NAD+ and Niacin Supplementation as Possible Treatments for Glaucoma and Age-Related Macular Degeneration: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
3. Overview of Oxidative Stress
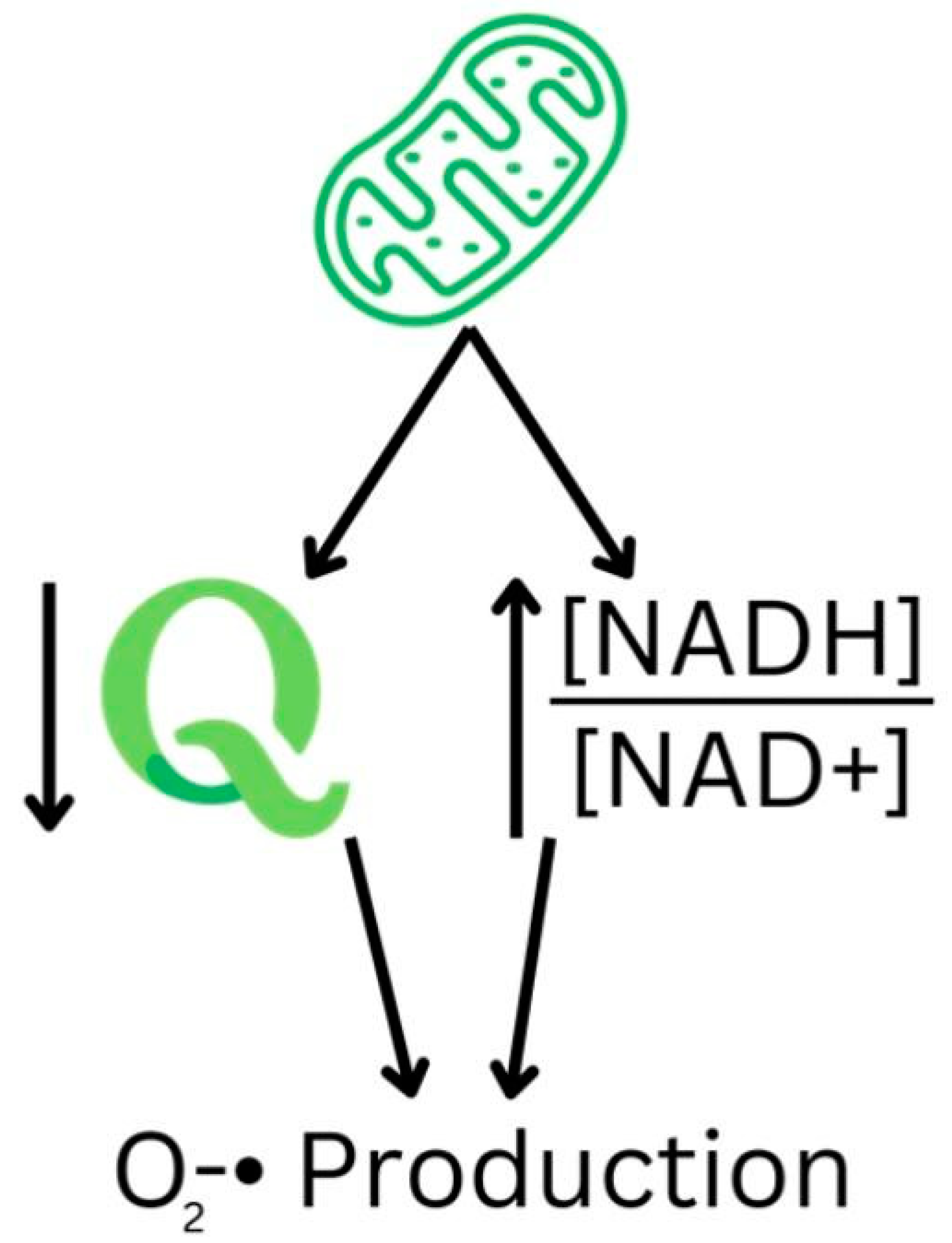
3.1. Reactive Oxygen Species Production and Pathogenesis
3.2. Role of NAD+ in NADPH Oxidase and ROS Production
3.3. Oxidative Stress and Retinal Diseases
4. Effect of Niacin on Glaucoma
5. Effect of Niacin on AMD
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wentz, S.M.; Kim, N.J.; Wang, J.; Amireskandari, A.; Siesky, B.; Harris, A. Novel therapies for open-angle glaucoma. F1000Prime Rep. 2014, 6, 102. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Cimaglia, G.; Votruba, M.; Morgan, J.E.; Andre, H.; Williams, P.A. Potential Therapeutic Benefit of NAD(+) Supplementation for Glaucoma and Age-Related Macular Degeneration. Nutrients 2020, 12, 2871. [Google Scholar] [CrossRef]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Cueva Vargas, J.L.; Di Polo, A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.A.; Higginbotham, E.J. Glaucoma and its treatment: A review. Am. J. Health Syst. Pharm. 2005, 62, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Kouassi Nzoughet, J.; Chao de la Barca, J.M.; Guehlouz, K.; Leruez, S.; Coulbault, L.; Allouche, S.; Bocca, C.; Muller, J.; Amati-Bonneau, P.; Gohier, P.; et al. Nicotinamide Deficiency in Primary Open-Angle Glaucoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2509–2514. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef]
- Hui, F.; Tang, J.; Williams, P.A.; McGuinness, M.B.; Hadoux, X.; Casson, R.J.; Coote, M.; Trounce, I.A.; Martin, K.R.; van Wijngaarden, P.; et al. Improvement in inner retinal function in glaucoma with nicotinamide (vitamin B3) supplementation: A crossover randomized clinical trial. Clin. Exp. Ophthalmol. 2020, 48, 903–914. [Google Scholar] [CrossRef]
- Metelitsina, T.I.; Grunwald, J.E.; DuPont, J.C.; Ying, G.S. Effect of niacin on the choroidal circulation of patients with age related macular degeneration. Br. J. Ophthalmol. 2004, 88, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- Taechameekietichai, T.; Chansangpetch, S.; Peerawaranun, P.; Lin, S.C. Association between Daily Niacin Intake and Glaucoma: National Health and Nutrition Examination Survey. Nutrients 2021, 13, 4263. [Google Scholar] [CrossRef]
- Dajani, H.M.; Lauer, A.K. Optical coherence tomography findings in niacin maculopathy. Can. J. Ophthalmol. 2006, 41, 197–200. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Verdin, E. NAD+ in aging, metabolism, and neurodegeneration. Science 2015, 350, 1208–1213. [Google Scholar] [CrossRef]
