | 3 | 3 |
| Fruta (fresca, enlatada o seca; sin incluir jugo) †† Fruit (fresh, canned or dried; not including juice) | 0 | 3 | 3 | 3 |
Appendix B
| Item | Daily | 2–3 Times per Week | 1 Time per Week | Never |
|---|---|---|---|---|
| Fast food | 0 | 3 | 19 | 9 |
| Breads | 8 | 9 | 9 | 5 |
| Snacks | 1 | 6 | 11 | 13 |
| Processed meats | 0 | 4 | 6 | 21 |
| Sugar-sweetened beverages | 2 | 10 | 3 | 16 |
| Nuts | 11 | 11 | 2 | 7 |
| Fish | 0 | 8 | 17 | 6 |
| Vegetables | 23 | 7 | 1 | 0 |
| Fruit | 20 | 9 | 2 | 0 |
References
- Micha, R.; Peñalvo, J.L.; Cudhea, F.; Imamura, F.; Rehm, C.D.; Mozaffarian, D. Association Between Dietary Factors and Mortality From Heart Disease, Stroke, and Type 2 Diabetes in the United States. JAMA 2017, 317, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Patnode, C.D.; Evans, C.V.; Senger, C.A.; Redmond, N.; Lin, J.S. Behavioral Counseling to Promote a Healthful Diet and Physical Activity for Cardiovascular Disease Prevention in Adults Without Known Cardiovascular Disease Risk Factors: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2017, 318, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; Depalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, e13–e115. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 5. Facilitating Behavior Change and Well-being to Improve Health Outcomes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S6–S82. [Google Scholar] [CrossRef]
- Vadiveloo, M.; Lichtenstein, A.H.; Anderson, C.; Aspry, K.; Foraker, R.; Griggs, S.; Hayman, L.L.; Johnston, E.; Stone, N.J.; Thorndike, A.N. Rapid Diet Assessment Screening Tools for Cardiovascular Disease Risk Reduction Across Healthcare Settings: A Scientific Statement From the American Heart Association. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e000094. [Google Scholar] [CrossRef]
- Frey, W.H. Mapping Americas Diversity with the 2020 Census; Brookings Institution: Wasington, DC, USA, 2021. [Google Scholar]
- Alemán, J.O.; Almandoz, J.P.; Frias, J.P.; Galindo, R.J. Obesity among Latinx people in the United States: A review. Obesity 2023, 31, 329–337. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Diabetes and Digestive and Kidney Diseases. Health Risks of Overweight & Obesity. Available online: https://www.niddk.nih.gov/health-information/weight-management/adult-overweight-obesity/health-risks (accessed on 23 August 2024).
- American Dietetic Association. Identifying patients at risk: ADA’s definitions for nutrition screening and nutrition assessment. J. Am. Diet Assoc. 1994, 94, 838–839. [Google Scholar] [CrossRef]
- Fernandez, S.; Olendzki, B.; Rosal, M.C. A dietary behaviors measure for use with low-income, Spanish-speaking Caribbean Latinos with type 2 diabetes: The Latino Dietary Behaviors Questionnaire. J. Am. Diet. Assoc. 2011, 111, 589–599. [Google Scholar] [CrossRef]
- England, C.; Andrews, R.; Jago, R.; Thompson, J. A systematic review of brief dietary questionnaires suitable for clinical use in the prevention and management of obesity, cardiovascular disease and type 2 diabetes. Eur. J. Clin. Nutr. 2015, 69, 977–1003. [Google Scholar] [CrossRef]
- Johnston, E.A.; Petersen, K.S.; Beasley, J.M.; Krussig, T.; Mitchell, D.C.; Van Horn, L.V.; Weiss, R.; Kris-Etherton, P.M. Relative validity and reliability of a diet risk score (DRS) for clinical practice. BMJ Nutr. Prev. Health 2020, 3, 263–269. [Google Scholar] [CrossRef]
