Comparison of the Nutritional Adequacy of Current Food-Based Very Low Energy Diets: A Review and Nutritional Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion/Exclusion Criteria
2.3. Selection of Menu Plans
2.4. Analysis of Menus
2.5. Assessment of Nutritional Adequacy
2.6. Comparison of Food-Based VLED to Formula VLED
2.7. Data Analysis
3. Results
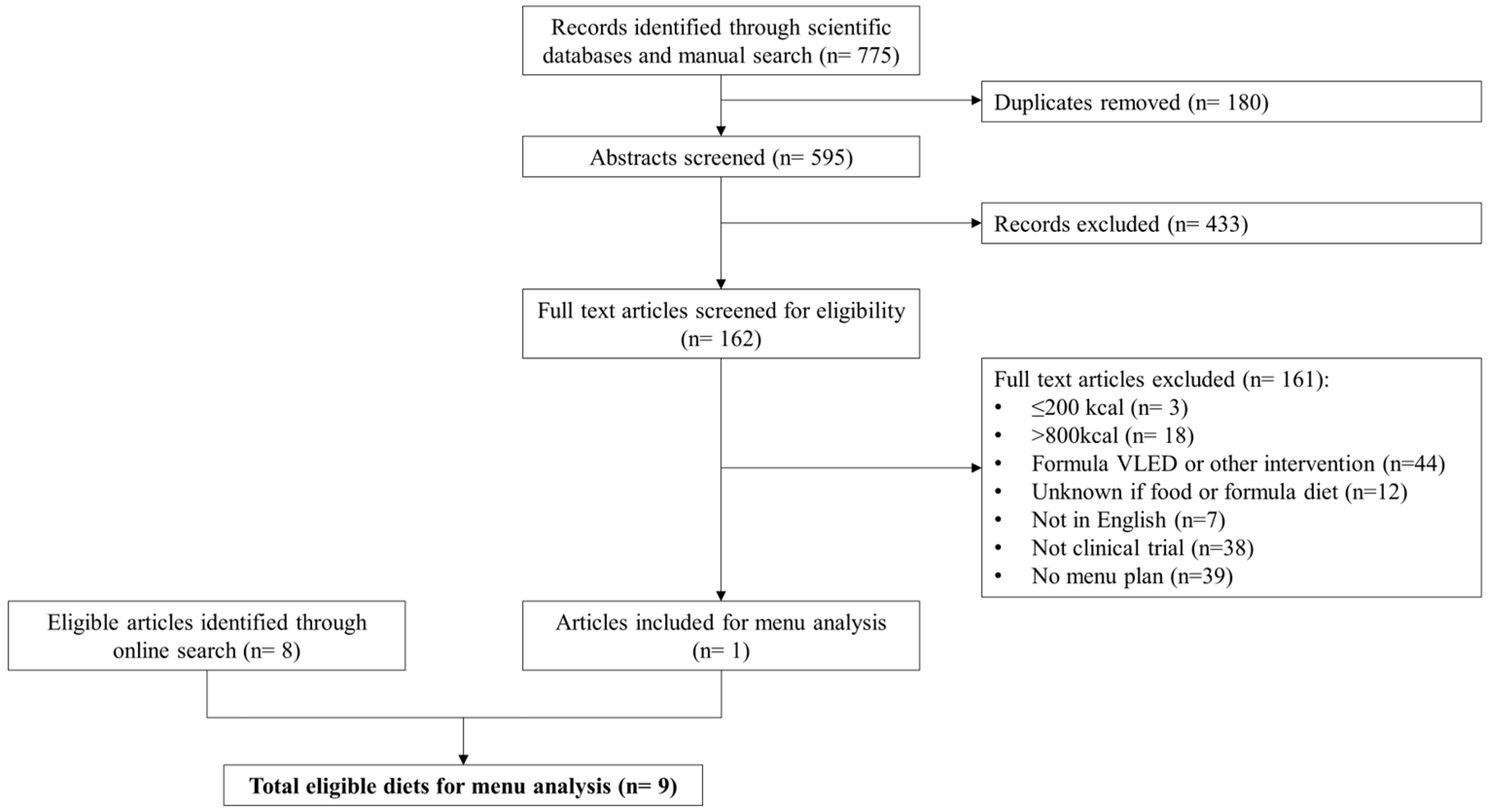
3.1. Diets Identified for Analysis
3.2. Nutrient Composition of the Diets
3.3. Nutritional Adequacy of the Diets (Comparison with Nutrient Reference Values)
3.3.1. Nutrients with EAR Values
3.3.2. Nutrients with AI Values
3.4. Comparison with Standards for Formula VLEDs
3.4.1. Comparison with Codex Alimentarius Standard (CXS 203-1995)
| Nutrient | Optifast® | Mosley 2015 [23] | Bailey 2016 [24] | Baldry 2017 [25] | Mosley 2019 [26] | Bailey 2019 [27] | Myers-Cooke 2020 [28] | Bailey 2021 [29] | Mosley 2021 [30] | Bailey 2022 [31] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | |
| Protein (g) | 8 | 23 | 10 | 25 | 17 | 32 | 25 | 40 | 3 | 18 | 9 | 24 | 0 | 15 | 11 | 26 | 12 | 27 | 17 | 32 |
| Thiamine (mg) | 0.6 | 0.7 | −0.2 | −0.1 | −0.2 | −0.1 | −0.1 | 0 | −0.2 | −0.1 | −0.2 | −0.1 | −0.2 | −0.1 | −0.4 | −0.3 | −0.2 | −0.1 | −0.2 | −0.1 |
| Riboflavin (mg) | 1.2 | 1.4 | −0.2 | 0 | −0.2 | 0 | 1.2 | 1.4 | −0.1 | 0.1 | −0.4 | −0.2 | −0.3 | −0.1 | −0.3 | −0.1 | 0 | 0.2 | −0.1 | 0.1 |
| Niacin equiv. (mg) | 16 | 17 | 19 | 20 | 20 | 21 | 19 | 20 | 11 | 12 | 18 | 19 | 10 | 11 | 21 | 22 | 13 | 14 | 15 | 16 |
| Vitamin C (mg) | 91 | 91 | 282 | 282 | 294 | 294 | 137 | 137 | 149 | 149 | 116 | 116 | 137 | 137 | 184 | 184 | 145 | 145 | 74 | 74 |
| Vitamin B6 (mg) | 1.8 | 1.8 | 1.3 | 1.3 | 0.7 | 0.7 | 0.1 | 0.1 | 0.6 | 0.6 | 0.3 | 0.3 | −0.1 | −0.1 | 0.3 | 0.3 | 0.5 | 0.5 | −0.1 | −0.1 |
| Vitamin B12 (µg) | 1.7 | 1.7 | 0.5 | 0.5 | 1 | 1 | 2.8 | 2.8 | 1.4 | 1.4 | 0.1 | 0.1 | −0.2 | −0.2 | 0.6 | 0.6 | 1.2 | 1.2 | 0.8 | 0.8 |
| Folate equiv. (µg) | 274 | 274 | 237 | 237 | 235 | 235 | 187 | 187 | 113 | 113 | −6 | −6 | 81 | 81 | −24 | −24 | 0 | 0 | 10 | 10 |
| Vitamin A equiv. (µg) | 414 | 539 | 1195 | 1320 | 781 | 906 | 320 | 445 | 177 | 302 | 443 | 568 | 697 | 822 | 238 | 363 | 95 | 220 | 156 | 281 |
| Magnesium (mg) | 116 | 201 | −22 | 63 | −78 | 7 | −57 | 28 | −126 | −41 | −146 | −61 | −150 | −65 | −159 | −74 | −119 | −34 | −144 | −59 |