- Magni, G.; Amici, A.; Emanuelli, M.; Orsomando, G.; Raffaelli, N.; Ruggieri, S. Enzymology of NAD+ homeostasis in man. Cell. Mol. Life Sci. (CMLS) 2004, 61, 19–34. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, C.; Feng, C.; Yan, C.; Yu, Y.; Chen, Z.; Guo, C.; Wang, X. Role of mitochondrial reactive oxygen species in homeostasis regulation. Redox Rep. 2022, 27, 45–52. [Google Scholar] [CrossRef]
- Emmert, H.; Fonfara, M.; Rodriguez, E.; Weidinger, S. NADPH oxidase inhibition rescues keratinocytes from elevated oxidative stress in a 2D atopic dermatitis and psoriasis model. Exp. Dermatol. 2020, 29, 749–758. [Google Scholar] [CrossRef]
- Benavente, C.A.; Jacobson, E.L. Niacin restriction upregulates NADPH oxidase and reactive oxygen species (ROS) in human keratinocytes. Free Radic. Biol. Med. 2008, 44, 527–537. [Google Scholar] [CrossRef]
- Yokota, H.; Narayanan, S.P.; Zhang, W.; Liu, H.; Rojas, M.; Xu, Z.; Lemtalsi, T.; Nagaoka, T.; Yoshida, A.; Brooks, S.E.; et al. Neuroprotection from retinal ischemia/reperfusion injury by NOX2 NADPH oxidase deletion. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8123–8131. [Google Scholar] [CrossRef] [PubMed]
- Revollo, J.R.; Grimm, A.A.; Imai, S.-I. The NAD Biosynthesis Pathway Mediated by Nicotinamide Phosphoribosyltransferase Regulates Sir2 Activity in Mammalian Cells. J. Biol. Chem. 2004, 279, 50754–50763. [Google Scholar] [CrossRef] [PubMed]
- Jadeja, R.N.; Powell, F.L.; Jones, M.A.; Fuller, J.; Joseph, E.; Thounaojam, M.C.; Bartoli, M.; Martin, P.M. Loss of NAMPT in aging retinal pigment epithelium reduces NAD+ availability and promotes cellular senescence. Aging 2018, 10, 1306–1323. [Google Scholar] [CrossRef]
- Lautrup, S.; Sinclair, D.A.; Mattson, M.P.; Fang, E.F. NAD+ in Brain Aging and Neurodegenerative Disorders. Cell Metab. 2019, 30, 630–655. [Google Scholar] [CrossRef]
- Cantó, C.; Keir; Auwerx, J. NAD+ Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015, 22, 31–53. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Kausar, S.; Wang, F.; Cui, H. The Role of Mitochondria in Reactive Oxygen Species Generation and Its Implications for Neurodegenerative Diseases. Cells 2018, 7, 274. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020, 79, 100858. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, S.G.; Boulton, M.E. Consequences of oxidative stress in age-related macular degeneration. Mol. Asp. Med. 2012, 33, 399–417. [Google Scholar] [CrossRef]
- Fisher, C.R.; Ferrington, D.A. Perspective on AMD Pathobiology: A Bioenergetic Crisis in the RPE. Investig. Opthalmol. Vis. Sci. 2018, 59, AMD41. [Google Scholar] [CrossRef]
- Dvoriantchikova, G.; Grant, J.; Santos, A.R.; Hernandez, E.; Ivanov, D. Neuronal NAD(P)H oxidases contribute to ROS production and mediate RGC death after ischemia. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2823–2830. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahsan, H. Biomarkers of inflammation and oxidative stress in ophthalmic disorders. J. Immunoass. Immunochem. 2020, 41, 257–271. [Google Scholar] [CrossRef]
