Dietary Fiber-Derived Butyrate Alleviates Piglet Weaning Stress by Modulating the TLR4/MyD88/NF-κB Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Analysis
2.2. Animals and Treatment
2.3. Growth Performance and Diarrhea Rate
2.4. Sample Collection
2.5. Measurement of Organ Indexes
2.6. Serum Parameters Analysis
2.7. Intestinal Morphology Analysis
2.8. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.9. Western Blotting
2.10. 16S rRNA Gene Sequencing and Analysis
2.11. SCFAs’ Measurement
2.12. Statistical Analysis
3. Results
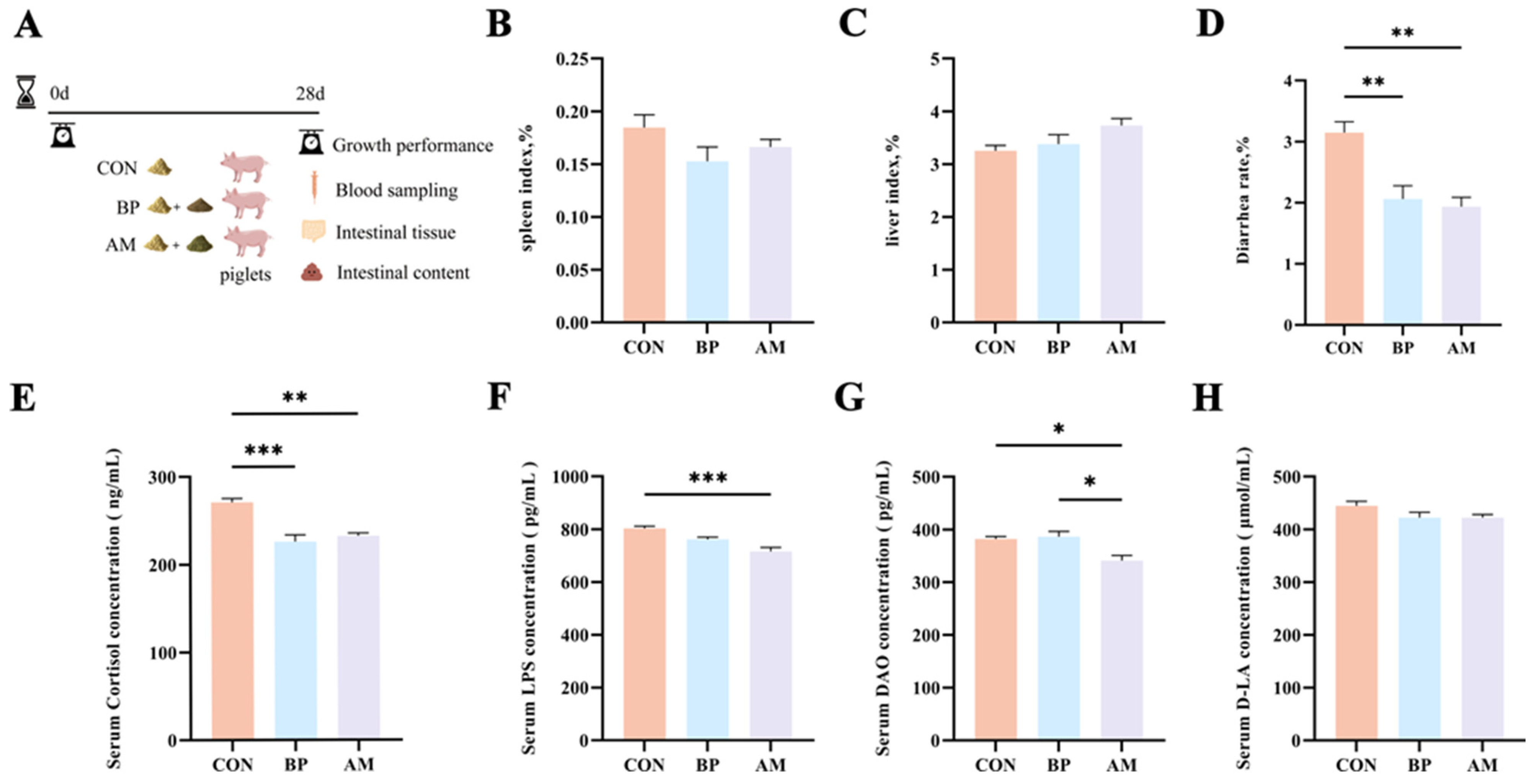
3.1. Effect of Different Sources of DF on Growth Performance and Weaning Stress
3.2. Different Sources of DF Improve Serum Immunity and Antioxidant Capacity
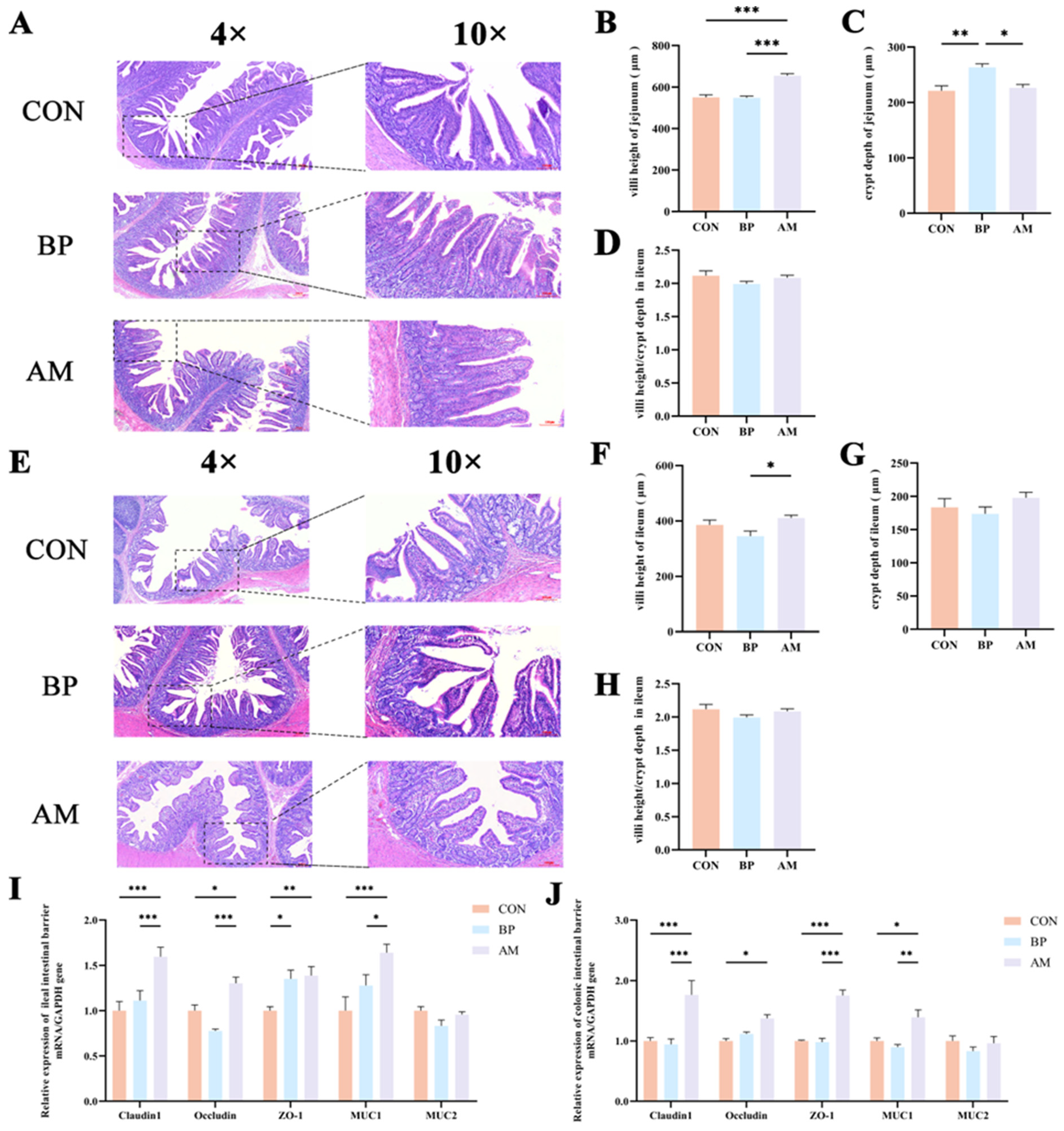
3.3. Effects of Different Sources of DF on Intestinal Development and Barrier Function
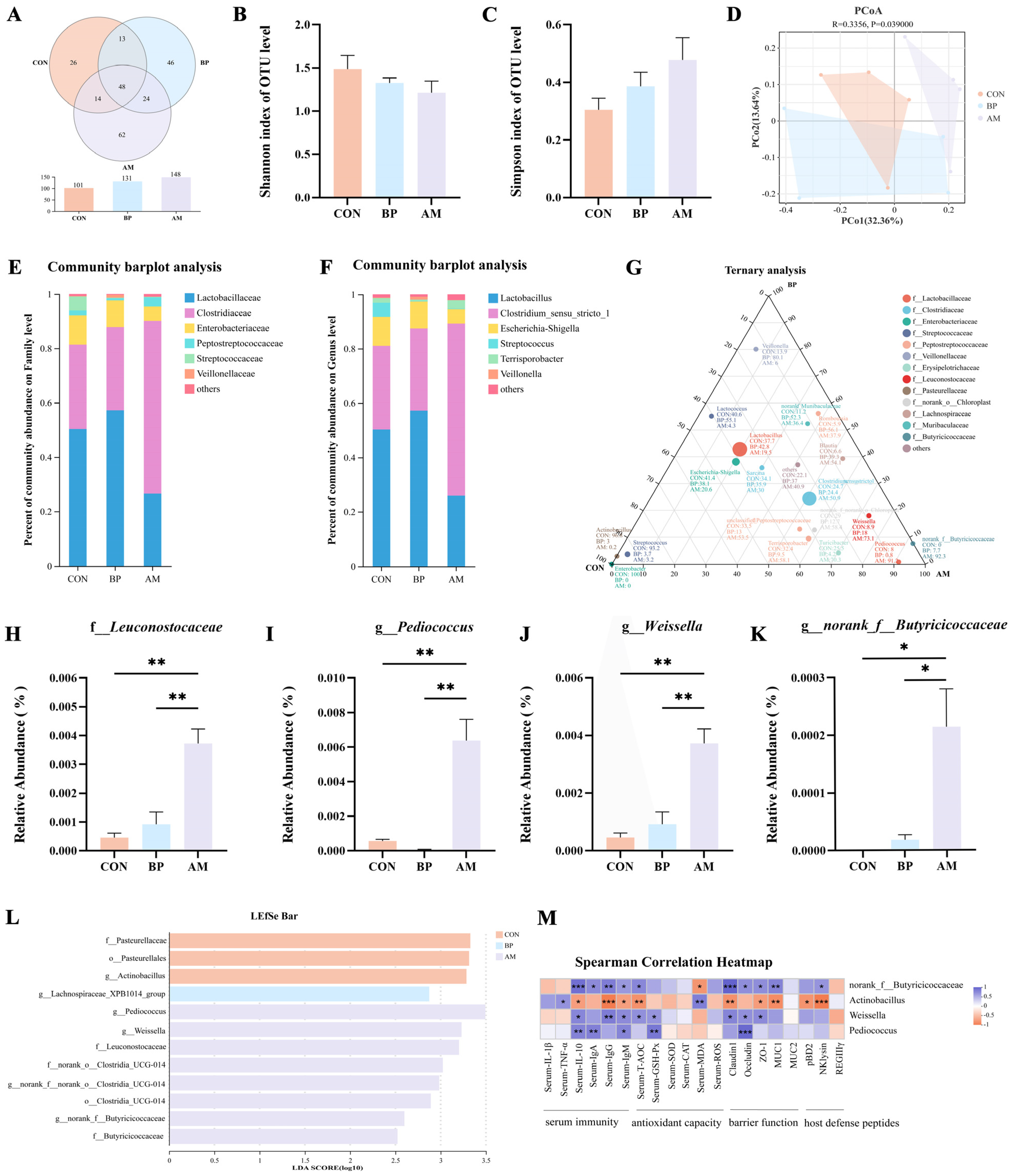
3.4. Effects of Different Sources of DF on Ileal Microbiota of Weaned Piglets
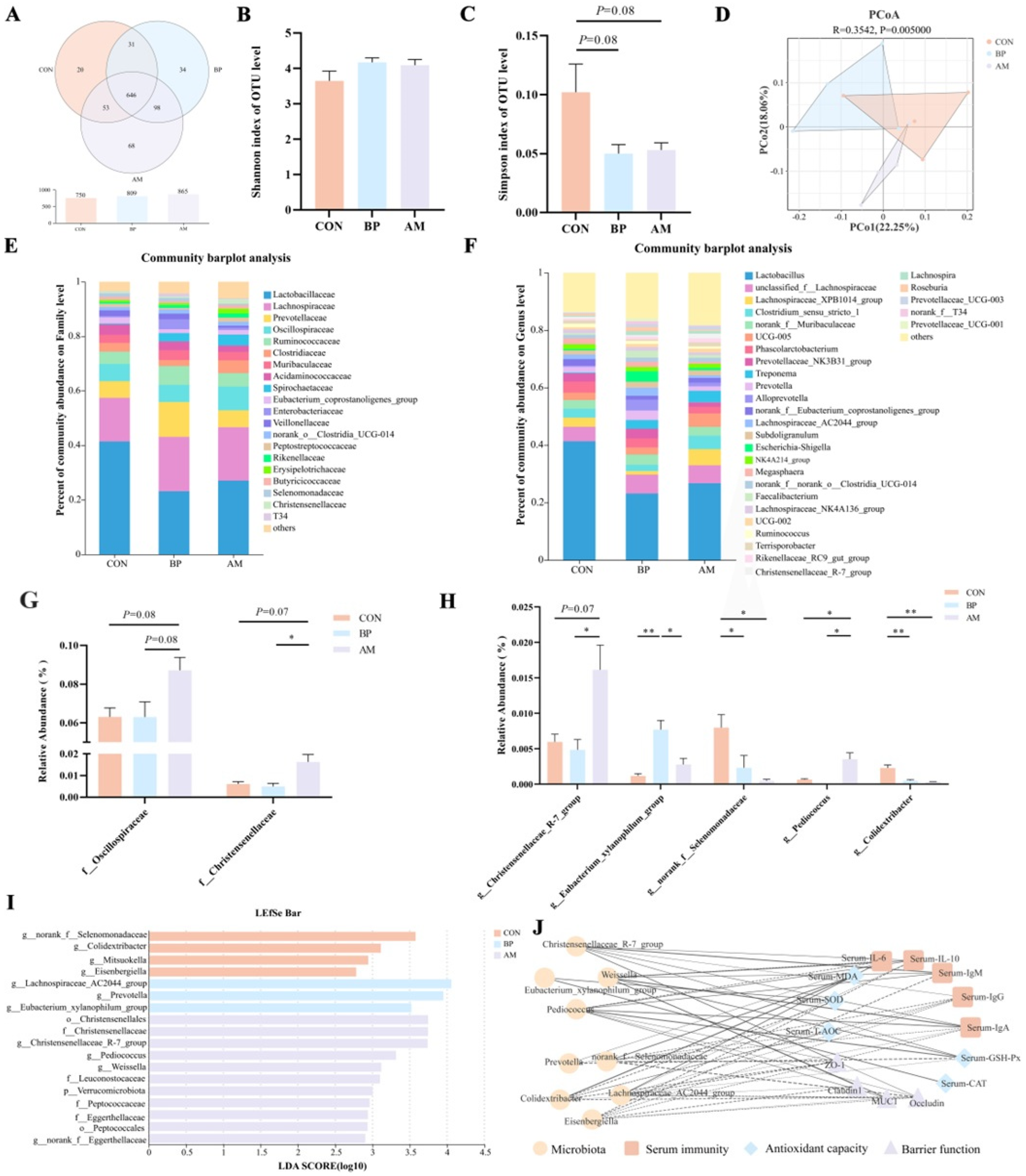
3.5. Effects of Different Sources of DF on Colonic Microbiota of Weaned Piglets
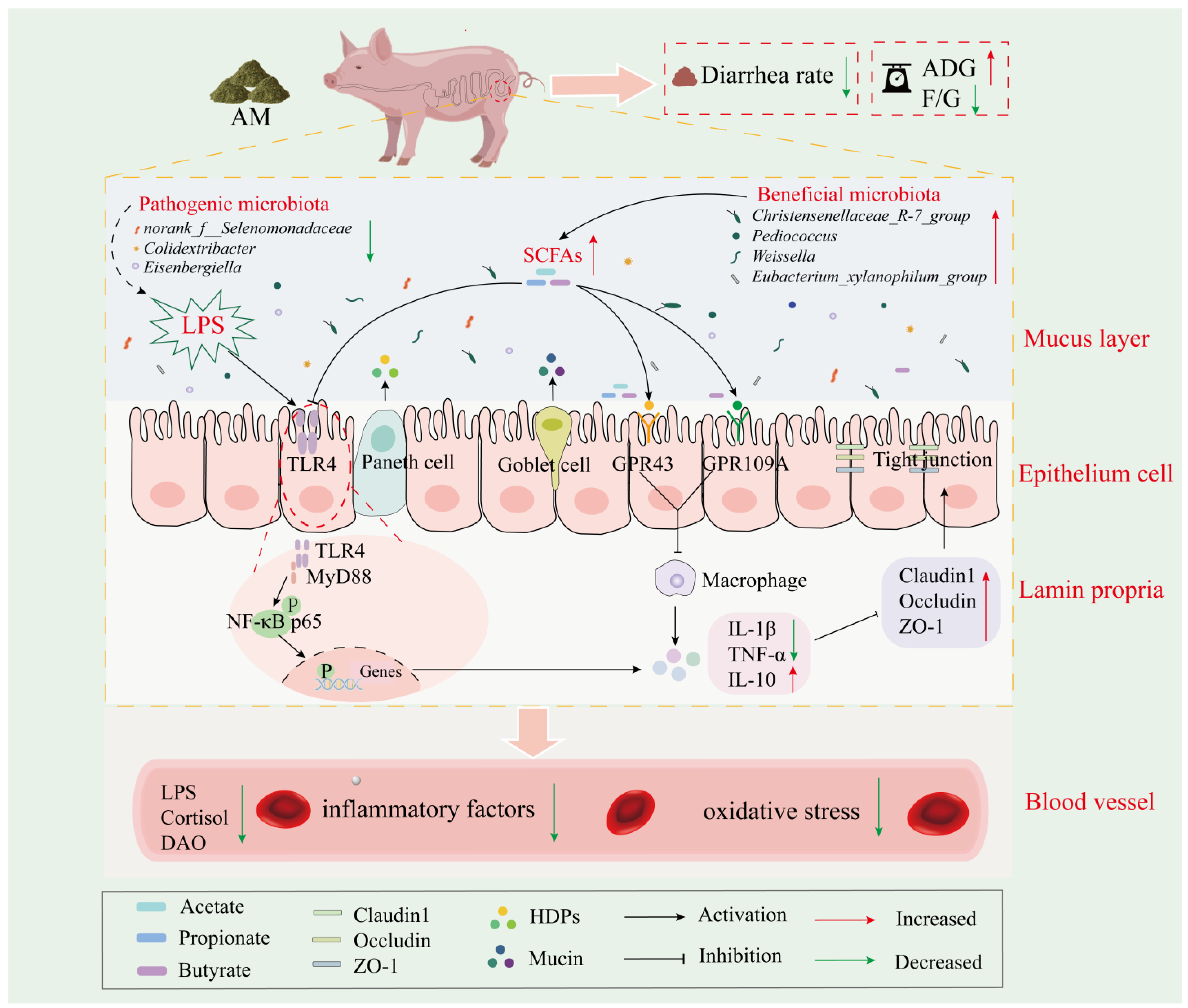
3.6. DF Alleviates Weaning Stress by Regulating the TLR/MyD88/NF-κB Pathway through Gut-Microbe-Derived SCFAs
4. Discussion
4.1. DF Relieves Stress and Improves Growth Performance in Weaned Piglets
4.2. DF Alleviates Gut Microbiota Dysbiosis in Weaned Piglets and Increases SCFAs-Producing Bacteria
4.3. DF Improves Gut Barrier Function by Alleviating Gut Inflammatory Pathways via Microbial-Derived SCFAs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Hu, H.; Zijlstra, R.T.; Zheng, J.; Gänzle, M.G. Metagenomic reconstructions of gut microbial metabolism in weanling pigs. Microbiome 2019, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Huang, X.; Wang, P.; Yan, Z.; Sun, W.; Zhao, S.; Gun, S. Longitudinal development of the gut microbiota in healthy and diarrheic piglets induced by age-related dietary changes. Microbiologyopen 2019, 8, e923. [Google Scholar] [CrossRef]
- Hughes, E.R.; Winter, M.G.; Duerkop, B.A.; Spiga, L.; Furtado de Carvalho, T.; Zhu, W.; Gillis, C.C.; Büttner, L.; Smoot, M.P.; Behrendt, C.L.; et al. Microbial Respiration and Formate Oxidation as Metabolic Signatures of Inflammation-Associated Dysbiosis. Cell Host Microbe 2017, 21, 208–219. [Google Scholar] [CrossRef]