| Calcium (mg) | 541 | 541 | −18 | −18 | −250 | −250 | 246 | 246 | −449 | −449 | −562 | −562 | −518 | −518 | −546 | −546 | −288 | −288 | −443 | −443 |
| Phosphorus (mg) | 647 | 647 | 595 | 595 | 478 | 478 | 777 | 777 | 378 | 378 | 275 | 275 | 190 | 190 | 331 | 331 | 443 | 443 | 433 | 433 |
| Iron (mg) | 17 | 15 | 13 | 11 | 4 | 2 | 1 | −1 | 4 | 2 | 1 | −1 | 3 | 1 | 1 | −1 | 2 | 0 | 2 | 0 |
| Zinc (mg) | 1.7 | 7.2 | −3.5 | 2 | −5.5 | 0 | −4.3 | 1.2 | −5.7 | −0.2 | −6.9 | −1.4 | −5.8 | −0.3 | −5.8 | −0.3 | −6 | −0.5 | −4.8 | 0.7 |
| Selenium (µg) | 52 | 62 | 12 | 22 | 16 | 26 | 1 | 11 | 6 | 16 | 4 | 14 | −23 | −13 | 6 | 16 | 5 | 15 | 0 | 10 |
| Iodine (µg) | 169 | 169 | −40 | −40 | −9 | −9 | 63 | 63 | 2 | 2 | −51 | −51 | −47 | −47 | −49 | −49 | −17 | −17 | −46 | −46 |
| Nutrient | Optifast® | Mosley 2015 [23] | Bailey 2016 [24] | Baldry 2017 [25] | Mosley 2019 [26] | Bailey 2019 [27] | Myers-Cooke 2020 [28] | Bailey 2021 [29] | Mosley 2021 [30] | Bailey 2022 [31] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | |
| Dietary fiber (g) | −17 | −12 | 1 | 6 | −7 | −2 | −9 | −4 | −12 | −7 | −18 | −13 | −10 | −5 | −13 | −8 | −10 | −5 | −15 | −10 |
| Vitamin E (mg) | 11 | 14 | 11 | 14 | 9 | 12 | −4 | −1 | 4 | 7 | 3 | 6 | 0 | 3 | 4 | 7 | 3 | 6 | 3 | 6 |
| Sodium (mg) | 449–909 | 449–909 | 679–1139 | 679–1139 | 1201–1661 | 1201–1661 | −460 | −460 | 919–1379 | 919–1379 | 122–582 | 122–582 | 39–499 | 39–499 | 591–1051 | 591–1051 | 696–1156 | 696–1156 | 998–1458 | 998–1458 |
| Potassium (mg) | −1084 | −84 | −603 | 397 | −887 | 113 | −681 | 319 | −1273 | −273 | −1735 | −735 | −1696 | −696 | −1605 | −605 | −1512 | −512 | −1556 | −556 |
| Linoleic acid | −11 | −6 | −3 | 2 | −6 | −1 | −10 | −5 | −7 | −2 | −8 | −3 | −9 | −4 | −8 | −3 | −5 | 0 | −5 | 0 |
| α linolenic acid | −0.7 | −0.2 | 1.6 | 2.1 | −0.5 | 0 | −1 | −0.5 | −0.2 | 0.3 | −0.4 | 0.1 | −1 | −0.5 | −0.6 | −0.1 | −0.1 | 0.4 | −0.2 | 0.3 |
| Nutrient | Criteria, Units Per Day | Optifast® | Mosley 2015 [23] | Bailey 2016 [24] | Baldry 2017 [25] | Mosley 2019 [26] | Bailey 2019 [27] | Myers-Cooke 2020 [28] | Bailey 2021 [29] | Mosley 2021 [30] | Bailey 2022 [31] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Energy | 450–800 kcal | 77 | 122 | 124 | 133 | 121 | 96 | 84 | 104 | 131 | 145 |
| Protein | >50 g | 120 | 125 | 137 | 154 | 111 | 122 | 104 | 127 | 128 | 137 |
| Carbohydrate | >50 g | 115 | 101 | 72 | 237 | 66 | 49 | 95 | 63 | 82 | 45 |
