The Anticancer Potential of Edible Mushrooms: A Review of Selected Species from Roztocze, Poland
Abstract
1. Introduction
2. Mushrooms from Polish Forests as a Potential Source of Anticancer Compounds: Current Research and Findings
2.1. Order Boletales
2.1.1. Boletus edulis
Polysaccharides
Proteins
Nanoparticles
Extracts
2.1.2. Boletus reticulatus Schaeff
Polysaccharides
2.1.3. Leccinum scabrum
Extracts
2.1.4. Suillus granulatus
Extracts
Small Molecules
2.1.5. Suillus luteus
Extracts
Small Molecules
2.2. Order Agaricales
2.2.1. Armillaria mellea
Polysaccharides
Small Molecules
2.2.2. Macrolepiota procera
Extracts
Small Molecules
2.2.3. Tricholoma equestre
Extracts
Small Molecules
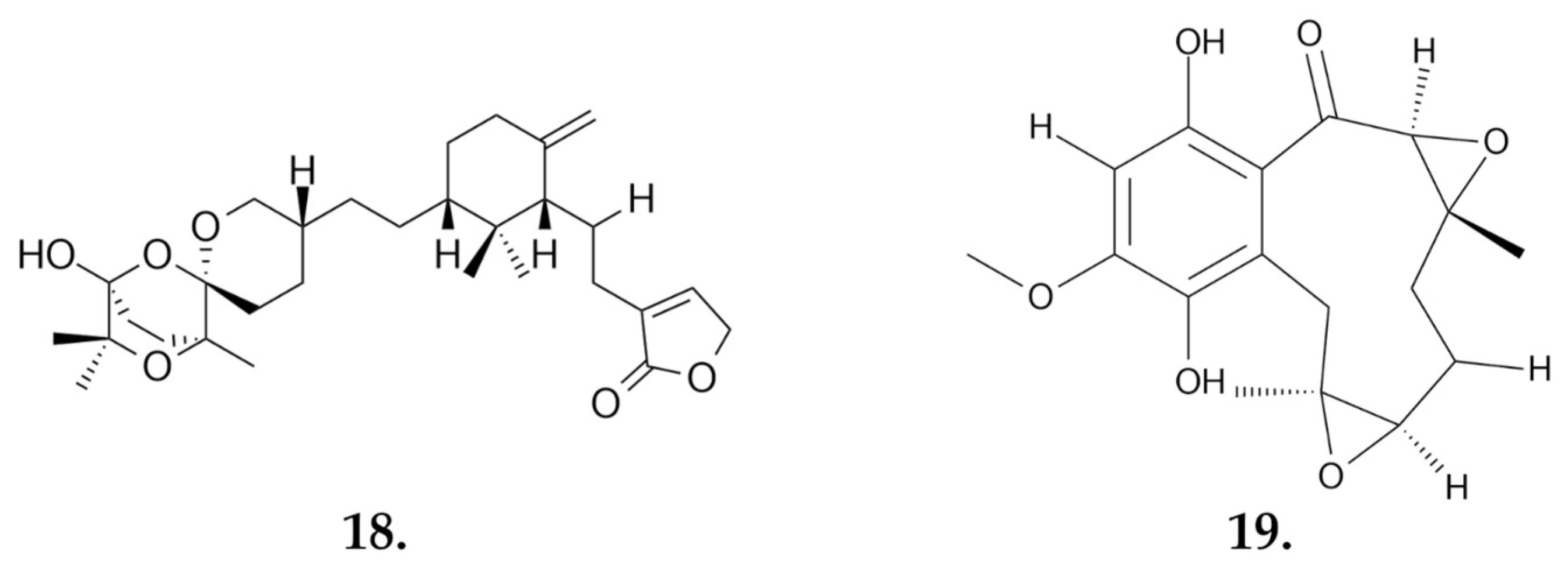
2.2.4. Tricholoma terreum
Extracts
Small Molecules
2.3. Order Cantharellales
2.3.1. Cantharellus cibarius
Polysaccharides
Extracts
2.4. Order Russulales
2.4.1. Lactarius deliciosus
Oligosaccharides
Polysaccharides
Extracts
3. The Scientific Literature Lacks Reports on the Anticancer Activity of Many Edible Fungi Commonly Found in Polish Forests
4. Limitations of the Use of Edible Mushrooms Compared to Anticancer Agents
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Z.; Eichler, B.; Klausner, E.A.; Duffy-Matzner, J.; Zheng, W. Lead/Drug Discovery from Natural Resources. Molecules 2022, 27, 8280. [Google Scholar] [CrossRef] [PubMed]
- Roupas, P.; Keogh, J.; Noakes, M.; Margetts, C.; Taylor, P. The Role of Edible Mushrooms in Health: Evaluation of the Evidence. J. Funct. Foods 2012, 4, 687–709. [Google Scholar] [CrossRef]
- Cerletti, C.; Esposito, S.; Iacoviello, L. Edible Mushrooms and Beta-Glucans: Impact on Human Health. Nutrients 2021, 13, 2195. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Bian, Z.; Xu, B. Beta-Glucans from Edible and Medicinal Mushrooms: Characteristics, Physicochemical and Biological Activities. J. Food Compos. Anal. 2015, 41, 165–173. [Google Scholar] [CrossRef]