- Hu, J.; Chen, J.; Xu, X.; Hou, Q.; Ren, J.; Yan, X. Gut microbiota-derived 3-phenylpropionic acid promotes intestinal epithelial barrier function via AhR signaling. Microbiome 2023, 11, 102. [Google Scholar] [CrossRef]
- Huting, A.M.S.; Middelkoop, A.; Guan, X.; Molist, F. Using Nutritional Strategies to Shape the Gastro-Intestinal Tracts of Suckling and Weaned Piglets. Animals 2021, 11, 402. [Google Scholar] [CrossRef]
- Han, X.; Ma, Y.; Ding, S.; Fang, J.; Liu, G. Regulation of dietary fiber on intestinal microorganisms and its effects on animal health. Anim. Nutr. 2023, 14, 356–369. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, P.; Wu, Y.; Guo, P.; Liu, L.; Ma, N.; Levesque, C.; Chen, Y.; Zhao, J.; Zhang, J.; et al. Dietary Fiber Increases Butyrate-Producing Bacteria and Improves the Growth Performance of Weaned Piglets. J. Agric. Food Chem. 2018, 66, 7995–8004. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.L.; Kim, H.S.; Hong, J.S.; Lee, J.H.; Han, Y.G.; Jin, Y.H.; Son, S.W.; Ha, S.H.; Kim, Y.Y. Effect of Dietary sugar beet pulp supplementation on growth performance, nutrient digestibility, fecal Microflora, blood profiles and Diarrhea incidence in weaning pigs. J. Anim. Sci. Technol. 2017, 59, 18. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Liu, H.; Wu, D.; Mahfuz, S.; Piao, X. Source of fiber influences growth, immune responses, gut barrier function and microbiota in weaned piglets fed antibiotic-free diets. Anim. Nutr. 2021, 7, 315–325. [Google Scholar] [CrossRef]
- Huang, S.; Cui, Z.; Hao, X.; Cheng, C.; Chen, J.; Wu, D.; Luo, H.; Deng, J.; Tan, C. Dietary fibers with low hydration properties exacerbate diarrhea and impair intestinal health and nutrient digestibility in weaned piglets. J. Anim. Sci. Biotechnol. 2022, 13, 142. [Google Scholar] [CrossRef]
- Ma, J.; Huangfu, W.; Yang, X.; Xu, J.; Zhang, Y.; Wang, Z.; Zhu, X.; Wang, C.; Shi, Y.; Cui, Y. “King of the forage”-Alfalfa supplementation improves growth, reproductive performance, health condition and meat quality of pigs. Front. Vet. Sci. 2022, 9, 1025942. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, W.; Zhu, X.; Sun, X.; Xiao, J.; Li, D.; Cui, Y.; Wang, C.; Shi, Y. Response of Gut Microbiota to Dietary Fiber and Metabolic Interaction with SCFAs in Piglets. Front. Microbiol. 2018, 9, 2344. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cui, Y.; Su, Y.; Gao, Z.; Diao, X.; Li, J.; Zhu, X.; Li, D.; Li, Z.; Wang, C.; et al. Dietary Fiber Ameliorates Lipopolysaccharide-Induced Intestinal Barrier Function Damage in Piglets by Modulation of Intestinal Microbiome. mSystems 2021, 6, e01374-20. