Abstract
Rice bran, which is abundant in dietary fiber and phytochemicals, provides multiple health benefits. Nonetheless, its effects on neuroinflammation and gut microbiota in postmenopausal conditions are still not well understood. This study investigated the effects of rice bran and/or tea seed oil supplementation in d-galactose-injected ovariectomized (OVX) old mice fed a fructose drink. The combination of d-galactose injection, ovariectomy, and fructose drink administration creates a comprehensive model that simulates aging in females under multiple metabolic stressors, including oxidative stress, estrogen deficiency, and high-sugar diets, and allows the study of their combined impact on metabolic disorders and related diseases. Eight-week-old and 6–8-month-old female C57BL/6 mice were used. The mice were divided into six groups: a sham + young mice, a sham + old mice, an OVX + soybean oil, an OVX + soybean oil with rice bran, an OVX + tea seed oil (TO), and an OVX + TO with rice bran diet group. The OVX groups were subcutaneously injected with d-galactose (100 mg/kg/day) and received a 15% (v/v) fructose drink. The rice bran and tea seed oil supplementation formed 10% of the diet (w/w). The results showed that the rice bran with TO diet increased the number of short-chain fatty acid (SCFA)-producing Clostridia and reduced the number of endotoxin-producing Tannerellaceae, which mitigated imbalances in the gut–liver–brain axis. Rice bran supplementation reduced the relative weight of the liver, levels of hepatic triglycerides and total cholesterol; aspartate transaminase and alanine aminotransferase activity; brain levels of proinflammatory cytokines, including interleukin-1β and tumor necrosis factor-α; and plasma 8-hydroxy-2-deoxyguanosine. This study concludes that rice bran inhibits hepatic fat accumulation, which mitigates peripheral metaflammation and oxidative damage and reduces neuroinflammation in the brain.
1. Introduction
The incidences of neurodegenerative diseases and metabolic syndrome are higher in women after menopause [1,2,3]. The changes in sex hormones that occur during menopause, including the decrease in the amount of estrogen produced by the ovaries, impairs the utilization of glucose in the brain and triggers neuroinflammation, leading to cognitive dysfunction [4,5,6]. The effects of estrogen in skeletal muscle, liver, adipose tissue, and immune cells are associated with insulin sensitivity and the prevention of lipid accumulation and inflammation [7]. An increasing number of studies have attempted to slow the progression of neurodegenerative diseases and metabolic syndrome by altering the composition of the gut microbiota and their metabolites, such as short-chain fatty acids (SCFAs) [8,9]. When low-grade inflammation is induced by metabolic diseases, which often occurs in women after menopause and is known as metaflammation, the dominant gut microbiota change, with the changes that occur being influenced by the specific disease, and intestinal permeability increases, enabling harmful bacteria and toxins from the lumen to translocate into the bloodstream more easily [10,11,12]. Once these toxins enter the bloodstream, they bind to cluster of differentiation 14 (CD14) on macrophages and monocytes and are recognized by toll-like receptor 4 (TLR4), which exacerbates metaflammation and promotes liver damage, leading to a vicious cycle of hyperglycemia and dyslipidemia [13,14,15,16]. Additionally, the free fatty acids and cytokines released because of abnormal blood sugar levels and a fatty liver cross the blood–brain barrier and trigger immune responses by the microglia and astrocytes in the brain, causing the chronic secretion of reactive oxidative species, NO, and proinflammatory cytokines, which further induce neural insulin resistance and synaptic loss and contribute to the impairment of cognition [15,17,18].
The Mediterranean–Dietary Approaches to Stop Hypertension (DASH) Intervention for Neurodegenerative Delay (MIND) diet was proposed in 2015. A high level of adherence to this diet among patients with an average age of 81.4 years was reported to have delayed the worsening of global cognitive function by 7.5 years [19]. The MIND diet score was also reported to be inversely associated with high-density lipoprotein levels and the risk of general obesity in Iranian adults [20]. A review of the association between dietary patterns and dyslipidemia also revealed that consumption of a DASH diet was associated with a lower risk of metabolic dyslipidemia [21]. Olive oil and whole grains are two of the ten categories of foods emphasized in the MIND. Olive oil and whole grain consumption hinders the progression of neurodegenerative diseases and metabolic syndrome [22,23,24]. Similarly to olive oil, tea seed oil is abundant in monounsaturated fatty acids (MUFAs) [25]. In an AlCl3-induced Alzheimer disease rat model, treatment with tea seed oil (1.5 or 3 mg/kg/day) through oral gavage for 8 weeks significantly improved cognitive performance in the Morris water maze by inhibiting the production of malondialdehyde, interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α. Additionally, the dominant gut microbiota shifted from Enterobacteriaceae to Lactobacillus after the tea seed oil intervention [26]. Our previous study demonstrated that tea seed oil consumption prevented obesity in ovariectomized (OVX) mice with high-fat diet (HFD)-induced obesity [27]. In addition, tea seed oil was discovered to relieve the effects of metabolic disorders and oxidative stress in rats fed an HFD or high-fructose diet [28]. Similarly to whole grains, rice bran contains numerous phytochemicals [29]. Among them, γ-oryzanol is most often used to ameliorate neuroinflammation and fat accumulation in the liver [30,31]. To date, no study on the effects of tea seed oil and rice bran on multifactorial menopausal disorders exists. Therefore, the present study investigated the effects of rice bran and tea seed oil supplementation in old d-galactose-injected OVX mice fed a fructose drink for 8 weeks.
2. Materials and Methods
2.1. Animals
Female C57BL/6 mice aged 8 weeks or 6–8 months were purchased from BioLASCO (Yilan, Taiwan). The mice were housed in a temperature- (22 ± 2 °C) and humidity-controlled (50 ± 10%) environment with a 12 h light/dark cycle and had free access to food and water. The experimental procedure was approved by the Institutional Animal Care and Use Committee of Taipei Medical University, Taipei, Taiwan (LAC-2020-0161).