- Asahi, T.; Wu, X.; Shimoda, H.; Hisaka, S.; Harada, E.; Kanno, T.; Nakamura, Y.; Kato, Y.; Osawa, T. A Mushroom-Derived Amino Acid, Ergothioneine, Is a Potential Inhibitor of Inflammation-Related DNA Halogenation. Biosci. Biotechnol. Biochem. 2016, 80, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Lam-Sidun, D.; Peters, K.M.; Borradaile, N.M. Mushroom-Derived Medicine? Preclinical Studies Suggest Potential Benefits of Ergothioneine for Cardiometabolic Health. Int. J. Mol. Sci. 2021, 22, 3246. [Google Scholar] [CrossRef]
- El Enshasy, H.A.; Hatti-Kaul, R. Mushroom Immunomodulators: Unique Molecules with Unlimited Applications. Trends Biotechnol. 2013, 31, 668–677. [Google Scholar] [CrossRef]
- Boh, B.; Berovic, M.; Zhang, J.; Zhi-Bin, L. Ganoderma lucidum and Its Pharmaceutically Active Compounds. In Biotechnology Annual Review; Elsevier: Amsterdam, The Netherlands, 2007; Volume 13, pp. 265–301. ISBN 978-0-444-53032-5. [Google Scholar]
- Ekiz, E.; Oz, E.; Abd El-Aty, A.M.; Proestos, C.; Brennan, C.; Zeng, M.; Tomasevic, I.; Elobeid, T.; Çadırcı, K.; Bayrak, M.; et al. Exploring the Potential Medicinal Benefits of Ganoderma lucidum: From Metabolic Disorders to Coronavirus Infections. Foods 2023, 12, 1512. [Google Scholar] [CrossRef]
- Habtemariam, S. Trametes versicolor (Synn. Coriolus versicolor) Polysaccharides in Cancer Therapy: Targets and Efficacy. Biomedicines 2020, 8, 135. [Google Scholar] [CrossRef]
- Standish, L.J.; Wenner, C.A.; Sweet, E.S.; Bridge, C.; Nelson, A.; Martzen, M.; Novack, J.; Torkelson, C. Trametes versicolor Mushroom Immune Therapy in Breast Cancer. J. Soc. Integr. Oncol. 2008, 6, 122–128. [Google Scholar]
- Tišma, M.; Žnidaršič-Plazl, P.; Šelo, G.; Tolj, I.; Šperanda, M.; Bucić-Kojić, A.; Planinić, M. Trametes versicolor in Lignocellulose-Based Bioeconomy: State of the Art, Challenges and Opportunities. Bioresour. Technol. 2021, 330, 124997. [Google Scholar] [CrossRef] [PubMed]
- Li, I.-C.; Chang, H.-H.; Lin, C.-H.; Chen, W.-P.; Lu, T.-H.; Lee, L.-Y.; Chen, Y.-W.; Chen, Y.-P.; Chen, C.-C.; Lin, D.P.-C. Prevention of Early Alzheimer’s Disease by Erinacine A-Enriched Hericium Erinaceus mycelia Pilot Double-Blind Placebo-Controlled Study. Front. Aging Neurosci. 2020, 12, 155. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.who.int/today/ (accessed on 15 August 2024).