| Thiamine | >0.8 mg | 206 | 100 | 100 | 107 | 94 | 95 | 103 | 81 | 94 | 101 |
| Riboflavin | >1.2 mg | 188 | 72 | 76 | 192 | 80 | 62 | 67 | 69 | 92 | 84 |
| Niacin equiv. | >11 mg | 251 | 284 | 289 | 279 | 209 | 273 | 200 | 296 | 223 | 248 |
| Vitamin E | >10 mg | 208 | 206 | 186 | 62 | 141 | 130 | 101 | 143 | 126 | 127 |
| Vitamin B6 | >2 mg | 147 | 122 | 89 | 58 | 83 | 71 | 51 | 72 | 80 | 48 |
| Vitamin B12 | >1 µg | 374 | 252 | 300 | 482 | 339 | 205 | 177 | 255 | 321 | 282 |
| Folate equiv. | >200 µg | 178 | 167 | 167 | 152 | 130 | 94 | 120 | 89 | 96 | 99 |
| Vitamin A equiv. | >600 µg | 173 | 303 | 234 | 157 | 134 | 178 | 220 | 144 | 120 | 130 |
| Sodium | >1000 mg | 137 | 160 | 212 | 81 | 184 | 104 | 96 | 151 | 162 | 192 |
| Potassium | >1600 mg | 170 | 200 | 182 | 195 | 158 | 129 | 131 | 137 | 143 | 140 |
| Magnesium | >350 mg | 133 | 94 | 78 | 84 | 64 | 58 | 57 | 55 | 66 | 59 |
| Calcium | >500 mg | 276 | 164 | 118 | 217 | 78 | 56 | 64 | 59 | 110 | 79 |
| Phosphorus | >500 mg | 245 | 235 | 212 | 271 | 192 | 171 | 154 | 182 | 205 | 203 |
| Iron | >16 mg | 147 | 116 | 61 | 45 | 64 | 43 | 55 | 44 | 52 | 51 |
| Zinc | >6 mg | 229 | 141 | 109 | 128 | 105 | 86 | 104 | 103 | 100 | 120 |
| Iodine | >140 µg | 192 | 43 | 65 | 117 | 73 | 35 | 38 | 37 | 59 | 39 |
| Linoleic acid | >3 g | 76 | 350 | 223 | 84 | 208 | 174 | 118 | 176 | 267 | 283 |
| α linolenic acid | <0.5 g | 123 | 585 | 164 | 70 | 214 | 173 | 64 | 139 | 240 | 218 |
3.4.2. Comparison with Optifast®
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delbridge, E.; Proietto, J. State of the science: VLED (Very Low Energy Diet) for obesity. Asia Pac. J. Clin. Nutr. 2006, 15, 49–54. [Google Scholar] [PubMed]
- Markovic, T.P.; Proietto, J.; Dixon, J.B.; Rigas, G.; Deed, G.; Hamdorf, J.M.; Bessell, E.; Kizirian, N.; Andrikopoulos, S.; Colagiuri, S. The Australian Obesity Management Algorithm: A simple tool to guide the management of obesity in primary care. Obes. Res. Clin. Pract. 2022, 16, 353–363. [Google Scholar] [CrossRef]
- National Health and Medical Research Council. Clinical Practice Guidelines for the Management of Overweight and Obesity in Adults, Adolescents and Children in Australia; National Health and Medical Research Council: Melbourne, VIC, Australia, 2013. Available online: http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/n57_obesity_guidelines_131204_0.pdf (accessed on 25 January 2023).
