From Vineyard to Vision: Efficacy of Maltodextrinated Grape Pomace Extract (MaGPE) Nutraceutical Formulation in Patients with Diabetic Retinopathy
Abstract
1. Introduction
2. Materials and Methods
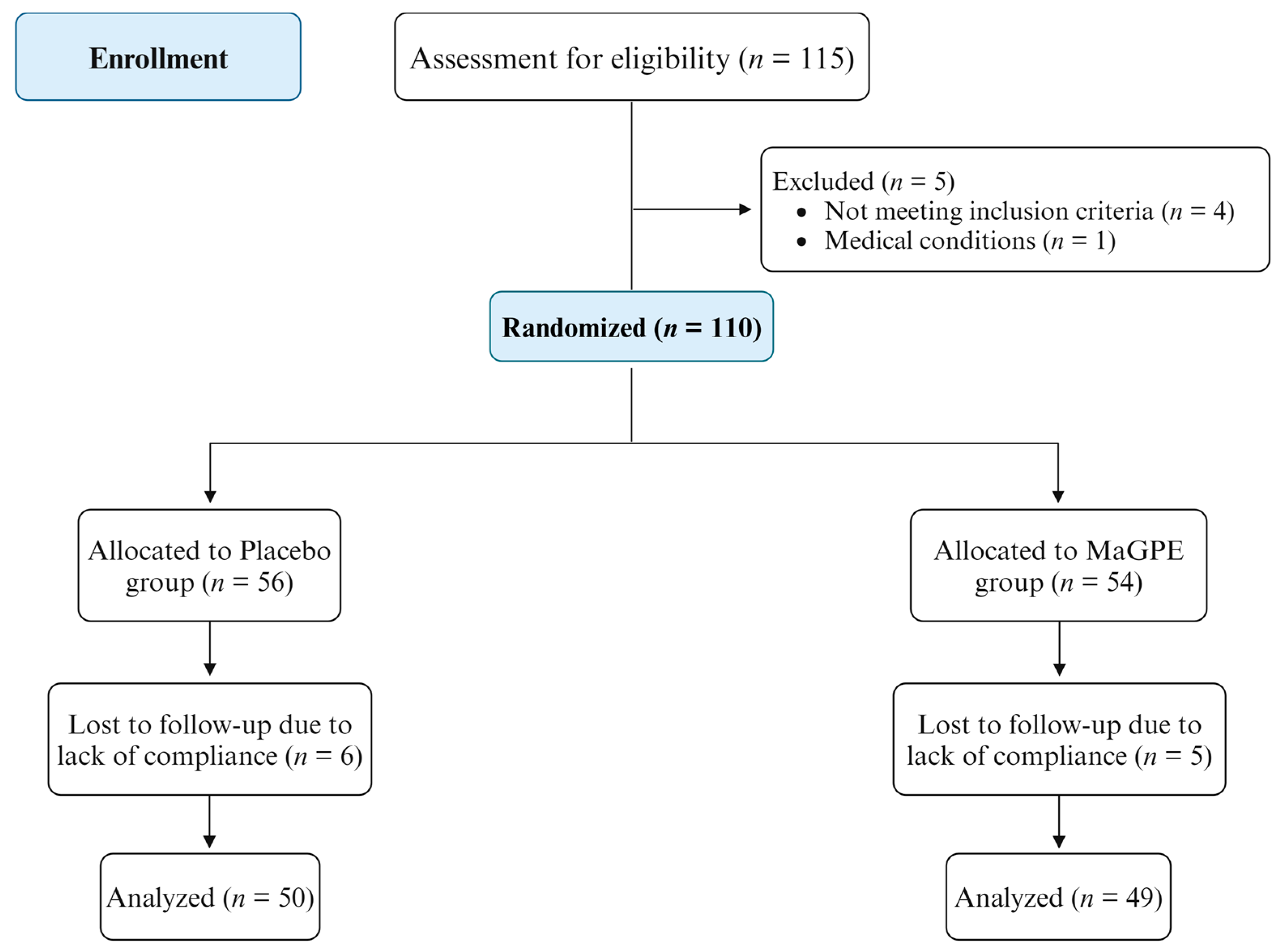
2.1. Study Population and Design
2.2. Formulation of MaGPE Supplement
2.3. Assessment of Ophthalmic Outcomes
2.4. Measurements of Biochemical and Oxidative Stress Biomarkers
2.5. Statistical Analysis
3. Results
3.1. Evaluation of Clinical and Biochemical Parameters in Placebo and MaGPE Groups
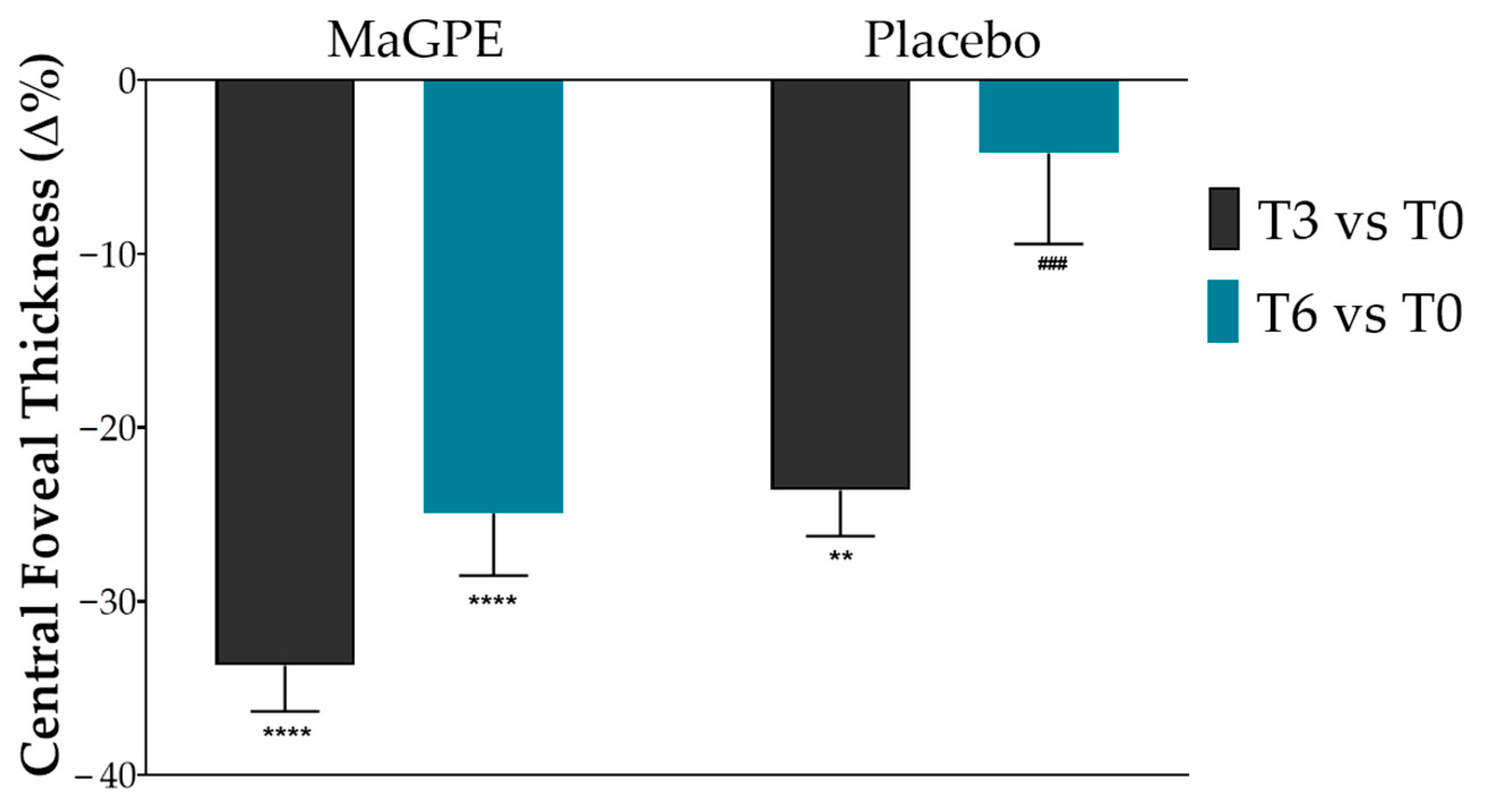
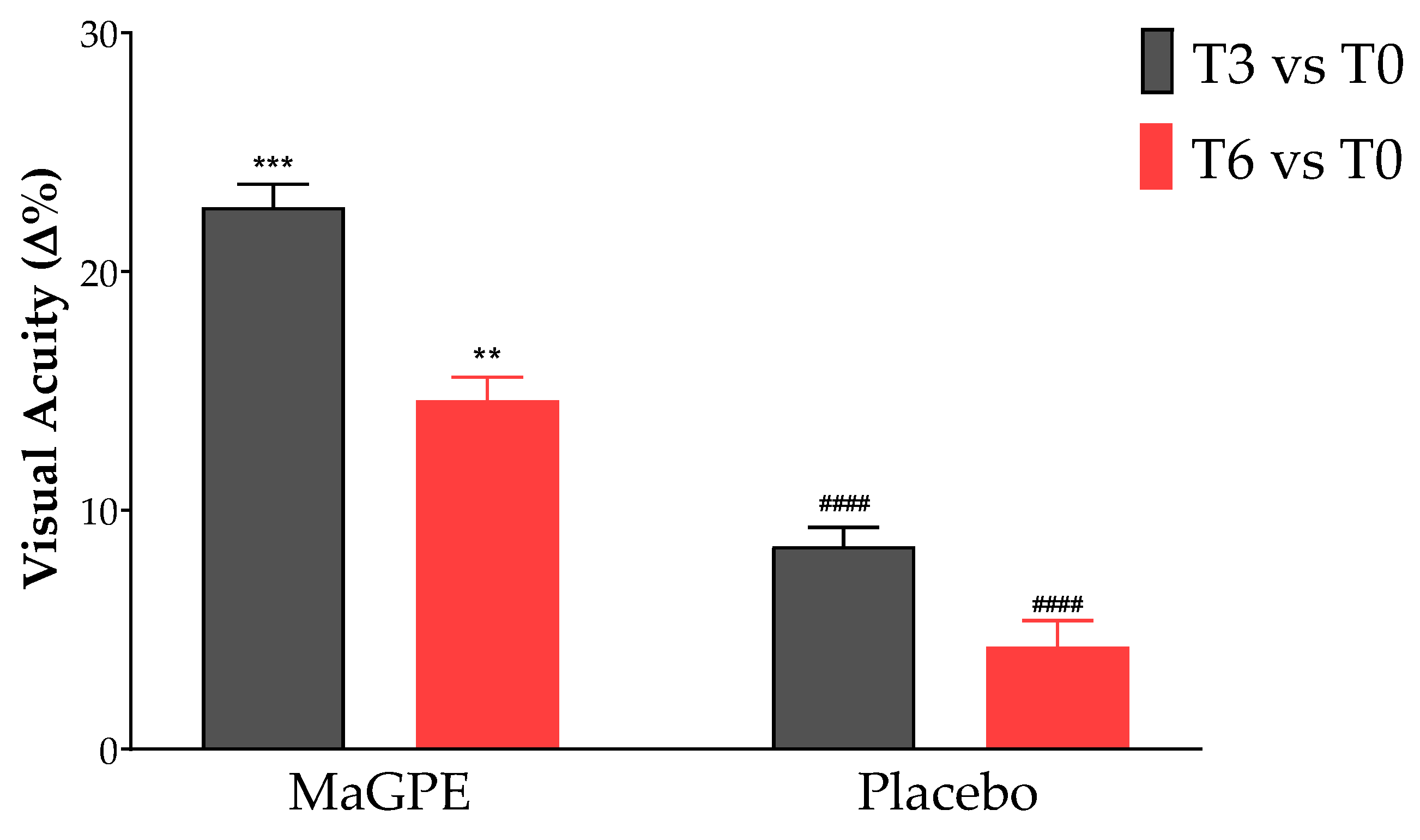
3.2. Changes in Ophthalmic Parameters in Placebo and MaGPE Groups
3.3. Oxidative Stress Biomarker Modulation by MaGPE Supplementation
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef]
- Kropp, M.; Golubnitschaja, O.; Mazurakova, A.; Koklesova, L.; Sargheini, N.; Vo, T.T.K.S.; de Clerck, E.; Polivka, J.; Potuznik, P.; Polivka, J.; et al. Diabetic retinopathy as the leading cause of blindness and early predictor of cascading complications—Risks and mitigation. EPMA J. 2023, 14, 21–42. [Google Scholar] [CrossRef]
- Bourne, R.R.A.; Steinmetz, J.D.; Saylan, M.; Mersha, A.M.; Weldemariam, A.H.; Wondmeneh, T.G.; Sreeramareddy, C.T.; Pinheiro, M.; Yaseri, M.; Yu, C.; et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef]
- Whitehead, M.; Wickremasinghe, S.; Osborne, A.; van Wijngaarden, P.; Martin, K.R. Diabetic retinopathy: A complex pathophysiology requiring novel therapeutic strategies. Expert Opin. Biol. Ther. 2018, 18, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.E.; Knudtson, M.D.; Tsai, M.Y.; Klein, R. The relation of markers of inflammation and endothelial dysfunction to the prevalence and progression of diabetic retinopathy: Wisconsin epidemiologic study of diabetic retinopathy. Arch. Ophthalmol. 2009, 127, 1175–1182. [Google Scholar] [CrossRef]