- Kayl, A.E.; Meyers, C.A. Side-Effects of Chemotherapy and Quality of Life in Ovarian and Breast Cancer Patients. Curr. Opin. Obstet. Gynecol. 2006, 18, 24–28. [Google Scholar] [CrossRef]
- Tan, Y.; Zeng, N.-K.; Xu, B. Chemical Profiles and Health-Promoting Effects of Porcini Mushroom (Boletus edulis): A Narrative Review. Food Chem. 2022, 390, 133199. [Google Scholar] [CrossRef]
- Lemieszek, M.K.; Cardoso, C.; Ferreira Milheiro Nunes, F.H.; Ramos Novo Amorim De Barros, A.I.; Marques, G.; Pożarowski, P.; Rzeski, W. Boletus edulis Biologically Active Biopolymers Induce Cell Cycle Arrest in Human Colon Adenocarcinoma Cells. Food Funct. 2013, 4, 575. [Google Scholar] [CrossRef] [PubMed]
- Lemieszek, M.K.; Ribeiro, M.; Marques, G.; Nunes, F.M.; Pożarowski, P.; Rzeski, W. New Insights into the Molecular Mechanism of Boletus edulis Ribonucleic Acid Fraction (BE3) Concerning Antiproliferative Activity on Human Colon Cancer Cells. Food Funct 2017, 8, 1830–1839. [Google Scholar] [CrossRef]
- Lemieszek, M.K.; Ribeiro, M.; Guichard Alves, H.; Marques, G.; Nunes, F.M.; Rzeski, W. Boletus edulis Ribonucleic Acid—A Potent Apoptosis Inducer in Human Colon Adenocarcinoma Cells. Food Funct. 2016, 7, 3163–3175. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Yu, S.; Ji, H.; Xu, X.; Liu, A. A Novel Acid Polysaccharide from Boletus edulis: Extraction, Characteristics and Antitumor Activities in Vitro. Glycoconj. J. 2021, 38, 13–24. [Google Scholar] [CrossRef]
- Wang, D.; Sun, S.-Q.; Wu, W.-Z.; Yang, S.-L.; Tan, J.-M. Characterization of a Water-Soluble Polysaccharide from Boletus edulis and Its Antitumor and Immunomodulatory Activities on Renal Cancer in Mice. Carbohydr. Polym. 2014, 105, 127–134. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, R.; Liu, F.; Ng, T.B. Purification and Characterization of a Novel Protein with Activity against Non-Small-Cell Lung Cancer in Vitro and in Vivo from the Edible Mushroom Boletus edulis. Int. J. Biol. Macromol. 2021, 174, 77–88. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, F.; Ng, T.B. Interrelationship among Paraptosis, Apoptosis and Autophagy in Lung Cancer A549 Cells Induced by BEAP, an Antitumor Protein Isolated from the Edible Porcini Mushroom Boletus edulis. Int. J. Biol. Macromol. 2021, 188, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Bovi, M.; Carrizo, M.E.; Capaldi, S.; Perduca, M.; Chiarelli, L.R.; Galliano, M.; Monaco, H.L. Structure of a Lectin with Antitumoral Properties in King Bolete (Boletus edulis) Mushrooms. Glycobiology 2011, 21, 1000–1009. [Google Scholar] [CrossRef]
- Bovi, M.; Cenci, L.; Perduca, M.; Capaldi, S.; Carrizo, M.E.; Civiero, L.; Chiarelli, L.R.; Galliano, M.; Monaco, H.L. BEL-Trefoil: A Novel Lectin with Antineoplastic Properties in King Bolete (Boletus edulis) Mushrooms. Glycobiology 2013, 23, 578–592. [Google Scholar] [CrossRef]
- Kaplan, Ö.; Gökşen Tosun, N.; Özgür, A.; Erden Tayhan, S.; Bilgin, S.; Türkekul, İ.; Gökce, İ. Microwave-Assisted Green Synthesis of Silver Nanoparticles Using Crude Extracts of Boletus edulis and Coriolus versicolor: Characterization, Anticancer, Antimicrobial and Wound Healing Activities. J. Drug Deliv. Sci. Technol. 2021, 64, 102641. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Rančić, A.; Stanojković, T. Evaluation of Metal Concentration and Antioxidant, Antimicrobial, and Anticancer Potentials of Two Edible Mushrooms Lactarius deliciosus and Macrolepiota procera. J. Food Drug Anal. 2016, 24, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Širić, I.; Ivica, K.; Bedeković, D.; Kaić, A.; Kasap, A. Heavy Metals in Edible Mushroom Boletus reticulatus Schaeff. Collected from Zrin Mountain, Croatia. Period. Biol. 2014, 116, 319–322. [Google Scholar]