2.2. Experimental Design
Eight mice aged 8 weeks and 8 mice aged 6–8 months underwent a sham operation, and 32 mice aged 6–8 months were OVX. After 3 weeks of recovery from surgery, the animals were divided into the following six groups: sham + young mice (SY), sham + old mice (SO), OVX + soybean oil diet (OS), OVX + soybean oil with rice bran diet (OSR), OVX + tea seed oil diet (OT), and OVX + tea seed oil with rice bran diet (OTR). When the intervention commenced, the mice were fed a modified AIN-93M diet that included soybean oil, soybean oil with rice bran, tea seed oil, or tea seed oil with rice bran for 8 weeks. The mice in the OVX groups were injected subcutaneously with d-galactose (100 mg/kg body weight; Sigma-Aldrich, St. Louis, MO, USA) and provided with a 15% (v/v) fructose drink for 8 weeks. Each group’s diet was a modified version of the AIN-93M diet with 10% (w/w) rice bran and/or tea seed oil added in the intervention groups, with the proportion of added bran or oil determined on the basis of other studies [32,33]. Rice bran is obtained from a blend of Koshihikari and Hitomebore semi-defatted rice bran from Japan. The rice bran contained 22% carbohydrates, 18.5% protein, 9.7% fat, and 34.6% dietary fiber. The active compounds in rice bran included total polyphenols (5.5 mg/g), ferulic acid (115 μg/g), and γ-oryzanol (119.1 μg/g). The soybean and tea seed (from Camellia Oleifera) oils were produced by Taiwan Sugar Corporation and purchased from the supermarket. The composition of the diets is presented in Supplementary Table S1.
2.3. Biochemical Assay and Enzyme-Linked Immunosorbent Assay
At the end of the experiment, the mice were anesthetized by the intraperitoneal injection of 40 mg/kg Zoletil (Virbac, Carros, France) plus 20 mg/kg Rompun (Bayer, Leverkusen, Germany) and sacrificed using cardiac puncture. Their brains, plasma, and livers were collected and stored at −80 °C until analysis. Plasma glucose, triglyceride (TG), total cholesterol (TC), aspartate transaminase (AST), and alanine aminotransferase (ALT) levels were determined using an automated biochemistry analyzer. The insulin and oxidative damage marker (8-hydroxy-2-deoxyguanosine (8-OHdG)) levels in the plasma, liver lipid (TG and TC) levels, and proinflammatory cytokine (IL-1β, IL-6, and TNF-α) levels in the frontal cortex of the brain were measured using a commercial enzyme-linked immunosorbent assay kit (insulin: Cat. No. 10-1249-01, Mercodia, Winston Salem, NC, USA; 8-OHdG: Lot No. D61YXN2J1T, Elabscience Biotechnology, Houston, TX, USA; TG: Lot No. 448536, Randox Laboratories, County Antrim, UK; TC: Lot No. 497567, Randox Laboratories, County Antrim, UK; IL-1β: Lot No. 8M9PKY4UB8, Elabscience Biotechnology, TX, USA; IL-6: Lot No. 8PZ7G6GLQ4, Elabscience Biotechnology, TX, USA; TNF-α: Lot No. QH1HWHCV1M, Elabscience Biotechnology, TX, USA). Insulin resistance was calculated using the homeostatic model assessment for insulin resistance (HOMA-IR) according to the following formula: glucose (mg/dL) × insulin (µg/L)]/405.
2.4. Morris Water Maze
The Morris water maze task was performed as previously described [34]. The setup included a circular pool (100 cm in diameter) filled with water that was maintained at 22 ± 2 °C. A transparent platform was positioned in the southwest quadrant of the pool and submerged 0.5–1 cm below the water surface. During the acquisition trial of the task, the mice were trained to locate the platform for 5 days and completed four trials per day, with each lasting 60 s. The time required for the mice to locate the platform was recorded as their escape latency. The mice that could not locate the platform within 60 s were placed by the experimenter on the platform, where they stayed for 15 s. On the sixth day, the platform was removed, and a probe trial was conducted. Each mouse was allowed to explore for 30 s before several parameters, including the time spent in and the path within the target quadrant, were measured.
2.5. 16S rRNA Sequencing
Extracted cecal DNA was subjected to amplification, which was completed using a universal V3–V4 primer and a thermocycler (Applied Biosystems 2720, Thermo Fisher Scientific, Vacaville, CA, USA) followed by PCR purification (Agencourt AMPure XP, Beckman Coulter, Illumina, San Diego, CA, USA). Microbiota were classified with reference to the SILVA v132 taxonomy database (https://www.arb-silva.de, accessed on 30 September 2022) and by using the DADA2 workflow (https://github.com/benjjneb/dada2, accessed on 30 September 2022). Differential features were identified using the linear discriminant analysis (LDA) effect size (LDA values of >3.0 were used as thresholds).
2.6. Short-Chain Fatty Acid Analysis
Fecal samples were suspended in 1 mL of water with 0.5% phosphoric acid (CAS No.: 7664-38-2, Honeywell Specialty Chemicals Seelze, Seelze, Germany) and extracted using ethyl acetate (CAS No.: 141-78-6, Macron Fine Chemicals, Radnor, PA, USA). The organic phase was analyzed using an apparatus comprising a gas chromatograph (7820A GC, Agilent, Santa Clara, CA, USA), a column (Nukol Capillary Column, Supelco, Bellefonte, PA, USA), a mass spectrometer (5977B EI MSD, Agilent, Santa Clara, CA, USA) and the Agilent MassHunter Workstation software version 10.0.368 (Agilent, Santa Clara, CA, USA). We injected 1 µL of the standard (Volatile Free Acid Mix, Lot No. LRAC0113, Sigma-Aldrich, St. Louis, MO, USA) or sample into the gas chromatograph, which was separated using nitrogen as the mobile phase. The separated substances were then broken into ionized fragments and passed through a detector for recognition based on their mass-to-charge ratios (m/z ratios). Short-chain fatty acids were identified and calculated on the basis of the retention times of the standard.
2.7. Statistical Analysis
Statistical analysis was performed using GraphPad Prism (Version 9, GraphPad Software, San Diego, CA, USA), and the data are presented as means ± standard errors of the mean. A t-test was used to compare the SO or OS group with the SY group, and one-way analysis of variance followed by Tukey’s test was used to identify significant differences between the OVX groups. Correlations of the brain and liver biomarkers with specific microbiota in the gut were analyzed using Pearson’s correlation. A p value of <0.05 indicated significance.