- Stolk, R.P.; van Schooneveld, M.J.; Cruickshank, J.K.; Hughes, A.D.; Stanton, A.; Lu, J.; Patel, A.; Thom, S.A.M.G.; Grobbee, D.E.; Vingerling, J.R. Retinal vascular lesions in patients of caucasian and asian origin with type 2 diabetes. Diabetes Care 2008, 31, 708–713. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cachafeiro, M.; Bemelmans, A.P.; Samardzija, M.; Afanasieva, T.; Pournaras, J.A.; Grimm, C.; Kostic, C.; Philippe, S.; Wenzel, A.; Arsenijevic, Y. Hyperactivation of retina by light in mice leads to photoreceptor cell death mediated by VEGF and retinal pigment epithelium permeability. Cell Death Dis. 2013, 4, e781. [Google Scholar] [CrossRef] [PubMed]
- Noma, H.; Yasuda, K.; Shimura, M. Involvement of cytokines in the pathogenesis of diabetic macular edema. Int. J. Mol. Sci. 2021, 22, 3472. [Google Scholar] [CrossRef]
- Bandello, F.; Battaglia Parodi, M.; Lanzetta, P.; Loewenstein, A.; Massin, P.; Menchini, F.; Veritti, D. Diabetic macular edema. Dev. Ophthalmol. 2017, 58, 102–138. [Google Scholar] [CrossRef] [PubMed]
- Citirik, M. The impact of central foveal thickness on the efficacy of subthreshold micropulse yellow laser photocoagulation in diabetic macular edema. Lasers Med. Sci. 2019, 34, 907–912. [Google Scholar] [CrossRef]
- Romero-Aroca, P. Managing diabetic macular edema: The leading cause of diabetes blindness. World J. Diabetes 2011, 2, 98. [Google Scholar] [CrossRef] [PubMed]
- Cefalu, W.T.; Andersen, D.K.; Arreaza-Rubín, G.; Pin, C.L.; Sato, S.; Bruce Verchere, C.; Woo, M.; Rosenblum, N.D. Heterogeneity of Diabetes: B-Cells, Phenotypes, and Precision Medicine: Proceedings of an International Symposium of the Canadian Institutes of Health Research’s Institute of Nutrition, Metabolism and Diabetes and the U.S. National Institutes of Health’s. Diabetes Care 2022, 45, 3–22. [Google Scholar] [CrossRef]