- Tezel, G. Oxidative stress in glaucomatous neurodegeneration: Mechanisms and consequences. Prog. Retin. Eye Res. 2006, 25, 490–513. [Google Scholar] [CrossRef]
- Qu, J.; Wang, D.; Grosskreutz, C.L. Mechanisms of retinal ganglion cell injury and defense in glaucoma. Exp. Eye Res. 2010, 91, 48–53. [Google Scholar] [CrossRef]
- Osborne, N.N. Mitochondria: Their role in ganglion cell death and survival in primary open angle glaucoma. Exp. Eye Res. 2010, 90, 750–757. [Google Scholar] [CrossRef]
- Kamel, K.; Farrell, M.; O’Brien, C. Mitochondrial dysfunction in ocular disease: Focus on glaucoma. Mitochondrion 2017, 35, 44–53. [Google Scholar] [CrossRef]
- Yildirim, Z.; Ucgun, N.I.; Yildirim, F. The role of oxidative stress and antioxidants in the pathogenesis of age-related macular degeneration. Clinics 2011, 66, 743–746. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, K.-K.; Tong, Y.; Zhou, Y.-L.; Wang, Y.-X.; Zhao, P.-Q.; Wang, Z.-Y. Exogenous NAD+ decreases oxidative stress and protects H2O2-treated RPE cells against necrotic death through the up-regulation of autophagy. Sci. Rep. 2016, 6, 26322. [Google Scholar] [CrossRef]
- Hyttinen, J.M.T.; Viiri, J.; Kaarniranta, K.; Błasiak, J. Mitochondrial quality control in AMD: Does mitophagy play a pivotal role? Cell. Mol. Life Sci. 2018, 75, 2991–3008. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Leung, K.W.; Zhang, Y.H.; Duan, S.; Zhong, X.F.; Jiang, R.Z.; Peng, Z.; Tombran-Tink, J.; Ge, J. Mitochondrial complex I defect induces ROS release and degeneration in trabecular meshwork cells of POAG patients: Protection by antioxidants. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1447–1458. [Google Scholar] [CrossRef]
- Nagaoka, T.; Kuo, L.; Ren, Y.; Yoshida, A.; Hein, T.W. C-reactive protein inhibits endothelium-dependent nitric oxide-mediated dilation of retinal arterioles via enhanced superoxide production. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2053–2060. [Google Scholar] [CrossRef]
- Al-Shabrawey, M.; Rojas, M.; Sanders, T.; Behzadian, A.; El-Remessy, A.; Bartoli, M.; Parpia, A.K.; Liou, G.; Caldwell, R.B. Role of NADPH oxidase in retinal vascular inflammation. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3239–3244. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.A.; Harder, J.M.; Foxworth, N.E.; Cochran, K.E.; Philip, V.M.; Porciatti, V.; Smithies, O.; John, S.W.M. Vitamin B <sub>3</sub> modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science 2017, 355, 756–760. [Google Scholar] [CrossRef]
- D’Angelo, A.; Vitiello, L.; Lixi, F.; Abbinante, G.; Coppola, A.; Gagliardi, V.; Pellegrino, A.; Giannaccare, G. Optic Nerve Neuroprotection in Glaucoma: A Narrative Review. J. Clin. Med. 2024, 13, 2214. [Google Scholar] [CrossRef] [PubMed]