3. Results
3.1. Effects of Rice Bran and Tea Seed Oil Supplementation on Body and Uterine Weight in Ovariectomized Old Mice Fed a Fructose Drink
With the exception of those in the SY group, the mice had similar body weights at baseline (Figure 1A). After d-galactose and the fructose drink treatment was administered for 8 weeks, the final average body weights in the OVX groups did not differ significantly (Figure 1B). However, the OSR mice exhibited greater proportional weight gain (52.3 ± 4.3%) than did the OT mice (33.5 ± 3.2%) at the end of the study (Figure 1C). As presented in Figure 1D, the relative uterine weight of the OVX groups decreased significantly, indicating that the bilateral removal of the ovaries was successful. Compared with those in the SY groups, the mice in the OVX groups had a lower relative uterine weight.
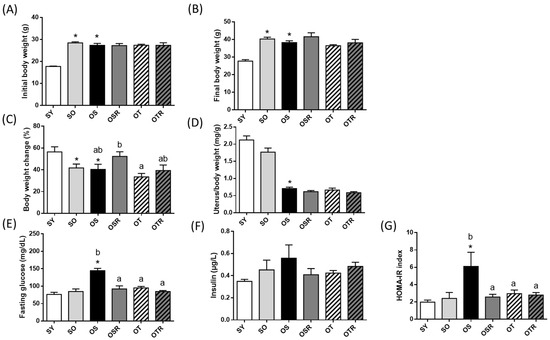
Figure 1.
Effects of rice bran and tea seed oil supplementation on body and uterine weight. (A) Initial body weight, (B) final body weight, (C) body weight change during the experiment, (D) relative uterine weight, (E) fasting glucose, (F) insulin, and (G) HOMA-IR index. HOMA-IR: homeostatic model assessment for insulin resistance; SY: sham + young mice; SO: sham + old mice; OS: OVX mice + soybean oil diet; OSR: OVX mice + soybean oil with rice bran diet; OT: OVX mice + tea seed oil diet; OTR: OVX + tea seed oil with rice bran diet; OVX: ovariectomized. Values are presented as the mean ± SEM (n = 8). Asterisks (*) indicate significant differences between the SO or OS group compared with the SY group. Different letters indicate significant differences between the OVX groups.
3.2. Effects of Rice Bran and Tea Seed Oil Supplementation on Glucose Utilization and Insulin Resistance in Ovariectomized Old Mice Fed a Fructose Drink
The fasting glucose levels in the OS group (144.4 ± 6.8 mg/dL) were higher than those in the two sham operation groups (76.3 ± 6.0 mg/dL for the SY group and 84.7 ± 7.5 mg/dL for the SO group). Supplementation with rice bran, tea seed oil, or both was associated with reductions in fasting glucose levels of 36.1%, 34.2%, and 41.3%, respectively (Figure 1E). However, insulin levels did not differ significantly between the groups (Figure 1F). The HOMA-IR index is generally used to determine the presence of insulin resistance [35]. The HOMA-IR index was significantly greater in the OS group (6.1 ± 1.6) than in the SY group (2.0 ± 0.2). Among the OVX groups, the OSR and OTR groups had lower HOMA-IR index values, indicating they had lower insulin resistance (2.6 ± 0.3 and 2.8 ± 0.3, respectively; Figure 1G).
3.3. Effects of Rice Bran and Tea Seed Oil Supplementation on Lipid Accumulation in Ovariectomized Old Mice Fed a Fructose Drink
Compared with the SY mice, the SO and OS groups had significantly lower plasma TG levels and significantly higher liver TG levels (Figure 2A,C). The liver TG levels were higher in the OT group than in the OS group (Figure 2C). The rice-bran-fed mice in the OSR and OTR groups had lower liver TG levels and lower relative liver weights than did those in the OS and OT groups, respectively (Figure 2C,E). When tea seed oil rather than soybean oil was administered for 8 weeks, hepatic TC level and AST and ALT activities were significantly higher in the groups fed tea seed oil than in those fed soybean oil (Figure 2D,F,G). Compared with the OT group, the OTR group had significantly lower levels of hepatic TG and TC and lower AST and ALT activity (Figure 2C,D,F,G).
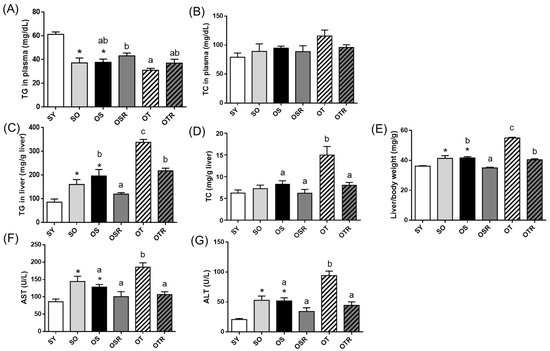
Figure 2.
Effects of rice bran and tea seed oil supplementation on lipid levels and liver function. Plasma (A) TG and (B) TC and hepatic (C) TG and (D) TC levels; (E) relative liver weight; and (F) AST and (G) ALT activities. SY: sham + young mice; SO: sham + old mice; OS: OVX mice + soybean oil diet; OSR: OVX mice + soybean oil with rice bran diet; OT: OVX mice + tea seed oil diet; OTR: OVX mice + tea seed oil with rice bran diet; TG: triglyceride; TC: total cholesterol; AST: aspartate transaminase; ALT: alanine transaminase; OVX: ovariectomized. Values are presented as the mean ± SEM (n = 8). Asterisks (*) indicate significant differences between the SO or OS group compared with the SY group. Different letters indicate significant differences between the OVX groups.