- Koc, H.; Alpay, A.; Ugurbas, S.H. Comparison of the efficacy of intravitreal Anti-VEGF versus intravitreal dexamethasone implant in treatment resistant diabetic Macular Edema. BMC Ophthalmol. 2023, 23, 97. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Garcia-Arumi, J.; Bandello, F.; Berg, K.; Chakravarthy, U.; Gerendas, B.S.; Jonas, J.; Larsen, M.; Tadayoni, R.; Loewenstein, A. Guidelines for the management of diabetic macular edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2017, 237, 185–222. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Medina, J.J.; Rubio-Velazquez, E.; Foulquie-Moreno, E.; Casaroli-Marano, R.P.; Pinazo-Duran, M.D.; Zanon-Moreno, V.; Del-Rio-vellosillo, M. Update on the effects of antioxidants on diabetic retinopathy: In vitro experiments, animal studies and clinical trials. Antioxidants 2020, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Schiano, E.; Annunziata, G.; Ciampaglia, R.; Iannuzzo, F.; Maisto, M.; Tenore, G.C.; Novellino, E. Bioactive Compounds for the Management of Hypertriglyceridemia: Evidence From Clinical Trials and Putative Action Targets. Front. Nutr. 2020, 7, 586178. [Google Scholar] [CrossRef]
- Fanaro, G.B.; Marques, M.R.; da Calaza, K.C.; Brito, R.; Pessoni, A.M.; Mendonça, H.R.; Lemos, D.E.d.A.; de Brito Alves, J.L.; de Souza, E.L.; Cavalcanti Neto, M.P. New Insights on Dietary Polyphenols for the Management of Oxidative Stress and Neuroinflammation in Diabetic Retinopathy. Antioxidants 2023, 12, 1237. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.B.; Hwang, J.W.; Kim, Y.S.; Kim, E.K.; Park, P.J. Ocular promoting activity of grape polyphenols—A review. Environ. Toxicol. Pharmacol. 2017, 50, 83–90. [Google Scholar] [CrossRef]
- Almanza-Oliveros, A.; Bautista-Hernández, I.; Castro-López, C.; Aguilar-Zárate, P.; Meza-Carranco, Z.; Rojas, R.; Michel, M.R.; Martínez-Ávila, G.C.G. Grape Pomace—Advances in Its Bioactivity, Health Benefits, and Food Applications. Foods 2024, 13, 580. [Google Scholar] [CrossRef]