- Mustajoki, P.; Pekkarinen, T. Very low energy diets in the treatment of obesity. Obes. Rev. 2001, 2, 61–72. [Google Scholar] [CrossRef]
- Codex Alimentarius International Food Standards. Standard for Formula Foods for Use in Very Low Energy Diets for Weight Reduction (CXS 203-1995); Food and Agriculture Organisation of the United Nations, World Health Organization: Rome, Italy, 1995; Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/es/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B203-1995%252FCXS_203e.pdf (accessed on 12 November 2021).
- Purcell, K.; Sumithran, P.; Prendergast, L.A.; Bouniu, C.J.; Delbridge, E.; Proietto, J. The effect of rate of weight loss on long-term weight management: A randomised controlled trial. Lancet Diabetes Endocrinol. 2014, 2, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Harper, C.; Maher, J.; Grunseit, A.; Seimon, R.V.; Sainsbury, A. Experiences of using very low energy diets for weight loss by people with overweight or obesity: A review of qualitative research. Obes. Rev. 2018, 19, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet 2018, 391, 541–551. [Google Scholar] [CrossRef]
- Gibson, A.; Franklin, J.; Pattinson, A.; Cheng, Z.; Samman, S.; Markovic, T.; Sainsbury, A. Comparison of Very Low Energy Diet Products Available in Australia and How to Tailor Them to Optimise Protein Content for Younger and Older Adult Men and Women. Healthcare 2016, 4, 71. [Google Scholar] [CrossRef]
- Scientific Advisory Committee on Nutrition. Carbohydrates and Health. Online: The Stationary Office. 2015. Available online: https://assets.publishing.service.gov.uk/media/5a7f7cc3ed915d74e622ac2a/SACN_Carbohydrates_and_Health.pdf (accessed on 1 May 2023).
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.-C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public. Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Lane, M.; Howland, G.; West, M.; Hockey, M.; Marx, W.; Loughman, A.; O’Hely, M.; Jacka, F.; Rocks, T. The effect of ultra-processed very low-energy diets on gut microbiota and metabolic outcomes in individuals with obesity: A systematic literature review. Obes. Res. Clin. Pract. 2020, 14, 197–204. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Obesity: Identification, Assessment and Management (Clinical Guideline CG189); National Institute for Health and Care Excellence: London, UK, 2014; Available online: https://www.nice.org.uk/guidance/cg189/chapter/Recommendations#dietary-approaches (accessed on 26 January 2023).
- Astrup, A.; Bügel, S. Overfed but undernourished: Recognizing nutritional inadequacies/deficiencies in patients with overweight or obesity. Int. J. Obes. 2019, 43, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.J.; Booth, A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Inf. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council (NHMRC). Australian Dietary Guidelines. Canberra: National Health and Medical Research Council. 2013. Available online: https://www.eatforhealth.gov.au/sites/default/files/content/n55_australian_dietary_guidelines.pdf (accessed on 3 July 2023).
- Food Standards Australia New Zealand. Food Standards Code Online: Food Standards Australia New Zealand. 2021. Available online: https://www.foodstandards.gov.au/code/Pages/default.aspx (accessed on 3 July 2023).
- Food Standards Australia New Zealand. Australian Food Composition Database Canberra; Food Standards: Kingston, ACT, Australia; Wellington, New Zealand, 2022. Available online: https://www.foodstandards.gov.au/science/monitoringnutrients/afcd/pages/default.aspx (accessed on 3 July 2023).
- Australian National Health and Medical Research Council (NHMRC), New Zealand Ministry of Health (MoH). Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes; Commonwealth of Australia: Canberra, NSW, Australia, 2006. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes: Applications in Dietary Assessment; The National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Nestlé Australia Ltd.; OPTIFAST Australia Bestsellers Online: Nestlé Australia Ltd. 2024. Available online: https://www.optifast.com.au/ (accessed on 25 January 2024).