3.4. Effects of Rice Bran and Tea Seed Oil Supplementation on Peripheral Oxidative Damage and Neuroinflammation in Ovariectomized Old Mice Fed a Fructose Drink
Peripheral oxidative damage was evaluated by measuring the levels of DNA oxidation products. The level of 8-OHdG was higher in the OS group (4.3 ± 0.6 ng/mL) than in the SY group (2.6 ± 0.4 ng/mL). Compared with the OT groups (6.5 ± 0.9 ng/mL), the OTR groups exhibited significantly lower 8-OHdG production (1.7 ± 0.5 ng/mL; Figure 3A). Compared with the SY group, the SO and OS groups had higher levels of brain IL-1β, IL-6, and TNF-α (SO: 1.7, 2.2, and 1.1 times higher, respectively; OS: 1.9, 2.4, and 1.2 times higher, respectively; Figure 3B–D). When tea seed oil rather than soybean oil was administered for 8 weeks, the levels of IL-1β, IL-6, and TNF-α in the OT group were 441%, 310%, and 299% higher, respectively, than those in the OS group (Figure 3B–D). The OTR group had lower levels of brain IL-1β and TNF-α than did the OT group (Figure 3B,D).
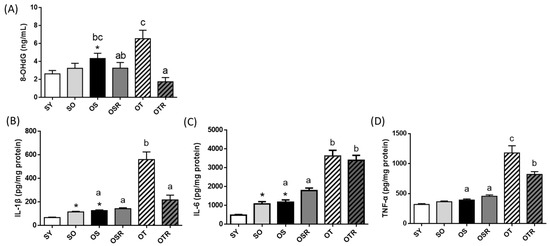
Figure 3.
Effects of rice bran and tea seed oil supplementation on the (A) concentration of 8-OHdG in plasma and the levels of (B) IL-1β, (C) IL-6, and (D) TNF-α in the frontal cortex of the brain. SY: sham + young mice; SO: sham + old mice; OS: OVX mice + soybean oil diet; OSR: OVX mice + soybean oil with rice bran diet; OT: OVX mice + tea seed oil diet; OTR: OVX mice + tea seed oil with rice bran diet; 8-OHdG: 8-hydroxy-2-deoxyguanosine; OVX: ovariectomized; IL: interleukin; TNF-α: tumor necrosis factor-α. Values are presented as the mean ± SEM (n = 6–7). Asterisks (*) indicate significant differences between the SO or OS group compared with the SY group. Different letters indicate significant differences between the OVX groups.
3.5. Effects of Rice Bran and Tea Seed Oil Supplementation on Cognition in Ovariectomized Old Mice Fed a Fructose Drink
In the Morris water maze acquisition trial, OS mice took longer to find the platform on day 4 (16.6 ± 2.6 s) compared with SY mice (5.6 ± 0.4 s). The escape latency during the acquisition trial did not differ significantly among the different OVX groups (Figure 4A). Mice aged 6–8 months may already be at an age where it is difficult to induce noticeable aging effects; this may explain why there was no significant difference in cognitive function compared with age-matched mice that did not undergo OVX and d-galactose treatment. During the probe trial, the time spent and the path lengths in the target quadrant did not differ significantly among the OVX groups (Figure 4B,C). There are two possible explanations. First, the natural aging process may have already triggered age-related changes in these mice, making it more difficult to enhance cognitive function through interventions. Second, the dosage used in this study might be insufficient to improve cognitive function in this multi-factor-induced animal model.
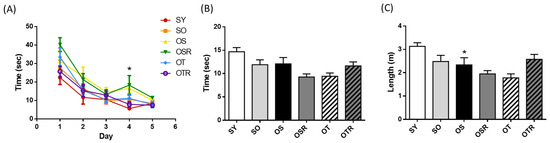
Figure 4.
Effects of rice bran and tea seed oil supplementation on cognitive performance in the Morris water maze. (A) Escape latency during the acquisition trial and the (B) time spent and (C) path length in the target quadrant during the probe trial after the platform removal. SY: sham + young mice; SO: sham + old mice; OS: OVX mice + soybean oil diet; OSR: OVX mice + soybean oil with rice bran diet; OT: OVX mice + tea seed oil diet; OTR: OVX mice + tea seed oil with rice bran diet; OVX: ovariectomized. Values are presented as the mean ± SEM (n =6–7). Asterisks (*) indicate significant differences between the SO or OS group compared with the SY group.
3.6. Effects of Rice Bran and Tea Seed Oil Supplementation on Gut Microbial Composition and Metabolites in Ovariectomized Old Mice Fed a Fructose Drink
We analyzed the gut microbiota composition of the mice. The alpha diversity index values of richness (ACE and Chao1) and richness and evenness (Shannon) were significantly lower in the SO and OS groups than in the SY group. The ACE, Chao1, and Shannon index values were significantly higher in the OSR and OTR groups than in the OS and OT groups, respectively (Figure 5A). The differences in the gut microbiota among the groups were analyzed. Regarding the relative abundance of the gut microbiome at the family level (Figure 5B), in the SO and OS groups, the relative abundance of Clostridia was lower than that in the SY group. The OSR and OTR groups exhibited a significantly higher relative abundance of Clostridia (60.0 ± 2.5% and 61.1 ± 2.0%, respectively) than did the OS and OT groups (47.8 ± 4.1% and 47.2 ± 1.9%, respectively; Figure 5D). Moreover, the OSR and OTR groups exhibited a lower relative abundance of Tannerellaceae than did the OS and OT groups, respectively (Figure 5C,D).