- Tribble, J.R.; Otmani, A.; Sun, S.; Ellis, S.A.; Cimaglia, G.; Vohra, R.; Jöe, M.; Lardner, E.; Venkataraman, A.P.; Domínguez-Vicent, A.; et al. Nicotinamide provides neuroprotection in glaucoma by protecting against mitochondrial and metabolic dysfunction. Redox Biol. 2021, 43, 101988. [Google Scholar] [CrossRef]
- Chou, T.-H.; Romano, G.L.; Amato, R.; Porciatti, V. Nicotinamide-Rich Diet in DBA/2J Mice Preserves Retinal Ganglion Cell Metabolic Function as Assessed by PERG Adaptation to Flicker. Nutrients 2020, 12, 1910. [Google Scholar] [CrossRef] [PubMed]
- Charng, J.; Ansari, A.S.; Bondonno, N.P.; Hunter, M.L.; O’Sullivan, T.A.; Louca, P.; Hammond, C.J.; Mackey, D.A. Association between dietary niacin and retinal nerve fibre layer thickness in healthy eyes of different ages. Clin. Exp. Ophthalmol. 2022, 50, 736–744. [Google Scholar] [CrossRef]
- Ramdas, W.; Schouten, J.; Webers, C. The Effect of Vitamins on Glaucoma: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 359. [Google Scholar] [CrossRef]
- Lee, S.; Van Bergen, N.J.; Kong, G.Y.; Chrysostomou, V.; Waugh, H.S.; O’Neill, E.C.; Crowston, J.G.; Trounce, I.A. Mitochondrial dysfunction in glaucoma and emerging bioenergetic therapies. Exp. Eye Res. 2011, 93, 204–212. [Google Scholar] [CrossRef]
- Katsyuba, E.; Romani, M.; Hofer, D.; Auwerx, J. NAD+ homeostasis in health and disease. Nat. Metab. 2020, 2, 9–31. [Google Scholar] [CrossRef]
- Williams, P.A.; Harder, J.M.; Cardozo, B.H.; Foxworth, N.E.; John, S.W.M. Nicotinamide treatment robustly protects from inherited mouse glaucoma. Commun. Integr. Biol. 2018, 11, e1356956. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, N.; Chrenek, M.A.; Girardot, P.E.; Wang, J.; Sellers, J.T.; Geisert, E.E.; Brenner, C.; Nickerson, J.M.; Boatright, J.H.; et al. Systemic Treatment with Nicotinamide Riboside Is Protective in Two Mouse Models of Retinal Ganglion Cell Damage. Pharmaceutics 2021, 13, 893. [Google Scholar] [CrossRef]
- Jung, K.; Kim, Y.; Park, C. Dietary Niacin and Open-Angle Glaucoma: The Korean National Health and Nutrition Examination Survey. Nutrients 2018, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, C.G.; John, S.W.M.; Williams, P.A.; Blumberg, D.M.; Cioffi, G.A.; Liebmann, J.M. Nicotinamide and Pyruvate for Neuroenhancement in Open-Angle Glaucoma. JAMA Ophthalmol. 2022, 140, 11. [Google Scholar] [CrossRef]
- Shin, H.-T.; Yoon, B.W.; Seo, J.H. Comparison of risk allele frequencies of single nucleotide polymorphisms associated with age-related macular degeneration in different ethnic groups. BMC Ophthalmol. 2021, 21, 97. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A. The Diagnosis and Treatment of Age-Related Macular Degeneration. Dtsch. Ärzteblatt Int. 2020, 117, 513. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Flaxel, C.J.; Adelman, R.A.; Bailey, S.T.; Fawzi, A.; Lim, J.I.; Vemulakonda, G.A.; Ying, G.-S. Age-Related Macular Degeneration Preferred Practice Pattern®. Ophthalmology 2020, 127, P1–P65. [Google Scholar] [CrossRef]