- Su, S.; Ding, X.; Hou, Y.; Liu, B.; Du, Z.; Liu, J. Structure Elucidation, Immunomodulatory Activity, Antitumor Activity and Its Molecular Mechanism of a Novel Polysaccharide from Boletus reticulatus Schaeff. Food Sci. Hum. Wellness 2023, 12, 647–661. [Google Scholar] [CrossRef]
- Gąsecka, M.; Siwulski, M.; Magdziak, Z.; Budzyńska, S.; Stuper-Szablewska, K.; Niedzielski, P.; Mleczek, M. The Effect of Drying Temperature on Bioactive Compounds and Antioxidant Activity of Leccinum scabrum (Bull.) Gray and Hericium erinaceus (Bull.) Pers. J. Food Sci. Technol. 2020, 57, 513–525. [Google Scholar] [CrossRef]
- Tubić Vukajlović, J. Evaluation of biological activities of acetone extract of the mushroom Leccinum scabrum. Farmacia 2021, 69, 974–979. [Google Scholar] [CrossRef]
- Stojanova, M.; Pantić, M.; Karadelev, M.; Ivanovski, V.; Nikšić, M. Determination of Biological Activity of Suillus granulatus Mushroom Extracts. J. Food Meas. Charact. 2022, 16, 4564–4572. [Google Scholar] [CrossRef]
- Tringali, C.; Geraci, C.; Nicolosi, G.; Verbist, J.-F.; Roussakis, C. An Antitutmor Principle from Suillus granulatus. J. Nat. Prod. 1989, 52, 844–845. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xiong, M.; Yang, X.; Yao, L.; Wang, Z.; Wang, L.; Li, Z.; Zhang, J.; Lv, J. Six New Polyphenolic Metabolites Isolated from the Suillus granulatus and Their Cytotoxicity against HepG2 Cells. Front. Nutr. 2024, 11, 1390256. [Google Scholar] [CrossRef]
- Chudzyński, K.; Jarzyńska, G.; Stefańska, A.; Falandysz, J. Mercury Content and Bio-Concentration Potential of Slippery Jack, Suillus luteus, Mushroom. Food Chem. 2011, 125, 986–990. [Google Scholar] [CrossRef]
- Jaworska, G.; Pogoń, K.; Bernaś, E.; Skrzypczak, A.; Kapusta, I. Vitamins, Phenolics and Antioxidant Activity of Culinary Prepared Suillus luteus (L.) Roussel Mushroom. LWT Food Sci. Technol. 2014, 59, 701–706. [Google Scholar] [CrossRef]
- Aytar, E.C.; Akata, İ.; Açik, L. Antioxidant, antimicrobial and anti-proliferative activity of Suillus luteus (L.) Roussel extracts. Ank. Univ. Eczaci. Fak. Derg. 2020, 44, 373–387. [Google Scholar] [CrossRef]
- Santos, T.D.; Tavares, C.; Sousa, D.; Vaz, J.A.; Calhelha, R.C.; Martins, A.; Almeida, G.M.; Ferreira, I.C.F.R.; Vasconcelos, M.H. Suillus luteus Methanolic Extract Inhibits Cell Growth and Proliferation of a Colon Cancer Cell Line. Food Res. Int. 2013, 53, 476–481. [Google Scholar] [CrossRef]
- Dos Santos, T.; Oliveira, M.; Sousa, D.; Lima, R.T.; Martins, A.; Ferreira, I.C.F.R.; Vasconcelos, M.H. Suillus luteus Methanolic Extract Inhibits Proliferation and Increases Expression of P-H2A.X in a Non-Small Cell Lung Cancer Cell Line. J. Funct. Foods 2014, 6, 100–106. [Google Scholar] [CrossRef]
- León, F.; Brouard, I.; Torres, F.; Quintana, J.; Rivera, A.; Estévez, F.; Bermejo, J. A New Ceramide from Suillus luteus and Its Cytotoxic Activity against Human Melanoma Cells. Chem. Biodivers. 2008, 5, 120–125. [Google Scholar] [CrossRef]