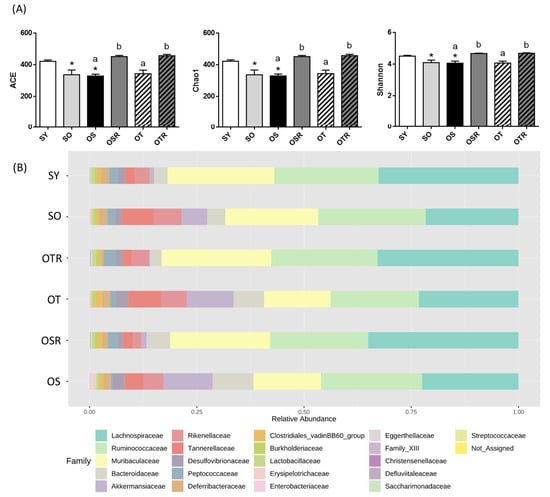
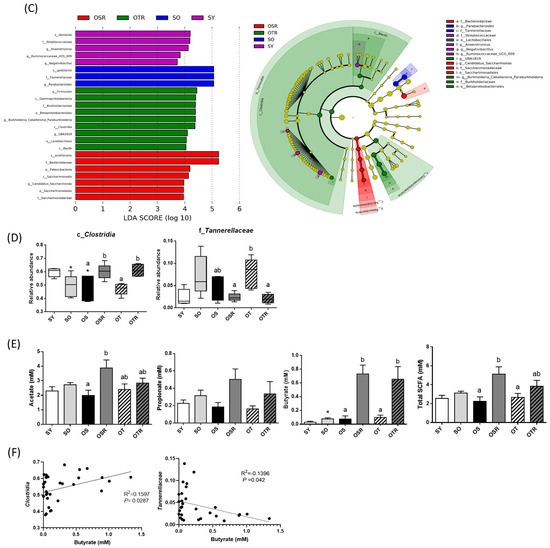
Figure 5.
Effects of rice bran and tea seed oil supplementation on gut microbial composition and short-chain fatty acid levels. (A) Alpha diversity of the gut microbiota. (B) The relative abundance of gut microbiota at the family level. (C) Greatest differences in the gut microbiota between the different groups. Only taxa with a significant LDA threshold value of >3 are shown. (D) Relative abundance of c_Clostridia and f_Tannerellaceae. (E) Short-chain fatty acids (acetate, propionate, and butyrate). (F) The correlation between butyrate and Clostridia and Tannerellaceae. LDA: linear discriminant analysis; SY: sham + young mice; SO: sham + old mice; OS: OVX mice + soybean oil diet; OSR: OVX mice + soybean oil with rice bran diet; OT: OVX mice + tea seed oil diet; OTR: OVX mice + tea seed oil with rice bran diet. Values are presented as the mean ± SEM (n = 5). Asterisks (*) indicate significant differences between the SO or OS group compared with the SY group. Different letters indicate significant differences between the OVX groups.
The primary metabolites that the intestinal microbiota ferment are SCFAs, mainly acetate, propionate, and butyrate [36]. Regarding the differences among the OVX groups, the OSR group had higher fecal acetate levels (3.9 ± 0.6 mM) than did the OS group (2.0 ± 0.4 mM; Figure 5E). Butyrate levels were higher in the OSR and OTR groups (0.73 ± 0.13 and 0.65 ± 0.18 mM, respectively) than in the OS and OT groups (0.08 ± 0.04 and 0.10 ± 0.03 mM, respectively; Figure 5E). Total SCFA levels were higher in the OSR and OTR groups than in the OS and OT groups, respectively (Figure 5E). The correlation between SCFAs and the gut microbiomes showed that butyrate levels were positively correlated with Clostridia (R2 = 0.1597; p = 0.0287) but negatively correlated with Tannerellaceae (R2 = −0.1396; p = 0.042).
3.7. Effects of Rice Bran and Tea Seed Oil Supplementation on the Gut–Liver–Brain Axis in Ovariectomized Old Mice Fed a Fructose Drink
Linear regression was performed to explore the correlations of brain and liver biomarkers with specific gut microbiota (Figure 6). Tannerellaceae abundance (greater in mice fed with tea seed oil) was significantly positively correlated with brain IL-1β and TNF-α levels, relative liver weight, and plasma liver function biomarker (AST and ALT) levels; Clostridia abundance (greater in mice fed with rice bran) was significantly negatively correlated with relative liver weight and liver AST and ALT levels. Levels of IL-1β and TNF-α in the brain significantly positively correlated with relative liver weight and hepatic TG and TC contents.
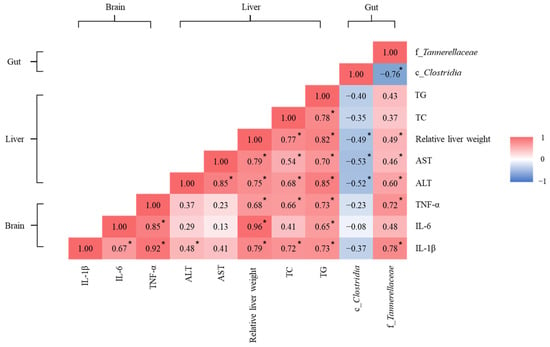
Figure 6.
Heatmap of linear regression for biomarkers of the brain and liver with specific gut microbiota. IL: interleukin; TNF-α: tumor necrosis factor-α; ALT: alanine transaminase; AST: aspartate transaminase; TC: total cholesterol; TG: triglyceride. Colors were assigned according to the distribution of the Pearson correlation coefficient: red and blue represent positive and negative correlations, respectively. * p < 0.05, significant correlations.
4. Discussion
Women who have undergone menopause exhibit higher rates of neurodegenerative diseases and metabolic syndrome than those who have not [1,2,3]. Because of the burden of these diseases on caregivers and the health-care system, dietary modification for such individuals has become increasingly crucial. Evidence links the MIND diet to beneficial effects on cognition and metabolic syndrome [19,37]. However, the mechanisms of the effects of MUFA-rich oil or dietary-fiber-rich whole grains on menopausal disorders remain unclear. To address this, we explored the effects of tea seed oil and rice bran treatment in a multifactorial menopausal disorder model. OVX, d-galactose-injected, and chronically fructose-fed mice exhibit similar physiological changes to those that are incurred through aging [38,39,40]. In our study, old female mice underwent ovariectomy and after were treated with d-galactose and fructose for 8 weeks, after which they developed peripheral oxidative damage and insulin resistance. However, the mice aged 6–8 months may be considered too old to easily induce noticeable aging effects compared with a group of mice of the same age range without OVX and d-galactose treatment.