- Chiuve, S.E.; Cook, N.R.; Shay, C.M.; Rexrode, K.M.; Albert, C.M.; Manson, J.E.; Willett, W.C.; Rimm, E.B. Lifestyle-based prediction model for the prevention of CVD: The healthy heart score. J. Am. Heart Assoc. 2014, 3, e000954. [Google Scholar] [CrossRef]
- Aaron, K.J.; Sanders, P.W. Role of dietary salt and potassium intake in cardiovascular health and disease: A review of the evidence. Mayo Clin. Proc. 2013, 88, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Buil-Cosiales, P.; Toledo, E.; Salas-Salvado, J.; Zazpe, I.; Farras, M.; Basterra-Gortari, F.J.; Diez-Espino, J.; Estruch, R.; Corella, D.; Ros, E. Association between dietary fibre intake and fruit, vegetable or whole-grain consumption and the risk of CVD: Results from the PREvencion con DIeta MEDiterranea (PREDIMED) trial. Br. J. Nutr. 2016, 116, 534–546. [Google Scholar] [CrossRef]
- Hu, D.; Huang, J.; Wang, Y.; Zhang, D.; Qu, Y. Fruits and vegetables consumption and risk of stroke: A meta-analysis of prospective cohort studies. Stroke 2014, 45, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Johnston, E.A.; Park, A.; Hu, L.; Yi, S.S.; Thorpe, L.E.; Rummo, P.E.; Beasley, J.M. Relative validity of a Diet Risk Score (DRS) for Chinese American adults. BMJ Nutr. Prev. Health 2023, 6, e000509. [Google Scholar] [CrossRef]
- Singh, G.M.; Danaei, G.; Farzadfar, F.; Stevens, G.A.; Woodward, M.; Wormser, D.; Kaptoge, S.; Whitlock, G.; Qiao, Q.; Lewington, S.; et al. The Age-Specific Quantitative Effects of Metabolic Risk Factors on Cardiovascular Diseases and Diabetes: A Pooled Analysis. PLoS ONE 2013, 8, e65174. [Google Scholar] [CrossRef]
- United State Department of Agriculture: Food Surveys Research Group. AMPM—USDA Automated Multiple-Pass Method; United State Department of Agriculture: Washington, DC, USA, 2024. [Google Scholar]
- Subar, A.F.; Kirkpatrick, S.I.; Mittl, B.; Zimmerman, T.P.; Thompson, F.E.; Bingley, C.; Willis, G.; Islam, N.G.; Baranowski, T.; McNutt, S. The automated self-administered 24-hour dietary recall (ASA24): A resource for researchers, clinicians and educators from the National Cancer Institute. J. Acad. Nutr. Diet. 2012, 112, 1134. [Google Scholar] [CrossRef] [PubMed]
- Northwestern Medicine. A Handy Guide to Serving Size [Infographic]. Available online: https://www.nm.org/healthbeat/healthy-tips/nutrition/handy-guide-to-serving-size (accessed on 8 June 2023).
- National Cancer Institute. Healthy Eating Index SAS Code. Available online: https://epi.grants.cancer.gov/hei/sas-code.html (accessed on 3 November 2023).
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the healthy eating index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef]
- US Department of Agriculture Food and Nutrition Service. Average Healthy Eating Index-2015 Scores for Adults by Age Groups; USDA: Washington, DC, USA, 2021. [Google Scholar]
- Siega-Riz, A.M.; Pace, N.D.; Butera, N.M.; Van Horn, L.; Daviglus, M.L.; Harnack, L.; Mossavar-Rahmani, Y.; Rock, C.L.; Pereira, R.I.; Sotres-Alvarez, D. How Well Do U.S. Hispanics Adhere to the Dietary Guidelines for Americans? Results from the Hispanic Community Health Study/Study of Latinos. Health Equity 2019, 3, 319–327. [Google Scholar] [CrossRef]
- Overcash, F.; Reicks, M. Diet Quality and Eating Practices among Hispanic/Latino Men and Women: NHANES 2011–2016. Int. J. Environ. Res. Public Health 2021, 18, 1302. [Google Scholar] [CrossRef] [PubMed]