- Mosley, M. The 8-Week Blood Sugar Diet (Australian and New Zealand Edition); Simon & Schuster Australia: Sydney, NSW, Australia, 2015. [Google Scholar]
- Bailey, C.; Schenker, S.; Mosley, M. The 8-Week Blood Sugar Diet Recipe Book; Simon & Schuster Australia: Sydney, NSW, Australia, 2016. [Google Scholar]
- Baldry, E.L.; Aithal, G.P.; Kaye, P.; Idris, I.R.; Bennett, A.; Leeder, P.C.; Macdonald, I.A. Effects of short-term energy restriction on liver lipid content and inflammatory status in severely obese adults: Results of a randomized controlled trial using 2 dietary approaches. Diabetes Obes. Metab. 2017, 19, 1179–1183. [Google Scholar] [CrossRef]
- Mosley, M. The Fast 800: How to Combine Rapid Weight Loss and Intermittent Fasting for Long-Term Health (Australian and New Zealand Edition); Simon & Schuster Australia: London, UK, 2019. [Google Scholar]
- Bailey, C.; Pattison, J. The Fast 800 Recipe Book (Australian and New Zealand Edition); Simon & Schuster Australia: Sydney, NSW, Australia, 2019. [Google Scholar]
- Myers-Cooke, B. The Fast Revolution: The Best of the Best Recipes from Australians #1 Food Site; Myers-Cooke, B., Ed.; HarperCollins Publishers Australia: Sydney, NSW, Australia, 2020. [Google Scholar]
- Bailey, C.; Pattison, J. The Fast 800 Easy (Australian and New Zealand Edition); Simon & Schuster Australia: Sydney, NSW, Australia, 2021. [Google Scholar]
- Mosley, M. The Fast 800 Keto: Eat Well, Burn Fat, Manage Your Weight Long Term; Hachette Australia: Sydney, NSW, Australia, 2021. [Google Scholar]
- Bailey, C. The Fast 800 Keto Recipe Book: Delicious Low-Carb Recipes for Rapid Weight Loss and Long-Term Health; Hachette Australia Pty Limited: Sydney, NSW, Australia, 2022; 255p. [Google Scholar]
- Bistrian, B.R.; Blackburn, G.L.; Flatt, J.P.; Sizer, J.; Scrimshaw, N.S.; Sherman, M. Nitrogen metabolism and insulin requirements in obese diabetic adults on a protein-sparing modified fast. Diabetes 1976, 25, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Palmblad, J.; Rössner, S.; Uden, A.M. Granulocyte functions during treatment of obesity. Int. J. Obes. 1979, 3, 119–122. [Google Scholar]
- Bosello, O.; Ferrari, F.; Tonon, M.; Cigolini, M.; Micciolo, R.; Renoffio, M. Serum thyroid hormone concentration during semi-starvation and physical exercise. Horm. Metab. Res. 1981, 13, 651–652. [Google Scholar] [CrossRef]
- Chan, J.C.; Bartter, F.C. Weight reduction. Renal mineral and hormonal excretion during semistarvation in obese patients. JAMA 1981, 245, 371–373. [Google Scholar] [CrossRef]
- Fisler, J.S.; Drenick, E.J.; Blumfield, D.E.; Swendseid, M.E. Nitrogen economy during very low calorie reducing diets: Quality and quantity of dietary protein. Am. J. Clin. Nutr. 1982, 35, 471–486. [Google Scholar] [CrossRef]
- Iselin, H.U.; Burckhardt, P. Balanced hypocaloric diet versus protein-sparing modified fast in the treatment of obesity: A comparative study. Int. J. Obes. 1982, 6, 175–181. [Google Scholar]
- Linet, O.I.; Butler, D.; Caswell, K.; Metzler, C.; Reele, S.B. Absence of cardiac arrhythmias during a very-low-calorie diet with high biological quality protein. Int. J. Obes. 1983, 7, 313–320. [Google Scholar]
- Bosello, O.; Cominacini, L.; Zocca, I.; Garbin, U.; Davoli, A.; Ferrari, F. High density lipoprotein subfractions during semistarvation in obese women. Ann. Nutr. Metab. 1985, 29, 381–386. [Google Scholar] [CrossRef]