Studies showed that both polyphenols and dietary fiber may modulate gut microbiota, bringing health benefits [41,42]. A study also showed that rice bran contains both components, which regulate the gut microbiota and influence the production of SCFAs [43]. The potential bioactive compound in rice bran is γ-oryzanol; oral gavage of γ-oryzanol (100 mg/kg) in mice for three weeks can revert LPS-induced cognitive and memory impairments by promoting brain anti-inflammatory molecular responses [44]. In this study, 100 g of rice bran contained 550 mg of polyphenols, of which 11.9 mg was γ-oryzanol. The γ-oryzanol intake of mice was approximately 1 mg/kg. Thus, we hypothesized that the reduction in brain inflammatory responses might have been attributable to the γ-oryzanol in rice bran. However, this dosage remains insufficient to improve cognitive function in this multifactor-induced animal model.
Abnormal metabolism in the liver and altered gut microbiota jointly influence brain function through metaflammation that is induced by hepatic steatosis and harmful toxins produced in the gut, and this influence is evidence of the gut–liver–brain axis [45]. In the present study, linear regression revealed that the presence of specific microbiota, including Tannerellaceae and Clostridia, was associated with the levels of several brain and liver biomarkers. Burz et al. identified a higher relative abundance of Tannerellaceae in the feces of patients with nonalcoholic fatty liver disease (NAFLD) than in those from healthy controls, indicating that NAFLD might correlate positively with the abundance of Tannerellaceae, which secretes endotoxins in the gut and accelerates the progression of liver damage [46,47]. In the current study, the OSR and OTR groups exhibited significantly lower Tannerellaceae abundance than did the OS and OT groups. This lower abundance may be associated with improved liver function. Clostridia is one of the main microbiota that ferments insoluble dietary fiber to produce SCFAs, producing butyrate for energy and other uses [48]. In our study, Clostridia abundance was higher in the OSR and OTR groups than in the OS and OT groups, which may have caused greater butyrate production.
In our study, the mice fed a tea-seed-oil-based diet exhibited significantly greater accumulation of fat in the liver than did those fed different diets. Previous studies have reported that replacing dietary saturated-fatty-acid-rich palm oil with MUFA-rich olive oil in an apolipoprotein E−/− mice model elevates the levels of adipose-differentiation-related proteins, which is strongly associated with the cellular TG content [49,50], and that for individuals with a MUFA-rich diet, the presence of fructose might enhance the accumulation of fat in the liver [51]. Fatty liver causes low-grade inflammation in the whole body and causes peripheral oxidative damage, which might increase the production of endotoxins and lead to aggravation of the proinflammatory response in the brain.
Although totally replacing dietary oil with tea seed oil can contribute to an imbalance in the gut–liver–brain axis, additional rice bran supplementation might alleviate the adverse effects of such a change. In our study, the ingestion of dietary-fiber-rich rice bran caused the relative abundance of Clostridia and the levels of SCFAs, especially butyrate, to increase. The delivery of butyrate-producing probiotics may regulate glucose and lipid metabolism in the liver by activating G protein-coupled receptor 41/43 or by inhibiting histone deacetylase, which would attenuate the progression of hepatic steatosis [52] and reduce the severity of metaflammation [15]. Consistently with previous studies, our data reveal that in mice fed tea seed oil and rice bran, the relative weight of the liver, the hepatic TG and TC content, AST and ALT activity, brain IL-1β and TNF-α levels, and 8-OHdG levels were significantly lower than those in mice fed other diets, indicating that rice bran supplementation has a protective effect against liver damage. SCFAs both reduce fat accumulation in the liver and directly or indirectly modulate the secretion of proinflammatory cytokines in the brain [53,54]. Our study demonstrated that in mice fed rice bran with tea seed oil, metaflammation and peripheral oxidative damage was reduced, and increased brain and liver SCFA levels enabled regulation of the gut–liver–brain axis, which led to reduced brain IL-1β and TNF-α levels.
The limitation of this study is that due to the high cost of 16S rRNA sequencing, we could only select and test five mice from each group out of a total of eight. Additionally, the OVX mice were already aged, and the feeding period may need to be longer. Consequently, no effects on spatial memory were observed in the water maze experiment.
5. Conclusions
Rice bran ameliorated the imbalance of the gut–liver–brain axis when administered with a tea-seed-oil–based diet by elevating the relative abundance of SCFA-producing Clostridia and reducing the relative abundance of endotoxin-producing Tannerellaceae. Rice bran supplementation inhibited the accumulation of hepatic fat, which reduced the severity of metaflammation and peripheral oxidative damage and led to decreased secretion of proinflammatory cytokines in the brain.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu16172980/s1, Table S1: Composition of the experimental diets.
Author Contributions
Conceptualization, W.-C.C., Y.-T.T. and S.-C.Y.; formal analysis, Y.-W.C., L.-H.S. and P.-Y.L.; data curation, Y.-W.C., Y.-T.T. and W.-C.C.; writing—original draft preparation, P.-Y.L.; writing—review and editing, Y.-T.T., W.-C.C. and S.-C.Y.; supervision, S.-C.Y., H.S. and W.-C.C.; project administration, Y.-W.C. and W.-C.C.; funding acquisition, W.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science and Technology Council, grant number 107-2320-B-038-015-MY3.