- Banna, J.C.; Townsend, M.S. Assessing factorial and convergent validity and reliability of a food behaviour checklist for Spanish-speaking participants in US Department of Agriculture nutrition education programmes. Public Health Nutr. 2011, 14, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Banna, J.C.; Becerra, L.E.V.; Kaiser, L.L.; Townsend, M.S. Using qualitative methods to improve questionnaires for Spanish speakers: Assessing face validity of a food behavior checklist. J. Am. Diet. Assoc. 2010, 110, 80–90. [Google Scholar] [CrossRef]
- Wakimoto, P.; Block, G.; Mandel, S.; Medina, N. Peer Reviewed: Development and Reliability of Brief Dietary Assessment Tools for Hispanics. Prev. Chronic Dis. 2006, 3, A95. [Google Scholar]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- García-Conesa, M.-T.; Philippou, E.; Pafilas, C.; Massaro, M.; Quarta, S.; Andrade, V.; Jorge, R.; Chervenkov, M.; Ivanova, T.; Dimitrova, D. Exploring the validity of the 14-item mediterranean diet adherence screener (Medas): A cross-national study in seven european countries around the mediterranean region. Nutrients 2020, 12, 2960. [Google Scholar] [CrossRef]
- American Medical Association. Health Promotion and Preventive Care. In AMA Code of Medical Ethics; AMA: Chicago, IL, USA, 2015. [Google Scholar]
- Aspry, K.E.; Van Horn, L.; Carson, J.A.S.; Wylie-Rosett, J.; Kushner, R.F.; Lichtenstein, A.H.; Devries, S.; Freeman, A.M.; Crawford, A.; Kris-Etherton, P. Medical Nutrition Education, Training, and Competencies to Advance Guideline-Based Diet Counseling by Physicians: A Science Advisory From the American Heart Association. Circulation 2018, 137, e821–e841. [Google Scholar] [CrossRef]
- Devries, S.; Agatston, A.; Aggarwal, M.; Aspry, K.E.; Esselstyn, C.B.; Kris-Etherton, P.; Miller, M.; O’Keefe, J.H.; Ros, E.; Rzeszut, A.K.; et al. A Deficiency of Nutrition Education and Practice in Cardiology. Am. J. Med. 2017, 130, 1298–1305. [Google Scholar] [CrossRef]
- Crowley, J.; Ball, L.; Hiddink, G.J. Nutrition in medical education: A systematic review. Lancet Planet. Health 2019, 3, e379–e389. [Google Scholar] [CrossRef]
- Wilson, A.D.; Childs, S.; Gonçalves-Bradley, D.C.; Irving, G.J. Interventions to Increase or Decrease the Length of Primary Care Physicians’ Consultation; Cochrane Database of Systematic Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Harkin, N.; Johnston, E.; Mathews, T.; Guo, Y.; Schwartzbard, A.; Berger, J.; Gianos, E. Physicians’ Dietary Knowledge, Attitudes, and Counseling Practices: The Experience of a Single Health Care Center at Changing the Landscape for Dietary Education. Am. J. Lifestyle Med. 2019, 13, 292–300. [Google Scholar] [CrossRef]
- Williams, A.R.; Hines, A.L.; Dow, A.W.; Sabo, R.T.; Thomson, M.D. Are primary care providers’ nutrition care and food insecurity screening practices associated with their perceptions of team-based care? Fam. Pract. 2022, 39, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Central Valley Food Access Working Group. Action Plan to Improve Food Access in the Central Valley; CDFA: Sacramento, CA, USA, 2016. [Google Scholar]
- American Osteopathic Association. Osteopathic Medical Profession Report; AOA: Chicago, IL, USA, 2022. [Google Scholar]
- Briggs Early, K.; Adams, K.M.; Kohlmeier, M. Analysis of Nutrition Education in Osteopathic Medical Schools. J. Biomed. Educ. 2015, 2015, 376041. [Google Scholar] [CrossRef]