- Chang, W.-H.; Huang, H.-L.; Huang, W.-P.; Chen, C.-C.; Chen, Y.-J. Armillaridin Induces Autophagy-Associated Cell Death in Human Chronic Myelogenous Leukemia K562 Cells. Tumor Biol. 2016, 37, 14291–14300. [Google Scholar] [CrossRef]
- Leu, Y.-S.; Chen, Y.-J.; Chen, C.-C.; Huang, H.-L. Induction of Autophagic Death of Human Hepatocellular Carcinoma Cells by Armillaridin from Armillaria mellea. Am. J. Chin. Med. 2019, 47, 1365–1380. [Google Scholar] [CrossRef]
- Wu, S.; Krings, U.; Zorn, H.; Berger, R. Volatile Compounds from the Fruiting Bodies of Beefsteak Fungus Fistulina hepatica (Schaeffer: Fr.) Fr. Food Chem. 2005, 92, 221–226. [Google Scholar] [CrossRef]
- Nowacka-Jechalke, N.; Kanak, S.; Moczulski, M.; Martyna, A.; Kubiński, K.; Masłyk, M.; Szpakowska, N.; Kaczyński, Z.; Nowak, R.; Olech, M. Crude Polysaccharides from Wild-Growing Armillaria mellea—Chemical Composition and Antidiabetic, Anti-Inflammatory, Antioxidant, and Antiproliferative Potential. Appl. Sci. 2023, 13, 3853. [Google Scholar] [CrossRef]
- Misiek, M.; Williams, J.; Schmich, K.; Hüttel, W.; Merfort, I.; Salomon, C.E.; Aldrich, C.C.; Hoffmeister, D. Structure and Cytotoxicity of Arnamial and Related Fungal Sesquiterpene Aryl Esters. J. Nat. Prod. 2009, 72, 1888–1891. [Google Scholar] [CrossRef]
- Yang, J.; Yuwu, C.; Xiaozhang, F.; Dequan, Y.; Xiaotian, L. Chemical Constituents of Armillaria mellea Mycelium I. Isolation and Characterization of Armillarin and Armillaridin. Planta Med. 1984, 50, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.-W.; Chen, C.-C.; Chen, Y.-J. Therapeutic and Radiosensitizing Effects of Armillaridin on Human Esophageal Cancer Cells. Evid. Based Complement. Alternat. Med. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Wu, S.-Y.; Chen, C.-C.; Tsao, Y.-L.; Hsu, N.-C.; Chou, Y.-C.; Huang, H.-L. Armillaria mellea Component Armillarikin Induces Apoptosis in Human Leukemia Cells. J. Funct. Foods 2014, 6, 196–204. [Google Scholar] [CrossRef]
- Huang, H.-L.; Chen, Y.-J.; Chen, C.-C. Induction of Apoptosis by Armillaria mellea Constituent Armillarikin in Human Hepatocellular Carcinoma. OncoTargets Ther. 2016, 9, 4773–4783. [Google Scholar] [CrossRef]
- Bohnert, M.; Miethbauer, S.; Dahse, H.-M.; Ziemen, J.; Nett, M.; Hoffmeister, D. In Vitro Cytotoxicity of Melleolide Antibiotics: Structural and Mechanistic Aspects. Bioorg. Med. Chem. Lett. 2011, 21, 2003–2006. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Jiang, B.; Li, W.; Zheng, L.; Yang, X.; Bao, Y.; Sun, L.; Huang, Y.; Li, Y. Structure, Cytotoxic Activity and Mechanism of Protoilludane Sesquiterpene Aryl Esters from the Mycelium of Armillaria mellea. J. Ethnopharmacol. 2016, 184, 119–127. [Google Scholar] [CrossRef]
- Baptista, P.; Ferreira, S.; Soares, E.; Coelho, V.; Bastos, M.D.L. Tolerance and Stress Response of Macrolepiota procera to Nickel. J. Agric. Food Chem. 2009, 57, 7145–7152. [Google Scholar] [CrossRef]
- Mleczek, M.; Szostek, M.; Siwulski, M.; Budka, A.; Kalač, P.; Budzyńska, S.; Kuczyńska-Kippen, N.; Niedzielski, P. Road Traffic and Abiotic Parameters of Underlying Soils Determine the Mineral Composition and Nutritive Value of the Mushroom Macrolepiota procera (Scop.) Singer. Chemosphere 2022, 303, 135213. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J.; Kunito, T.; Kubota, R.; Gucia, M.; Mazur, A.; Falandysz, J.J.; Tanabe, S. Some Mineral Constituents of Parasol Mushroom (Macrolepiota procera). J. Environ. Sci. Health Part B 2008, 43, 187–192. [Google Scholar] [CrossRef]