Institutional Review Board Statement
The animal study was performed per the National Institutes of Health guidelines. The animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Taipei Medical University, Taipei, Taiwan (LAC-2020-0161, 6 May 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors acknowledge the technical support provided by the Taipei Medical University Core Facility.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lobo, R.A. Metabolic syndrome after menopause and the role of hormones. Maturitas 2008, 60, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Vegeto, E.; Poletti, A.; Maggi, A. Estrogens, Neuroinflammation, and Neurodegeneration. Endocr. Rev. 2016, 37, 372–402. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.; Raval, A.P. The peri-menopause in a woman’s life: A systemic inflammatory phase that enables later neurodegenerative disease. J. Neuroinflamm. 2020, 17, 317. [Google Scholar] [CrossRef] [PubMed]
- Burger, H.G.; Hale, G.E.; Robertson, D.M.; Dennerstein, L. A review of hormonal changes during the menopausal transition: Focus on findings from the Melbourne Women’s Midlife Health Project. Hum. Reprod. Update 2007, 13, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Brinton, R.D. Inflammation: Bridging Age, Menopause and APOEε4 Genotype to Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 312. [Google Scholar] [CrossRef] [PubMed]
- Scheyer, O.; Rahman, A.; Hristov, H.; Berkowitz, C.; Isaacson, R.S.; Diaz Brinton, R.; Mosconi, L. Female Sex and Alzheimer’s Risk: The Menopause Connection. J. Prev. Alzheimer’s Dis. 2018, 5, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef]
- Fröhlich, E.E.; Farzi, A.; Mayerhofer, R.; Reichmann, F.; Jačan, A.; Wagner, B.; Zinser, E.; Bordag, N.; Magnes, C.; Fröhlich, E.; et al. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav. Immun. 2016, 56, 140–155. [Google Scholar] [CrossRef]
- Luczynski, P.; McVey Neufeld, K.A.; Oriach, C.S.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Growing up in a Bubble: Using Germ-Free Animals to Assess the Influence of the Gut Microbiota on Brain and Behavior. Int. J. Neuropsychopharmacol. 2016, 19, pyw020. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Tilg, H.; Zmora, N.; Adolph, T.E.; Elinav, E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 2020, 20, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.M.; Costanzo, A.; Gareau, M.G.; Armando, A.M.; Quehenberger, O.; Jameson, J.M.; Olefsky, J.M. High fat diet causes depletion of intestinal eosinophils associated with intestinal permeability. PLoS ONE 2015, 10, e0122195. [Google Scholar] [CrossRef] [PubMed]
- Sampath, V. Bacterial endotoxin-lipopolysaccharide; structure, function and its role in immunity in vertebrates and invertebrates. Agric. Nat. Resour. 2018, 52, 115–120. [Google Scholar] [CrossRef]
- Harte, A.L.; da Silva, N.F.; Creely, S.J.; McGee, K.C.; Billyard, T.; Youssef-Elabd, E.M.; Tripathi, G.; Ashour, E.; Abdalla, M.S.; Sharada, H.M.; et al. Elevated endotoxin levels in non-alcoholic fatty liver disease. J. Inflamm. 2010, 7, 15. [Google Scholar] [CrossRef]
- Gehrke, N.; Schattenberg, J.M. Metabolic Inflammation-A Role for Hepatic Inflammatory Pathways as Drivers of Comorbidities in Nonalcoholic Fatty Liver Disease? Gastroenterology 2020, 158, 1929–1947.e26. [Google Scholar] [CrossRef]
- Prattichizzo, F.; De Nigris, V.; Spiga, R.; Mancuso, E.; La Sala, L.; Antonicelli, R.; Testa, R.; Procopio, A.D.; Olivieri, F.; Ceriello, A. Inflammageing and metaflammation: The yin and yang of type 2 diabetes. Ageing Res. Rev. 2018, 41, 1–17. [Google Scholar] [CrossRef]
- Varatharaj, A.; Galea, I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wanrooy, B.J.; Kumar, K.P.; Wen, S.W.; Qin, C.X.; Ritchie, R.H.; Wong, C.H.Y. Distinct contributions of hyperglycemia and high-fat feeding in metabolic syndrome-induced neuroinflammation. J. Neuroinflamm. 2018, 15, 293. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND diet slows cognitive decline with aging. Alzheimer’s Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef]
- Mohammadpour, S.; Ghorbaninejad, P.; Janbozorgi, N.; Shab-Bidar, S. Associations between adherence to MIND diet and metabolic syndrome and general and abdominal obesity: A cross-sectional study. Diabetol. Metab. Syndr. 2020, 12, 101. [Google Scholar] [CrossRef]
- Omar, S.H. Mediterranean and MIND Diets Containing Olive Biophenols Reduces the Prevalence of Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 2797. [Google Scholar] [CrossRef] [PubMed]
- Amtul, Z.; Westaway, D.; Cechetto, D.F.; Rozmahel, R.F. Oleic acid ameliorates amyloidosis in cellular and mouse models of Alzheimer’s disease. Brain Pathol. 2011, 21, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Shipley, M.; Kivimaki, M.; Singh-Manoux, A.; Brunner, E.J. Dietary pattern, inflammation and cognitive decline: The Whitehall II prospective cohort study. Clin. Nutr. 2017, 36, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Meydani, M. A Mediterranean-style diet and metabolic syndrome. Nutr. Rev. 2005, 63, 312–314. [Google Scholar] [CrossRef]
- Ahmed, H.O.A.; Wang, C. Determination of tea saponin in camellia seed oil with UV and HPLC analysis. World J. Eng. Technol. 2015, 3, 30–37. [Google Scholar] [CrossRef]
- Weng, M.H.; Chen, S.Y.; Li, Z.Y.; Yen, G.C. Camellia oil alleviates the progression of Alzheimer’s disease in aluminum chloride-treated rats. Free Radic. Biol. Med. 2020, 152, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.T.; Hsu, Y.J.; Chien, Y.W.; Huang, C.C.; Huang, W.C.; Chiu, W.C. Tea Seed Oil Prevents Obesity, Reduces Physical Fatigue, and Improves Exercise Performance in High-Fat-Diet-Induced Obese Ovariectomized Mice. Molecules 2019, 24, 980. [Google Scholar] [CrossRef]