- Hargrove, E.J.; Berryman, D.E.; Yoder, J.M.; Beverly, E.A. Assessment of Nutrition Knowledge and Attitudes in Preclinical Osteopathic Medical Students. J. Am. Osteopath. Assoc. 2017, 117, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Sastre, L.R.; Van Horn, L.T. Family medicine physicians’ report strong support, barriers and preferences for Registered Dietitian Nutritionist care in the primary care setting. Fam. Pract. 2021, 38, 25–31. [Google Scholar] [CrossRef] [PubMed]
| Variable | Mean | 95% CI |
|---|---|---|
| Age (±SD) | 58 years (±8.4) | |
| Sex | 52% female (n = 16) | |
| Reported total energy (ASA-24; calories) | 3282 | 2965, 3600 |
| DRS-S Fast food | 0.8 | 0.6, 1.1 |
| DRS-S Bread | 1.6 | 1.2, 2.0 |
| DRS-S Snacks | 0.9 | 0.6, 1.2 |
| DRS-S Processed meat | 0.9 | 0.4, 1.4 |
| DRS-S Sugar-sweetened beverages | 1 | 0.6, 1.4 |
| DRS-S Nuts | 0.8 | 0.3, 1.3 |
| DRS-S Fish | 1.1 | 0.7, 1.5 |
| DRS-S Veg | 0.8 | 0.3, 1.3 |
| DRS-S Fruit | 1.2 | 0.6, 1.7 |
| DRS-S Total Score | 9 | 7.4, 10.5 |
| HEI-2015 Total Vegetables * | 4.7 | 4.4, 5 |
| HEI-2015 Greens/beans * | 4.6 | 4.1, 5 |
| HEI-2015 Total fruit * | 3.1 | 2.3, 3.9 |
| HEI-2015 Whole fruit * | 3.4 | 2.7, 4.2 |
| HEI-2015 Whole grains ** | 2.8 | 1.5, 4.1 |
| HEI-2015 Dairy ** | 3.5 | 2.4, 4.6 |
| HEI-2015 Total protein * | 4.9 | 4.8, 5 |
| HEI-2015 Seafood/plant proteins * | 4.9 | 4.7, 5 |
| HEI-2015 Fatty acids ** | 8.1 | 7.2, 9 |
| HEI-2015 Sodium ** | 4.3 | 3.2, 5.5 |
| HEI-2015 Refined grains ** | 4.1 | 2.6, 5.5 |
| HEI-2015 Fatty acids (SFA) ** | 7.8 | 7, 8.6 |
| HEI-2015 Added sugars ** | 9.5 | 9.2, 9.8 |
| HEI-2015 Total Score | 65.7 | 62.2, 69.3 |
| DRS-S Component | HEI-2015 Component | Correlation | p Value |
|---|---|---|---|
| Fast food | Sodium | 0.20 | 0.27 |
| Bread | −0.27 | 0.13 | |
| Snacks | 0.15 | 0.41 | |
| Processed meats | −0.10 | 0.56 | |
| Saturated fat | 0.09 | 0.59 | |
| Sugar-sweetened beverages | Added sugars | −0.03 | 0.86 |
| Nuts | Seafood/plant protein | −0.24 | 0.18 |
| Fish | −0.008 | 0.96 | |
| Fruit | Total fruit | −0.45 | 0.01 |
| Whole fruits | −0.41 | 0.02 | |
| Vegetables | Total vegetables | 0.06 | 0.72 |
| Green vegetables, beans | 0.01 | 0.93 | |
| Total DRS-S | Total HEI-2015 score | −0.44 | 0.01 |
| HEI Scores by DRS-S Tertile | |
|---|---|
| DRS-S Tertile | HEI Scores (95% CI) |
| 1 (0–9) | 70.8 (65.5, 76.1) * |
| 2 (10–18) | 66.1 (61.0, 71.2) |
| 3 (19–27) | 58.2 (52.0, 64.5) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnston, E.A.; Torres, M.; Hansen, J.; Ochoa, K.; Mortenson, D.; De Leon, E.; Beasley, J.M. Implementing a Diet Risk Score (DRS) for Spanish-Speaking Adults in a Clinical Setting: A Feasibility Study. Nutrients 2024, 16, 2992. https://doi.org/10.3390/nu16172992
Johnston EA, Torres M, Hansen J, Ochoa K, Mortenson D, De Leon E, Beasley JM. Implementing a Diet Risk Score (DRS) for Spanish-Speaking Adults in a Clinical Setting: A Feasibility Study. Nutrients. 2024; 16(17):2992. https://doi.org/10.3390/nu16172992
Chicago/Turabian StyleJohnston, Emily A., Maria Torres, John Hansen, Kimberly Ochoa, Daniel Mortenson, Elaine De Leon, and Jeannette M. Beasley. 2024. "Implementing a Diet Risk Score (DRS) for Spanish-Speaking Adults in a Clinical Setting: A Feasibility Study" Nutrients 16, no. 17: 2992. https://doi.org/10.3390/nu16172992
APA StyleJohnston, E. A., Torres, M., Hansen, J., Ochoa, K., Mortenson, D., De Leon, E., & Beasley, J. M. (2024). Implementing a Diet Risk Score (DRS) for Spanish-Speaking Adults in a Clinical Setting: A Feasibility Study. Nutrients, 16(17), 2992. https://doi.org/10.3390/nu16172992