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J.A.; Liu, H. Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation. Anim. Nutr. 2022, 8, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Chen, Y.; Deng, F.; Yan, X.; Zhong, R.; Meng, Q.; Liu, L.; Zhao, Y.; Zhang, S.; Chen, L.; et al. Xylooligosaccharide-mediated gut microbiota enhances gut barrier and modulates gut immunity associated with alterations of biological processes in a pig model. Carbohydr. Polym. 2022, 294, 119776. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.X.; Shah, B.R.; Li, J.; Liang, H.S.; Zhan, F.C.; Geng, F.; Li, B. A critical review on interplay between dietary fibers and gut microbiota. Trends Food Sci. Technol. 2022, 124, 237–249. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, J.; Lin, Z.; Liu, C.; Zhang, Y.; Zhang, S.; Zhou, M.; Zhao, J.; Liu, H.; Ma, X. Clostridium butyricum alleviates weaned stress of piglets by improving intestinal immune function and gut microbiota. Food Chem. 2023, 405, 135014. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, J.; Zheng, W.; Yang, T.; Wang, X.; Xie, C.; Yan, X. Lactobacillus frumenti mediates energy production via fatty acid β-oxidation in the liver of early-weaned piglets. J. Anim. Sci. Biotechnol. 2019, 10, 95. [Google Scholar] [CrossRef]
- Liu, B.; Cui, Y.; Ali, Q.; Zhu, X.; Li, D.; Ma, S.; Wang, Z.; Wang, C.; Shi, Y. Gut Microbiota Modulate Rabbit Meat Quality in Response to Dietary Fiber. Front. Nutr. 2022, 9, 849429. [Google Scholar] [CrossRef]
- Adams, S.; Kong, X.; Jiang, H.; Qin, G.; Sossah, F.L.; Che, D. Prebiotic effects of alfalfa (Medicago sativa) fiber on cecal bacterial composition, short-chain fatty acids, and diarrhea incidence in weaning piglets. RSC Adv. 2019, 9, 13586–13599. [Google Scholar] [CrossRef]
- Shang, Q.; Ma, X.; Liu, H.; Liu, S.; Piao, X. Effect of fibre sources on performance, serum parameters, intestinal morphology, digestive enzyme activities and microbiota in weaned pigs. Arch. Anim. Nutr. 2020, 74, 121–137. [Google Scholar] [CrossRef]
- Jayaraman, B.; Nyachoti, C.M. Husbandry practices and gut health outcomes in weaned piglets: A review. Anim. Nutr. 2017, 3, 205–211. [Google Scholar] [CrossRef]