- Sun, Y.; Xiu, C.; Liu, W.; Tao, Y.; Wang, J.; Qu, Y. Grape seed proanthocyanidin extract protects the retina against early diabetic injury by activating the Nrf2 pathway. Exp. Ther. Med. 2016, 11, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Zhu, W.; Wu, Y.; Long, Y.; Liu, H.; Wan, W.; Wan, G.; Yu, J. Grape seed proanthocyanidin extract moderated retinal pigment epithelium cellular senescence through nampt/sirt1/NLRP3 pathway. J. Inflamm. Res. 2021, 14, 3129–3143. [Google Scholar] [CrossRef]
- Annunziata, G.; Solimeo, A.; de Angelis, V.; de Rosa, P.; Ciampaglia, R.; Guerra, F.; Maisto, M.; Tenore, G.C.; Novellino, E. Grape pomace polyphenols improve ophthalmic outcomes in patients with age-related macular degeneration (Amd) via reducing tmao serum levels and oxidative stress: Preliminary results from a pilot clinical trial. Pharmacologyonline 2021, 2, 1479–1498. [Google Scholar]
- Wu, L. Classification of diabetic retinopathy and diabetic macular edema. World J. Diabetes 2013, 4, 290. [Google Scholar] [CrossRef] [PubMed]
- The Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch. Ophthalmol. 1985, 103, 1796–1806. [Google Scholar] [CrossRef]
- Carnevali, A.; Vaccaro, S.; Borselli, M.; Bousyf, S.; Lamonica, L.; Randazzo, G.; Giannaccare, G.; Scorcia, V. Anatomical and Functional Effects of an Oral Supplementation of Bromelain and Curcugreen in Patients with Focal Diabetic Macular Edema. J. Clin. Med. 2023, 12, 7138. [Google Scholar] [CrossRef]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Tenore, G.C.; Novellino, E. Effects of grape pomace polyphenolic extract (Taurisolo®) in reducing tmao serum levels in humans: Preliminary results from a randomized, placebo-controlled, cross-over study. Nutrients 2019, 11, 139. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Hassan, S.T.S.; Tenore, G.C.; Novellino, E. Effect of grape pomace polyphenols with or without pectin on TMAO serum levels assessed by LC/MS-based assay: A preliminary clinical study on overweight/obese subjects. Front. Pharmacol. 