- Jadeja, R.N.; Thounaojam, M.C.; Bartoli, M.; Martin, P.M. Implications of NAD+ Metabolism in the Aging Retina and Retinal Degeneration. Oxidative Med. Cell. Longev. 2020, 2020, 2692794. [Google Scholar] [CrossRef]
- Pawlowska, E.; Szczepanska, J.; Koskela, A.; Kaarniranta, K.; Blasiak, J. Dietary Polyphenols in Age-Related Macular Degeneration: Protection against Oxidative Stress and Beyond. Oxidative Med. Cell. Longev. 2019, 2019, 9682318. [Google Scholar] [CrossRef]
- Mills, K.F.; Yoshida, S.; Stein, L.R.; Grozio, A.; Kubota, S.; Sasaki, Y.; Redpath, P.; Migaud, M.E.; Apte, R.S.; Uchida, K.; et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016, 24, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.-K.; Kim, Y.A.; Park, U.C.; Kwon, S.-H.; Lee, Y.; Yoo, H.J.; Seo, J.H.; Yu, H.G. Vitreous Fatty Amides and Acyl Carnitines Are Altered in Intermediate Age-Related Macular Degeneration. Investig. Opthalmol. Vis. Sci. 2023, 64, 28. [Google Scholar] [CrossRef]
- Agrón, E.; Mares, J.; Clemons, T.E.; Swaroop, A.; Chew, E.Y.; Keenan, T.D.L. Dietary Nutrient Intake and Progression to Late Age-Related Macular Degeneration in the Age-Related Eye Disease Studies 1 and 2. Ophthalmology 2021, 128, 425–442. [Google Scholar] [CrossRef]
- Merle, B.M.J.; Barthes, S.; Féart, C.; Cougnard-Grégoire, A.; Korobelnik, J.-F.; Rougier, M.-B.; Delyfer, M.-N.; Delcourt, C. B Vitamins and Incidence of Advanced Age-Related Macular Degeneration: The Alienor Study. Nutrients 2022, 14, 2821. [Google Scholar] [CrossRef]
- Kráľová, J.Š.; Kolář, P.; Kapounová, Z.; Veselý, P.; Derflerová Brázdová, Z. Dietary habits and dietary nutrient intake in patients with age-related macular degeneration: A case-control study. Cent. Eur. J. Public Health 2023, 31, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.R.; Metelitsina, T.I.; Dupont, J.C.; Grunwald, J.E. Effect of Niacin on Retinal Vascular Diameter in Patients with Age-Related Macular Degeneration. Curr. Eye Res. 2006, 31, 629–634. [Google Scholar] [CrossRef]
- Evans, J.R.; Lawrenson, J.G. Antioxidant vitamin and mineral supplements for preventing age-related macular degeneration. Cochrane Database Syst. Rev. 2017, 2017. [Google Scholar] [CrossRef]
| Effect | Study Type | Details/Findings |
|---|---|---|
| Protection against retinal ganglion cell (RGC) loss | Animal studies | Nicotinamide (NAM) supplementation maintains RGC density and function in glaucoma-prone mice [49,50,51,52]. |
| Preservation of mitochondrial function | Animal studies | NAM supplementation transiently increases mitochondrial size and motility, assisting oxidative phosphorylation [49,50]. |
| Reduction in ROS production | In vitro and animal studies | NADPH oxidase inhibition or NAD+ replenishment reduces ROS production and oxidative stress [9,55]. |