- Hramiak, I.M.; Nisker, J.A. Decreased serum reverse triiodothyronine levels with major weight loss in obese women. Am. J. Obstet. Gynecol. 1985, 151, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Palgi, A.; Read, J.L.; Greenberg, I.; Hoefer, M.A.; Bistrian, B.R.; Blackburn, G.L. Multidisciplinary treatment of obesity with a protein-sparing modified fast: Results in 668 outpatients. Am. J. Public Health 1985, 75, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Wadden, T.A.; Stunkard, A.J.; Brownell, K.D.; Day, S.C. A comparison of two very-low-calorie diets: Protein-sparing-modified fast versus protein-formula-liquid diet. Am. J. Clin. Nutr. 1985, 41, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, R.; Baraldi, G.; Capelli, M.; Patrono, D.; Melchionda, N. Interrelationships between dietary carbohydrates, B cell function and rate of ketogenesis during underfeeding in obese patients. Ann. Nutr. Metab. 1987, 31, 219–230. [Google Scholar] [CrossRef]
- Vandewoude, M.G.; Van Gaal, L.; De Leeuw, I. Changes in vitamin E status during obesity treatment. Ann. Nutr. Metab. 1987, 31, 185–190. [Google Scholar] [CrossRef]
- Wadden, T.A.; Stunkard, A.J.; Day, S.C.; Gould, R.A.; Rubin, C.J. Less food, less hunger: Reports of appetite and symptoms in a controlled study of a protein-sparing modified fast. Int. J. Obes. 1987, 11, 239–249. [Google Scholar]
- Vansant, G.; Van Gaal, L.; Van Acker, K.; De Leeuw, I. Short and long term effects of a very low calorie diet on resting metabolic rate and body composition. Int. J. Obes. 1989, 13 (Suppl. S2), 87–89. [Google Scholar]
- Vermeulen, A. Effects of a short-term (4 weeks) protein-sparing modified fast on plasma lipids and lipoproteins in obese women. Ann. Nutr. Metab. 1990, 34, 133–142. [Google Scholar] [CrossRef]
- Vermeulen, A. Plasma lipid and lipoprotein levels in obese post-menopausal women: Effects of a short-term low-protein diet and exercise. Maturitas 1990, 12, 121–126. [Google Scholar] [CrossRef]
- Phinney, S.D.; Davis, P.G.; Johnson, S.B.; Holman, R.T. Obesity and weight loss alter serum polyunsaturated lipids in humans. Am. J. Clin. Nutr. 1991, 53, 831–838. [Google Scholar] [CrossRef]
- Kawamura, I.I.; Chen, C.C.; Yamazaki, K.; Miyazawa, Y.; Isono, K. A Clinical Study of Protein Sparing Modified Fast (PSMF) Administered Preoperatively to Morbidly Obese Patients: Comparison of PSMF with natural food products to originally prepared PSMF. Obes. Surg. 1992, 2, 33–40. [Google Scholar] [CrossRef]
- Piatti, P.M.; Monti, F.; Fermo, I.; Baruffaldi, L.; Nasser, R.; Santambrogio, G.; Librenti, M.C.; Galli-Kienle, M.; Pontiroli, A.E.; Pozza, G. Hypocaloric high-protein diet improves glucose oxidation and spares lean body mass: Comparison to hypocaloric high-carbohydrate diet. Metabolism 1994, 43, 1481–1487. [Google Scholar] [CrossRef]
- Sakata, T. A very-low-calorie conventional Japanese diet: Its implications for prevention of obesity. Obes. Res. 1995, 3 (Suppl. S2), 233s–239s. [Google Scholar] [CrossRef]
- Summerbell, C.D.; Watts, C.; Higgins, J.P.T.; Garrow, J.S. Randomised controlled trial of novel, simple, and well supervised weight reducing diets in outpatients. BMJ 1998, 317, 1487–1489. [Google Scholar] [CrossRef] [PubMed]
- Bistrian, B.R. Clinical use of a protein-sparing modified fast. JAMA 1978, 240, 2299–2302. [Google Scholar] [CrossRef]
- Chang, J.; Kashyap, S.R. The protein-sparing modified fast for obese patients with type 2 diabetes: What to expect. Cleve Clin. J. Med. 2014, 81, 557–565. [Google Scholar] [CrossRef]
- Shlisky, J.; Mandlik, R.; Askari, S.; Abrams, S.; Belizan, J.M.; Bourassa, M.W.; Cormick, G.; Driller-Colangelo, A.; Gomes, F.; Khadilkar, A.; et al. Calcium deficiency worldwide: Prevalence of inadequate intakes and associated health outcomes. Ann. N. Y. Acad. Sci. 2022, 1512, 10–28. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, R. Magnesium metabolism and its disorders. Clin. Biochem. Rev. 2003, 24, 47–66. [Google Scholar] [PubMed]