2019, 10, 575. [Google Scholar] [CrossRef]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Bahr, T.A.; Bakri, S.J. Update on the Management of Diabetic Retinopathy: Anti-VEGF Agents for the Prevention of Complications and Progression of Nonproliferative and Proliferative Retinopathy. Life 2023, 13, 1098. [Google Scholar] [CrossRef]
- Bocsan, I.C.; Măgureanu, D.C.; Pop, R.M.; Levai, A.M.; Macovei, Ș.O.; Pătrașca, I.M.; Chedea, V.S.; Buzoianu, A.D. Antioxidant and Anti-Inflammatory Actions of Polyphenols from Red and White Grape Pomace in Ischemic Heart Diseases. Biomedicines 2022, 10, 2337. [Google Scholar] [CrossRef] [PubMed]
- Schiano, E.; Novellino, E.; Gámez Fernández, M.M.; Tiekou Lorinczova, H.; Tenore, G.C.; Iannuzzo, F.; Patel, V.B.; Somavarapu, S.; Zariwala, M.G. Antioxidant and Antidiabetic Properties of a Thinned-Nectarine-Based Nanoformulation in a Pancreatic β-Cell Line. Antioxidants 2024, 13, 63. [Google Scholar] [CrossRef]
- Hammes, H.P. Diabetic retinopathy: Hyperglycaemia, oxidative stress and beyond. Diabetologia 2018, 61, 29–38. [Google Scholar] [CrossRef]
- Campos, F.; Peixoto, A.F.; Fernandes, P.A.R.; Coimbra, M.A.; Mateus, N.; de Freitas, V.; Fernandes, I.; Fernandes, A. The Antidiabetic Effect of Grape Pomace Polysaccharide-Polyphenol Complexes. Nutrients 2021, 13, 4495. [Google Scholar] [CrossRef]
- Kumar, B.; Gupta, S.K.; Nag, T.C.; Srivastava, S.; Saxena, R.; Jha, K.A.; Srinivasan, B.P. Retinal neuroprotective effects of quercetin in streptozotocin-induced diabetic rats. Exp. Eye Res. 2014, 125, 193–202. [Google Scholar] [CrossRef]
- Abu-Amero, K.K.; Kondkar, A.A.; Chalam, K.V. Resveratrol and ophthalmic diseases. Nutrients 2016, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, S.; Ying, J.; Shi, T.; Wang, P. Resveratrol prevents ROS-induced apoptosis in high glucose-treated retinal capillary endothelial cells via the activation of AMPK/Sirt1/PGC-1 α pathway. Oxidative Med. Cell. Longev. 