- Pinthong, W.; Suanarunsawat, T. Tea seed oil alleviates metabolic derangement and oxidative stress in rats fed with high fat and high fructose diet. Chiang Mai Univ. J. Nat. Sci. 2020, 20, 19. [Google Scholar] [CrossRef]
- Burlando, B.; Cornara, L. Therapeutic properties of rice constituents and derivatives (Oryza sativa L.): A review update. Trends Food Sci. Technol. 2014, 40, 82–98. [Google Scholar] [CrossRef]
- Rungratanawanich, W.; Cenini, G.; Mastinu, A.; Sylvester, M.; Wilkening, A.; Abate, G.; Bonini, S.A.; Aria, F.; Marziano, M.; Maccarinelli, G.; et al. γ-Oryzanol Improves Cognitive Function and Modulates Hippocampal Proteome in Mice. Nutrients 2019, 11, 753. [Google Scholar] [CrossRef]
- Wang, O.; Liu, J.; Cheng, Q.; Guo, X.; Wang, Y.; Zhao, L.; Zhou, F.; Ji, B. Effects of ferulic acid and γ-oryzanol on high-fat and high-fructose diet-induced metabolic syndrome in rats. PLoS ONE 2015, 10, e0118135. [Google Scholar] [CrossRef]
- Islam, J.; Koseki, T.; Watanabe, K.; Budijanto, S.; Oikawa, A.; Alauddin, M.; Goto, T.; Aso, H.; Komai, M.; Shirakawa, H. Dietary Supplementation of Fermented Rice Bran Effectively Alleviates Dextran Sodium Sulfate-Induced Colitis in Mice. Nutrients 2017, 9, 747. [Google Scholar] [CrossRef] [PubMed]
- Pitozzi, V.; Jacomelli, M.; Catelan, D.; Servili, M.; Taticchi, A.; Biggeri, A.; Dolara, P.; Giovannelli, L. Long-term dietary extra-virgin olive oil rich in polyphenols reverses age-related dysfunctions in motor coordination and contextual memory in mice: Role of oxidative stress. Rejuvenation Res. 2012, 15, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Fact. 2017, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Bennett, D.A.; Aggarwal, N.T. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimer’s Dement. 2015, 11, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Azman, K.F.; Zakaria, R. D-Galactose-induced accelerated aging model: An overview. Biogerontology 2019, 20, 763–782. [Google Scholar] [CrossRef]
- Cisternas, P.; Salazar, P.; Serrano, F.G.; Montecinos-Oliva, C.; Arredondo, S.B.; Varela-Nallar, L.; Barja, S.; Vio, C.P.; Gomez-Pinilla, F.; Inestrosa, N.C. Fructose consumption reduces hippocampal synaptic plasticity underlying cognitive performance. Biochim. Biophys. Acta 2015, 1852, 2379–2390. [Google Scholar] [CrossRef]
- Djiogue, S.; Djiyou Djeuda, A.B.; Seke Etet, P.F.; Ketcha Wanda, G.J.M.; Djikem Tadah, R.N.; Njamen, D. Memory and exploratory behavior impairment in ovariectomized Wistar rats. Behav. Brain Funct. 2018, 14, 14. [Google Scholar] [CrossRef]
- Mithul Aravind, S.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.X.; Yeh, C.L.; Yang, S.C.; Shirakawa, H.; Chang, C.L.; Chen, L.H.; Chiu, Y.S.; Chiu, W.C. Rice Bran Supplementation Ameliorates Gut Dysbiosis and Muscle Atrophy in Ovariectomized Mice Fed with a High-Fat Diet. Nutrients 2023, 15, 3514. [Google Scholar] [CrossRef]
- Mastinu, A.; Bonini, S.A.; Rungratanawanich, W.; Aria, F.; Marziano, M.; Maccarinelli, G.; Abate, G.; Premoli, M.; Memo, M.; Uberti, D. Gamma-oryzanol Prevents LPS-induced Brain Inflammation and Cognitive Impairment in Adult Mice. Nutrients 2019, 11, 728. [Google Scholar] [CrossRef] [PubMed]
- Higarza, S.G.; Arboleya, S.; Gueimonde, M.; Gómez-Lázaro, E.; Arias, J.L.; Arias, N. Neurobehavioral dysfunction in non-alcoholic steatohepatitis is associated with hyperammonemia, gut dysbiosis, and metabolic and functional brain regional deficits. PLoS ONE 2019, 14, e0223019. [Google Scholar] [CrossRef] [PubMed]
- Burz, S.D.; Monnoye, M.; Philippe, C.; Farin, W.; Ratziu, V.; Strozzi, F.; Paillarse, J.M.; Chêne, L.; Blottière, H.M.; Gérard, P. Fecal Microbiota Transplant from Human to Mice Gives Insights into the Role of the Gut Microbiota in Non-Alcoholic Fatty Liver Disease (NAFLD). Microorganisms 2021, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Shin, N.R.; Lim, S.K.; Im, U.; Song, E.J.; Nam, Y.D.; Kim, H. Diet Control More Intensively Disturbs Gut Microbiota Than Genetic Background in Wild Type and ob/ob Mice. Front. Microbiol. 2019, 10, 1292. [Google Scholar] [CrossRef] [PubMed]
- Ayua, E.O.; Kazem, A.E.; Hamaker, B.R. Whole grain cereal fibers and their support of the gut commensal Clostridia for health. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100245. [Google Scholar] [CrossRef]
- Arbones-Mainar, J.M.; Ross, K.; Rucklidge, G.J.; Reid, M.; Duncan, G.; Arthur, J.R.; Horgan, G.W.; Navarro, M.A.; Carnicer, R.; Arnal, C.; et al. Extra virgin olive oils increase hepatic fat accumulation and hepatic antioxidant protein levels in APOE−/− mice. J. Proteome. Res. 2007, 6, 4041–4054. [Google Scholar] [CrossRef]
- Magnusson, B.; Asp, L.; Boström, P.; Ruiz, M.; Stillemark-Billton, P.; Lindén, D.; Borén, J.; Olofsson, S.O. Adipocyte differentiation-related protein promotes fatty acid storage in cytosolic triglycerides and inhibits secretion of very low-density lipoproteins. Arterioscler Thromb. Vasc. Biol. 2006, 26, 1566–1571. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, X.; Wang, O.; Zhang, H.; Wang, Y.; Zhou, F.; Liu, J.; Ji, B. Fructose and glucose combined with free fatty acids induce metabolic disorders in HepG2 cell: A new model to study the impacts of high-fructose/sucrose and high-fat diets in vitro. Mol. Nutr. Food Res. 2016, 60, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Fan, J.G. Microbial metabolites in non-alcoholic fatty liver disease. World J. Gastroenterol. 2019, 25, 2019–2028. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Matt, S.M.; Allen, J.M.; Lawson, M.A.; Mailing, L.J.; Woods, J.A.; Johnson, R.W. Butyrate and Dietary Soluble Fiber Improve Neuroinflammation Associated With Aging in Mice. Front. Immunol. 2018, 9, 1832. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).