- Jarrett, S.; Ashworth, C.J. The role of dietary fibre in pig production, with a particular emphasis on reproduction. J. Anim. Sci. Biotechnol. 2018, 9, 59. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Patience, J.F.; Rossoni-Serão, M.C.; Gutiérrez, N.A. A review of feed efficiency in swine: Biology and application. J. Anim. Sci. Biotechnol. 2015, 6, 33. [Google Scholar] [CrossRef]
- Pouillart, P.R.; Dépeint, F.; Abdelnour, A.; Deremaux, L.; Vincent, O.; Mazière, J.C.; Madec, J.Y.; Chatelain, D.; Younes, H.; Wils, D.; et al. Nutriose, a prebiotic low-digestible carbohydrate, stimulates gut mucosal immunity and prevents TNBS-induced colitis in piglets. Inflamm. Bowel Dis. 2010, 16, 783–794. [Google Scholar] [CrossRef]
- Che, D.; Adams, S.; Wei, C.; Gui-Xin, Q.; Atiba, E.M.; Hailong, J. Effects of Astragalus membranaceus fiber on growth performance, nutrient digestibility, microbial composition, VFA production, gut pH, and immunity of weaned pigs. Microbiologyopen 2019, 8, e00712. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, F.; Yin, Y.; Huang, P.; Jiang, Q.; Liu, Z.; Yin, Y.; Chen, J. Dietary Litsea cubeba essential oil supplementation improves growth performance and intestinal health of weaned piglets. Anim. Nutr. 2023, 13, 9–18. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, X.; Cui, Y.; Wang, W.; Liu, H.; Li, Z.; Guo, Z.; Ma, S.; Li, D.; Wang, C.; et al. Consumption of Dietary Fiber from Different Sources during Pregnancy Alters Sow Gut Microbiota and Improves Performance and Reduces Inflammation in Sows and Piglets. mSystems 2021, 6, e00591-20. [Google Scholar] [CrossRef]
- Lallès, J.P.; Boudry, G.; Favier, C.; Le Floc’h, N.; Lurona, I.; Montagne, L.; Oswald, I.P.; Pié, S.; Piel, C.; Sève, B. Gut function and dysfunction in young pigs: Physiology. Anim. Res. 2004, 53, 301–316. [Google Scholar] [CrossRef]
- Planchais, C.; Mouquet, H. Easy pan-detection of human IgA immunoglobulins. J. Immunol. Methods 2020, 484, 112833. [Google Scholar] [CrossRef]
- Huang, T.; Che, Q.; Chen, X.; Chen, D.; Yu, B.; He, J.; Chen, H.; Yan, H.; Zheng, P.; Luo, Y.; et al. Apple Polyphenols Improve Intestinal Antioxidant Capacity and Barrier Function by Activating the Nrf2/Keap1 Signaling Pathway in a Pig Model. J. Agric. Food Chem. 2022, 70, 7576–7585. [Google Scholar] [CrossRef]
- Świątkiewicz, M.; Zimniewska, M.; Różańska, W.; Gryszczyńska, A.; Kołodziej, J.; Młocek, W.; Czech, A. Assessment of flax and hemp fibres in terms of their impact on the growth performance and health status of weaned piglets. Animal 2022, 16, 100677. [Google Scholar] [CrossRef]