2017, 2017, 7584691. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, L.; Huang, K.; Zheng, L. Endoplasmic reticulum stress in retinal vascular degeneration: Protective role of resveratrol. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3241–3249. [Google Scholar] [CrossRef]
- Losso, J.N.; Truax, R.E.; Richard, G. Trans -Resveratrol inhibits hyperglycemia-induced inflammation and connexin downregulation in retinal pigment epithelial cells. J. Agric. Food Chem. 2010, 58, 8246–8252. [Google Scholar] [CrossRef]
| Parameters | Placebo Group (n = 50) | MaGPE Group (n = 49) | |||
|---|---|---|---|---|---|
| T0 | T6 | T0 | T6 | ||
| Male, n° (%) | 34 (68.0) | - | 35 (71.4) | - | ns |
| Age (years) | 66.4 ± 8.1 | - | 67.5 ± 8.7 | - | ns |
| HbA1c (%) | 7.9 ± 1.5 | 7.2 ± 4.1 | 6.8 ± 0.4 | 7.3 ± 3.1 | ns |
| HDL-C (mg/dL) | 36.3 ± 10.4 | 35.4 ± 11.2 | 37.0 ± 14.2 | 35.9 ± 9.6 | ns |
| LDL-C (mg/dL) | 98.3 ± 38.7 | 94.1 ± 33.1 | 108.9 ± 42.7 | 91.5 ± 32.1 | ns |
| TG (mg/dL) | 148.7 ± 64.5 | 158.5 ± 77.4 | 157.5 ± 63.6 | 153.5 ± 85.2 | ns |
| TC (mg/dL) | 158.5 ± 34.8 | 164.7± 29.4 | 177.4 ± 44.2 | 158.1 ± 27.7 | ns |
| ALT (UI/L) | 18.8 ± 7.6 | 17.4 ± 9.4 | 16.7 ± 10.6 | 17.3 ± 10.8 | ns |
| AST (UI/L) | 28.6 ± 13.8 | 30.0 ± 15.2 | 29.6 ± 17.8 | 31.0 ± 16.4 | ns |
| Uric acid (mg/dL) | 8.6 ± 6.2 | 8.4 ± 6.7 | 8.8 ± 7.2 | 8.2 ± 6.2 | ns |
| Cre (mg/dL) | 1.0 ± 0.5 | 1.1 ± 0.4 | 1.2 ± 0.5 | 1.3 ± 0.6 | ns |
| Parameters | Placebo Group (n = 50) | MaGPE Group (n = 49) | ||||
|---|---|---|---|---|---|---|
| T0 | T3 | T6 | T0 | T3 | T6 | |
| CRT (%) | 395.0 ± 130.2 | 302.1 ± 73.4 ** | 379.4 ± 144.4 | 492.6 ± 107.1 | 326.8 ± 99.1 **** | 369.8 ± 134.7 **** |
| BCVA (letters) | 0.287 ± 0.19 | 0.311 ± 0.19 | 0.299± 0.26 | 0.274 ± 0.20 | 0.337 ± 0.23 *** | 0.314 ± 0.20 ** |
| VP (%) | 38.9 ± 5.9 | 37.2 ± 7.0 | 36.7 ± 6.8 | 37.9 ± 5.8 | 39.0 ± 5.7 | 38.9 ± 7.5 |