- Zimmermann, M.B.; Jooste, P.L.; Pandav, C.S. Iodine-deficiency disorders. Lancet 2008, 372, 1251–1262. [Google Scholar] [CrossRef]
- Stiles, L.I.; Ferrao, K.; Mehta, K.J. Role of zinc in health and disease. Clin. Exp. Med. 2024, 24, 38. [Google Scholar] [CrossRef]
- Saper, R.B.; Rash, R. Zinc: An essential micronutrient. Am. Fam. Physician 2009, 79, 768–772. [Google Scholar]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Black, C.J.; Staudacher, H.M.; Ford, A.C. Efficacy of a low FODMAP diet in irritable bowel syndrome: Systematic review and network meta-analysis. Gut 2022, 71, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
| D/SDD Criterion | Probability of Correct Conclusion | Interpretation 1 |
|---|---|---|
| >2.00 | 98% | High (A) |
| >1.65 | 95% | High (A) |
| >1.50 | 93% | High (A) |
| >1.00 | 85% | High (A) |
| >0.05 | 70% | Moderate (A) |
| >0.00 | 50% | Low (A) or (I) |
| <−0.50 | 70% | Moderate (I) |
| <−1.00 | 85% | High (I) |
| <−1.50 | 93% | High (I) |
| <−1.65 | 95% | High (I) |
| <−2.00 | 98% | High (I) |
| Author | Title | Description |
|---|---|---|
| Mosley 2015 [23] | The 8-Week Blood Sugar Diet (Australian and New Zealand Edition) | Self-described as a low-carbohydrate Mediterranean style diet. Minimize or avoid sugar, treats, desserts, sweet drinks; white starchy grains/cereals (e.g., bread, pasta, potatoes, rice); tropical fruits and margarine. Swap these for wholegrain varieties, low-carbohydrate fruits (e.g., berries, apples) and butter. Encourage full-fat dairy. Allow wine or spirits in moderation but not beer [23]. |
| Bailey 2016 [24] | The 8-Week Blood Sugar Diet Recipe Book | Recipes and menus based on [23]. |
| Baldry 2017 [25] | Derby Teaching Hospitals, NHS Foundation Trust Standard Pre-Op Diet for Bariatric Surgery | Three meals per day. No sugar or oils/fats allowed. All foods need to be weighed. |
| Mosley 2019 [26] | The Fast 800: How to Combine Rapid Weight Loss and Intermittent Fasting for Long-Term Health (Australian and New Zealand Edition) | Self-described as a low-carbohydrate Mediterranean style diet. Minimize or avoid white starchy grains/cereals (e.g., bread, pasta, potatoes, rice), tropical fruits and processed foods. Swap these for wholegrains and pulses, low-carbohydrate fruits (e.g., berries, apples). Encourage “natural healthy fats” (e.g., olive oil, salmon, tuna, full-fat dairy, avocado, nuts and seeds) and plenty of green and colored vegetables. Discourage snacks and grazing. No alcohol [26]. |
| Bailey 2019 [27] | The Fast 800 Recipe Book (Australian and New Zealand Edition) | Recipes and suggested menus based on [26]. |
| Myers-Cooke 2020 [28] | The Fast Revolution: the best of the best recipes from Australians’ #1 food site | A compilation of recipes categorized as 100 kcal, 250 kcal and 500 kcal. Main focus is caloric content; no particular dietary pattern or nutritional focus otherwise. Readers create their own menu plans from combinations of the recipe categories, e.g., Option A: 500 kcal + 250 kcal + 100 kcal; Option B: 250 kcal + 250 kcal + 250 kcal |
| Bailey 2021 [29] | The Fast 800 Easy (Australian and New Zealand Edition) | Self-described as a “lowish” carbohydrate Mediterranean style diet using easily accessible foods found in pantry or freezer, not necessarily fresh produce. Discourages starchy and processed foods, sugars, sweeteners. Encourages protein, non-starchy vegetables, low-carbohydrate fruits, plant-based fats (e.g., canola, olive oil), full-fat dairy and grass-fed meats. |
| Mosley 2021 [30] | The Fast 800 Keto: Eat Well, Burn Fat, Manage Your Weight Long-Term | Similar to [23,26], except aims for protein >50 g/day and carbohydrate <50 g/day. Protein at every meal, in moderation, 2 or 3 meals per day. |