- Li, Z.; Chi, H.; Zhu, W.; Yang, G.; Song, J.; Mo, L.; Zhang, Y.; Deng, Y.; Xu, F.; Yang, J.; et al. Cadmium induces renal inflammation by activating the NLRP3 inflammasome through ROS/MAPK/NF-κB pathway in vitro and in vivo. Arch. Toxicol. 2021, 95, 3497–3513. [Google Scholar] [CrossRef]
- Xun, W.; Ji, M.; Ma, Z.; Deng, T.; Yang, W.; Hou, G.; Shi, L.; Cao, T. Dietary emodin alleviates lipopolysaccharide-induced intestinal mucosal barrier injury by regulating gut microbiota in piglets. Anim. Nutr. 2023, 14, 152–162. [Google Scholar] [CrossRef]
- van Hees, H.M.J.; Chiers, K.; den Hartog, L.A.; van Kempen, T.; Maes, D.; Millet, S.; Janssens, G.P.J. Supplementing oat hulls to the diet of suckling piglets altered their intestinal tract and colonic microbiota development. Anim. Nutr. 2023, 12, 284–296. [Google Scholar] [CrossRef]
- McCarty, M.F.; Lerner, A. Perspective: Prospects for Nutraceutical Support of Intestinal Barrier Function. Adv. Nutr. 2021, 12, 316–324. [Google Scholar] [CrossRef]
- Wu, J.; Ma, N.; Johnston, L.J.; Ma, X. Dietary Nutrients Mediate Intestinal Host Defense Peptide Expression. Adv. Nutr. 2020, 11, 92–102. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Han, Q.; Guo, Y.; Zhang, B.; D’Inca, R. Dietary live yeast and mannan-oligosaccharide supplementation attenuate intestinal inflammation and barrier dysfunction induced by Escherichia coli in broilers. Br. J. Nutr. 2016, 116, 1878–1888. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, J.; Xiong, B.; Zhang, C.; Kang, B.; Gao, Y.; Li, Z.; Ge, W.; Cheng, S.; Hao, Y.; et al. Microbiota from alginate oligosaccharide-dosed mice successfully mitigated small intestinal mucositis. Microbiome 2020, 8, 112. [Google Scholar] [CrossRef]
- Teixeira, C.G.; Fusieger, A.; Milião, G.L.; Martins, E.; Drider, D.; Nero, L.A.; de Carvalho, A.F. Weissella: An Emerging Bacterium with Promising Health Benefits. Probiotics Antimicrob. Proteins 2021, 13, 915–925. [Google Scholar] [CrossRef]
- Ren, C.; Wang, Y.; Lin, X.; Song, H.; Zhou, Q.; Xu, W.; Shi, K.; Chen, J.; Song, J.; Chen, F.; et al. A Combination of Formic Acid and Monolaurin Attenuates Enterotoxigenic Escherichia coli Induced Intestinal Inflammation in Piglets by Inhibiting the NF-κB/MAPK Pathways with Modulation of Gut Microbiota. J. Agric. Food Chem. 2020, 68, 4155–4165. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, Y.; Liu, L.; Zheng, B.; Zhang, Y.; Zeng, H. Effect of Lotus Seed Resistant Starch on Lactic Acid Conversion to Butyric Acid Fermented by Rat Fecal Microbiota. J. Agric. Food Chem. 2022, 70, 1525–1535. [Google Scholar] [CrossRef]