| Prevention of RGC apoptosis | In vitro studies | NADPH oxidase inhibition protects RGCs from oxidative stress and apoptosis [17,18,19,20,25]. |
| Inverse correlation with glaucoma prevalence | Population-based studies | A high dietary niacin intake is associated with a lower risk of developing glaucoma [11,54]. |
| Improvement in visual field sensitivity | Randomized controlled trial | High-dose NAM and pyruvate supplementation improves visual field sensitivity in patients with glaucoma [19,20]. |
| Reported side effects of NAM supplementation | Randomized controlled trial | NAM supplementation may cause mild gastrointestinal discomfort, with a dose-dependent relationship [9,55]. |
| Effect | Study Type | Details/Findings |
|---|---|---|
| Improvement in rod cell function | Animal studies | Nicotinamide supplementation prevented deficits in rod cell function in aged mice [62]. |
| Protection against oxidative stress | In vitro and animal studies | NAD+ supplementation may protect retinal cells from oxidative stress and improve RPE health [29,60]. |
| Increases in choroidal blood flow | Human studies | Niacin transiently increased choroidal blood volume in patients with AMD [10]. |
| Lower niacin levels in AMD patients | Case–control studies | Patients with AMD had a lower dietary intake of niacin compared to controls [63,66]. |
| Potential retinal arteriole dilation | Human studies | Niacin may assist in dilating retinal arterioles, potentially mitigating ischemic damage in AMD [64,65]. |
| No association with AMD progression | Population-based studies | Some studies found no association between dietary niacin intake and a decreased risk of AMD progression [67]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gemae, M.R.; Bassi, M.D.; Wang, P.; Chin, E.K.; Almeida, D.R.P. NAD+ and Niacin Supplementation as Possible Treatments for Glaucoma and Age-Related Macular Degeneration: A Narrative Review. Nutrients 2024, 16, 2795. https://doi.org/10.3390/nu16162795
Gemae MR, Bassi MD, Wang P, Chin EK, Almeida DRP. NAD+ and Niacin Supplementation as Possible Treatments for Glaucoma and Age-Related Macular Degeneration: A Narrative Review. Nutrients. 2024; 16(16):2795. https://doi.org/10.3390/nu16162795
Chicago/Turabian StyleGemae, Mohamed R., Mario D. Bassi, Patrick Wang, Eric K. Chin, and David R.P. Almeida. 2024. "NAD+ and Niacin Supplementation as Possible Treatments for Glaucoma and Age-Related Macular Degeneration: A Narrative Review" Nutrients 16, no. 16: 2795. https://doi.org/10.3390/nu16162795
APA StyleGemae, M. R., Bassi, M. D., Wang, P., Chin, E. K., & Almeida, D. R. P. (2024). NAD+ and Niacin Supplementation as Possible Treatments for Glaucoma and Age-Related Macular Degeneration: A Narrative Review. Nutrients, 16(16), 2795. https://doi.org/10.3390/nu16162795