| Parameters | Placebo Group (n = 50) | MaGPE Group (n = 49) | ||||
|---|---|---|---|---|---|---|
| T0 | T3 | T6 | T0 | T3 | T6 | |
| dROMs (UCARR) | 1112.5 ± 450.2 | 1095.0 ± 465.4 | 1115.0 ± 464.3 | 1100.6 ± 430.1 | 974.8 ± 390.2 | 930.6 ± 310.3 * |
| ox-LDL (µEq/L) | 974.8 ± 208.3 | 962. ± 213.4 | 978.0 ± 201.5 | 953.9 ± 212.4 | 867. ± 209.5 * | 735.0 ± 213.7 **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiano, E.; Vaccaro, S.; Scorcia, V.; Carnevali, A.; Borselli, M.; Chisari, D.; Guerra, F.; Iannuzzo, F.; Tenore, G.C.; Giannaccare, G.; et al. From Vineyard to Vision: Efficacy of Maltodextrinated Grape Pomace Extract (MaGPE) Nutraceutical Formulation in Patients with Diabetic Retinopathy. Nutrients 2024, 16, 2850. https://doi.org/10.3390/nu16172850
Schiano E, Vaccaro S, Scorcia V, Carnevali A, Borselli M, Chisari D, Guerra F, Iannuzzo F, Tenore GC, Giannaccare G, et al. From Vineyard to Vision: Efficacy of Maltodextrinated Grape Pomace Extract (MaGPE) Nutraceutical Formulation in Patients with Diabetic Retinopathy. Nutrients. 2024; 16(17):2850. https://doi.org/10.3390/nu16172850
Chicago/Turabian StyleSchiano, Elisabetta, Sabrina Vaccaro, Vincenzo Scorcia, Adriano Carnevali, Massimiliano Borselli, Domenico Chisari, Fabrizia Guerra, Fortuna Iannuzzo, Gian Carlo Tenore, Giuseppe Giannaccare, and et al. 2024. "From Vineyard to Vision: Efficacy of Maltodextrinated Grape Pomace Extract (MaGPE) Nutraceutical Formulation in Patients with Diabetic Retinopathy" Nutrients 16, no. 17: 2850. https://doi.org/10.3390/nu16172850
APA StyleSchiano, E., Vaccaro, S., Scorcia, V., Carnevali, A., Borselli, M., Chisari, D., Guerra, F., Iannuzzo, F., Tenore, G. C., Giannaccare, G., & Novellino, E. (2024). From Vineyard to Vision: Efficacy of Maltodextrinated Grape Pomace Extract (MaGPE) Nutraceutical Formulation in Patients with Diabetic Retinopathy. Nutrients, 16(17), 2850. https://doi.org/10.3390/nu16172850