- Tavella, T.; Rampelli, S.; Guidarelli, G.; Bazzocchi, A.; Gasperini, C.; Pujos-Guillot, E.; Comte, B.; Barone, M.; Biagi, E.; Candela, M.; et al. Elevated gut microbiome abundance of Christensenellaceae, Porphyromonadaceae and Rikenellaceae is associated with reduced visceral adipose tissue and healthier metabolic profile in Italian elderly. Gut Microbes 2021, 13, 1880221. [Google Scholar] [CrossRef]
- Shi, H.; Li, X.; Hou, C.; Chen, L.; Zhang, Y.; Li, J. Effects of Pomegranate Peel Polyphenols Combined with Inulin on Gut Microbiota and Serum Metabolites of High-Fat-Induced Obesity Rats. J. Agric. Food Chem. 2023, 71, 5733–5744. [Google Scholar] [CrossRef]
- Xia, X.; Wei, H.; Hu, L.; Peng, J. Hydratability and improved fermentability in vitro of guar gum by combination of xanthan gum. Carbohydr. Polym. 2021, 258, 117625. [Google Scholar] [CrossRef]
- Gao, J.; Dong, J.; Sun, Z.; Wang, T.; Guan, Y.; Sun, Y.; Qin, G.; Zhang, X.; Zhen, Y. Effects of antimicrobial peptide and tributyrin on fecal microflora and blood indices of female calves. Food Sci. Nutr. 2023, 11, 5248–5257. [Google Scholar] [CrossRef]
- Guo, W.L.; Cao, Y.J.; You, S.Z.; Wu, Q.; Zhang, F.; Han, J.Z.; Lv, X.C.; Rao, P.F.; Ai, L.Z.; Ni, L. Ganoderic acids-rich ethanol extract from Ganoderma lucidum protects against alcoholic liver injury and modulates intestinal microbiota in mice with excessive alcohol intake. Curr. Res. Food Sci. 2022, 5, 515–530. [Google Scholar] [CrossRef]
- Hu, R.; Wu, S.; Li, B.; Tan, J.; Yan, J.; Wang, Y.; Tang, Z.; Liu, M.; Fu, C.; Zhang, H.; et al. Dietary ferulic acid and vanillic acid on inflammation, gut barrier function and growth performance in lipopolysaccharide-challenged piglets. Anim. Nutr. 2022, 8, 144–152. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Zheng, G.; Li, Z.; Mei, J. Resveratrol-loaded selenium/chitosan nano-flowers alleviate glucolipid metabolism disorder-associated cognitive impairment in Alzheimer’s disease. Int. J. Biol. Macromol. 2023, 239, 124316. [Google Scholar] [CrossRef]
- Yang, T.; Yang, S.; Zhao, J.; Wang, P.; Li, S.; Jin, Y.; Liu, Z.; Zhang, X.; Zhang, Y.; Zhao, Y.; et al. Comprehensive Analysis of Gut Microbiota and Fecal Bile Acid Profiles in Children with Biliary Atresia. Front. Cell Infect. Microbiol. 2022, 12, 914247. [Google Scholar] [CrossRef]