| Bailey 2022 [31] | The Fast 800 Keto Recipe Book: Delicious Low-Carb Recipes for Rapid Weight Loss And Long-Term Health | Recipes and suggested menus based on [30]. |
| Nutrient | Optifast® | Mosley 2015 [23] | Bailey 2016 [24] | Baldry 2017 [25] | Mosley 2019 [26] | Bailey 2019 [27] | Myers-Cooke 2020 [28] | Bailey 2021 [29] | Mosley 2021 [30] | Bailey 2022 [31] |
|---|---|---|---|---|---|---|---|---|---|---|
| Energy (MJ) | 2.6 | 4.1 | 4.2 | 4.4 | 4.1 | 3.2 | 2.8 | 3.5 | 4.4 | 4.8 * |
| Energy (kcal) | 619 | 975 | 991 | 1062 | 969 | 771 | 676 | 829 | 1044 | 1157 * |
| Protein (g) | 60 | 62 | 69 | 77 * | 55 | 61 | 52 | 63 | 64 | 69 |
| Total fat (g) | 15 | 49 | 59 | 24 | 64 | 45 | 26 | 46 | 65 | 85 * |
| - saturated (g) | 4 | 12 | 16 | 8 | 20 | 11 | 7 | 10 | 20 | 24 * |
| - poly (g) | 1 | 14 * | 8 | 4 | 8 | 7 | 4 | 7 | 10 | 10 |
| - mono (g) | 2 | 19 | 29 | 9 | 31 | 23 | 12 | 25 | 29 | 45 * |
| Carbohydrate (g) | 57 | 51 | 36 | 118 * | 33 | 25 | 47 | 32 | 41 | 23 |
| Dietary fiber (g) | 13 | 31 * | 23 | 21 | 18 | 12 | 20 | 17 | 20 | 15 |
| Thiamine (mg) | 1.6 * | 0.8 | 0.8 | 0.9 | 0.8 | 0.8 | 0.8 | 0.6 | 0.8 | 0.8 |
| Riboflavin (mg) | 2.3 * | 0.9 | 0.9 | 2.3 * | 1 | 0.7 | 0.8 | 0.8 | 1.1 | 1 |
| Niacin equiv. (mg) | 28 | 31 | 32 | 31 | 23 | 30 | 22 | 33 * | 25 | 27 |
| Vitamin C (mg) | 121 | 312 | 324 * | 167 | 179 | 146 | 167 | 214 | 175 | 104 |
| Vitamin E (mg) | 21 * | 21 * | 19 | 6 | 14 | 13 | 10 | 14 | 13 | 13 |
| Vitamin B6 (mg) | 2.9 * | 2.4 | 1.8 | 1.2 | 1.7 | 1.4 | 1 | 1.4 | 1.6 | 1 |
| Vitamin B12 (µg) | 3.7 | 2.5 | 3 | 4.8 * | 3.4 | 2.1 | 1.8 | 2.6 | 3.2 | 2.8 |
| Folate equiv. (µg) | 594 * | 557 | 555 | 507 | 433 | 314 | 401 | 296 | 320 | 330 |
| Vitamin A equiv. (µg) | 1039 | 1820 * | 1406 | 945 | 802 | 1068 | 1322 | 863 | 720 | 781 |
| Sodium (mg) | 1369 | 1599 | 2121 * | 813 | 1839 | 1042 | 959 | 1511 | 1616 | 1918 |
| Potassium (mg) | 2716 | 3197 * | 2913 | 3119 | 2527 | 2065 | 2104 | 2195 | 2288 | 2244 |
| Magnesium (mg) | 466 * | 328 | 272 | 293 | 224 | 204 | 200 | 191 | 231 | 206 |
| Calcium (mg) | 1381 * | 822 | 590 | 1086 | 391 | 278 | 322 | 294 | 552 | 397 |
| Phosphorus (mg) | 1227 | 1175 | 1058 | 1357 * | 958 | 855 | 770 | 911 | 1023 | 1013 |
| Iron (mg) | 23 * | 19 | 10 | 7 | 10 | 7 | 9 | 7 | 8 | 8 |
| Zinc (mg) | 13.7 * | 8.5 | 6.5 | 7.7 | 6.3 | 5.1 | 6.2 | 6.2 | 6 | 7.2 |
| Selenium (µg) | 112 * | 72 | 76 | 61 | 66 | 64 | 37 | 66 | 65 | 60 |
| Iodine (µg) | 269 * | 60 | 91 | 163 | 102 | 49 | 53 | 51 | 83 | 54 |
| Linoleic acid(g) | 2 | 10 * | 7 | 3 | 6 | 5 | 4 | 5 | 8 | 8 |
| α linolenic acid (g) | 0.6 | 2.9 * | 0.8 | 0.3 | 1.1 | 0.9 | 0.3 | 0.7 | 1.2 | 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poon, S.W.Y.; Brown, R.M.; Sumithran, P. Comparison of the Nutritional Adequacy of Current Food-Based Very Low Energy Diets: A Review and Nutritional Analysis. Nutrients 2024, 16, 2993. https://doi.org/10.3390/nu16172993
Poon SWY, Brown RM, Sumithran P. Comparison of the Nutritional Adequacy of Current Food-Based Very Low Energy Diets: A Review and Nutritional Analysis. Nutrients. 2024; 16(17):2993. https://doi.org/10.3390/nu16172993
Chicago/Turabian StylePoon, Shirley Wing Yan, Robyn Mary Brown, and Priya Sumithran. 2024. "Comparison of the Nutritional Adequacy of Current Food-Based Very Low Energy Diets: A Review and Nutritional Analysis" Nutrients 16, no. 17: 2993. https://doi.org/10.3390/nu16172993
APA StylePoon, S. W. Y., Brown, R. M., & Sumithran, P. (2024). Comparison of the Nutritional Adequacy of Current Food-Based Very Low Energy Diets: A Review and Nutritional Analysis. Nutrients, 16(17), 2993. https://doi.org/10.3390/nu16172993