- Wu, X.; Xu, N.; Ye, Z.; Zhao, Q.; Liu, J.; Li, J.; Wu, M.; Zheng, Y.; Li, X.; Li, W.; et al. Polysaccharide from Scutellaria barbata D. Don attenuates inflammatory response and microbial dysbiosis in ulcerative colitis mice. Int. J. Biol. Macromol. 2022, 206, 1–9. [Google Scholar] [CrossRef]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef]
- Mizuta, K.; Matoba, A.; Shibata, S.; Masaki, E.; Emala, C.W., Sr. Obesity-induced asthma: Role of free fatty acid receptors. Jpn. Dent. Sci. Rev. 2019, 55, 103–107. [Google Scholar] [CrossRef]
- Thangaraju, M.; Cresci, G.A.; Liu, K.; Ananth, S.; Gnanaprakasam, J.P.; Browning, D.D.; Mellinger, J.D.; Smith, S.B.; Digby, G.J.; Lambert, N.A.; et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009, 69, 2826–2832. [Google Scholar] [CrossRef]
- Aragonès, G.; González-García, S.; Aguilar, C.; Richart, C.; Auguet, T. Gut Microbiota-Derived Mediators as Potential Markers in Nonalcoholic Fatty Liver Disease. Biomed. Res. Int. 2019, 2019, 8507583. [Google Scholar] [CrossRef]
- Yuan, Y.; Lu, L.; Bo, N.; Yang, C.; Haiyang, Y. Allicin Ameliorates Intestinal Barrier Damage via Microbiota-Regulated Short-Chain Fatty Acids-TLR4/MyD88/NF-κB Cascade Response in Acrylamide-Induced Rats. J. Agric. Food Chem. 2021, 69, 12837–12852. [Google Scholar] [CrossRef]
- Tang, J.; Xu, L.; Zeng, Y.; Gong, F. Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int. Immunopharmacol. 2021, 91, 107272. [Google Scholar] [CrossRef]
- Lin, M.Y.; de Zoete, M.R.; van Putten, J.P.; Strijbis, K. Redirection of Epithelial Immune Responses by Short-Chain Fatty Acids through Inhibition of Histone Deacetylases. Front. Immunol. 2015, 6, 554. [Google Scholar] [CrossRef]
- Fu, R.; Liang, C.; Chen, D.; Tian, G.; Zheng, P.; He, J.; Yu, J.; Mao, X.; Luo, Y.; Luo, J.; et al. Yeast hydrolysate attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier damage in weaned piglets. J. Anim. Sci. Biotechnol. 2023, 14, 44. [Google Scholar] [CrossRef]
- Pedersen, S.S.; Prause, M.; Williams, K.; Barrès, R.; Billestrup, N. Butyrate inhibits IL-1β-induced inflammatory gene expression by suppression of NF-κB activity in pancreatic beta cells. J. Biol. Chem. 2022, 298, 102312. [Google Scholar] [CrossRef]


| Item | CON | BP | AM |
|---|---|---|---|
| Ingredients | |||
| Corn | 58.40 | 53.23 | 53.27 |
| Soybean-puffed | 12.00 | 11.53 | 11.85 |
| Fermented soybean meal | 5.40 | 5.58 | 3.99 |
| Soybean meal | 10.00 | 10.00 | 10.00 |
| Beet pulp meal | - | 5.00 | - |
| Alfalfa meal | - | - | 5.00 |
| Fish meal | 4.00 | 4.12 | 4.36 |
| Dried whey | 5.00 | 5.00 | 5.00 |
| Soybean oil | 1.50 | 1.99 | 3.15 |
| Salt | 0.35 | 0.32 | 0.33 |
| Limestone | 0.68 | 0.63 | 0.60 |
| Calcium hydrogen phosphate | 1.00 | 0.95 | 0.79 |
| L-Lysine | 0.32 | 0.30 | 0.32 |
| DL-Methionine | 0.14 | 0.15 | 0.14 |
| Zinc oxide | 0.20 | 0.20 | 0.20 |
| * Premix | 1.00 | 1.00 | 1.00 |
| Total | 100.00 | 100.00 | 100.00 |
| Nutrient levels | |||
| Digestion energy (MJ/kg) | 14.61 | 14.61 | 14.61 |
| Crude protein | 19.05 | 19.05 | 19.05 |
| Crude fiber | 2.83 | 3.76 | 3.76 |
| Lysine | 1.38 | 1.38 | 1.38 |
| Methionine + Cystine | 0.76 | 0.76 | 0.76 |
| Calcium | 0.76 | 0.76 | 0.76 |
| Available phosphorous | 0.39 | 0.39 | 0.39 |
| Item | CON | BP | AM | p Value |
|---|---|---|---|---|
| Initial body weight, kg | 8.74 ± 0.09 | 8.75 ± 0.26 | 8.75 ± 0.18 | 1.00 |
| Final body weight, kg | 25.15 ± 0.35 ab | 24.32 ± 0.27 b | 26.31 ± 0.59 a | 0.03 |
| ADFI, kg/d | 0.85 ± 0.01 a | 0.79 ± 0.01 b | 0.83 ± 0.01 a | 0.01 |
| ADG, kg/d | 0.47 ± 0.01 b | 0.44 ± 0.01 b | 0.50 ± 0.01 a | 0.01 |
| F:G | 1.81 ± 0.04 a | 1.77 ± 0.01 a | 1.66 ± 0.02 b | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huangfu, W.; Ma, J.; Zhang, Y.; Liu, M.; Liu, B.; Zhao, J.; Wang, Z.; Shi, Y. Dietary Fiber-Derived Butyrate Alleviates Piglet Weaning Stress by Modulating the TLR4/MyD88/NF-κB Pathway. Nutrients 2024, 16, 1714. https://doi.org/10.3390/nu16111714
Huangfu W, Ma J, Zhang Y, Liu M, Liu B, Zhao J, Wang Z, Shi Y. Dietary Fiber-Derived Butyrate Alleviates Piglet Weaning Stress by Modulating the TLR4/MyD88/NF-κB Pathway. Nutrients. 2024; 16(11):1714. https://doi.org/10.3390/nu16111714
Chicago/Turabian StyleHuangfu, Weikang, Jixiang Ma, Yan Zhang, Mengqi Liu, Boshuai Liu, Jiangchao Zhao, Zhichang Wang, and Yinghua Shi. 2024. "Dietary Fiber-Derived Butyrate Alleviates Piglet Weaning Stress by Modulating the TLR4/MyD88/NF-κB Pathway" Nutrients 16, no. 11: 1714. https://doi.org/10.3390/nu16111714
APA StyleHuangfu, W., Ma, J., Zhang, Y., Liu, M., Liu, B., Zhao, J., Wang, Z., & Shi, Y. (2024). Dietary Fiber-Derived Butyrate Alleviates Piglet Weaning Stress by Modulating the TLR4/MyD88/NF-κB Pathway. Nutrients, 16(11), 1714. https://doi.org/10.3390/nu16111714