- Chen, H.-P.; Zhao, Z.-Z.; Li, Z.-H.; Huang, Y.; Zhang, S.-B.; Tang, Y.; Yao, J.-N.; Chen, L.; Isaka, M.; Feng, T.; et al. Anti-Proliferative and Anti-Inflammatory Lanostane Triterpenoids from the Polish Edible Mushroom Macrolepiota procera. J. Agric. Food Chem. 2018, 66, 3146–3154. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Klimaszyk, P. Is the Yellow Knight Mushroom Edible or Not? A Systematic Review and Critical Viewpoints on the Toxicity of Tricholoma Equestre. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1309–1324. [Google Scholar] [CrossRef]
- Muszyńska, B.; Kała, K.; Radović, J.; Sułkowska-Ziaja, K.; Krakowska, A.; Gdula-Argasińska, J.; Opoka, W.; Kundaković, T. Study of Biological Activity of Tricholoma equestre Fruiting Bodies and Their Safety for Human. Eur. Food Res. Technol. 2018, 244, 2255–2264. [Google Scholar] [CrossRef]
- Bézivin, C.; Lohézic, F.; Sauleau, P.; Amoros, M.; Boustie, J. Cytotoxic Activity of Tricholomatales Determined with Murine and Human Cancer Cell Lines. Pharm. Biol. 2002, 40, 196–199. [Google Scholar] [CrossRef]
- Pachón-Peña, G.; Reyes-Zurita, F.J.; Deffieux, G.; Azqueta, A.; De Cerain, A.L.; Centelles, J.J.; Creppy, E.E.; Cascante, M. Antiproliferative Effect of Flavomannin-6,6′-Dimethylether from Tricholoma equestre on Caco-2 Cells. Toxicology 2009, 264, 192–197. [Google Scholar] [CrossRef]
- Kibar, B.; Peksen, A. Nutritional and Environmental Requirements for Vegetative Growth of Edible Ectomycorrhizal Mushroom Tricholoma terreum. Zemdirbyste 2011, 98, 409–414. [Google Scholar]
- Yuvali, G.; Onbasli, D. Cytotoxicity of Sarcosphaera Crassa and Tricholoma terreum Extracts on Colon Cancer Cell Line (HT-29) in Conjunction with Their Antioxidant Properties. Int. J. Environ. Health Res. 2022, 32, 2286–2297. [Google Scholar] [CrossRef]
- Feng, T.; He, J.; Ai, H.-L.; Huang, R.; Li, Z.-H.; Liu, J.-K. Three New Triterpenoids from European Mushroom Tricholoma terreum. Nat. Prod. Bioprospect. 2015, 5, 205–208. [Google Scholar] [CrossRef]
- Yin, X.; Feng, T.; Li, Z.-H.; Dong, Z.-J.; Li, Y.; Liu, J.K. Highly Oxygenated Meroterpenoids from Fruiting Bodies of the Mushroom Tricholoma terreum. J. Nat. Prod. 2013, 76, 1365–1368. [Google Scholar] [CrossRef]
- Frichert, A.; Jones, P.G.; Brönstrup, M.; Lindel, T. Oxidation of the Meroterpenoid (−)-Terreumol C from the Mushroom Tricholoma terreum: Discovery of Cytotoxic Analogues. J. Nat. Prod. 2017, 80, 2652–2658. [Google Scholar] [CrossRef]
- Muszyńska, B.; Kała, K.; Firlej, A.; Sułkowska-Ziaja, K. Cantharellus cibarius—Culinary-Medicinal Mushroom Content and Biological Activity. Acta Pol. Pharm. 2016, 73, 589–598. [Google Scholar]
- Lemieszek, M.K.; Marques, P.S.; Ribeiro, M.; Ferreira, D.; Marques, G.; Chaves, R.; Pożarowski, P.; Nunes, F.M.; Rzeski, W. Mushroom Small RNAs as Potential Anticancer Agents: A Closer Look at Cantharellus cibarius Proapoptotic and Antiproliferative Effects in Colon Cancer Cells. Food Funct. 2019, 10, 2739–2751. [Google Scholar] [CrossRef]
- Nowacka-Jechalke, N.; Nowak, R.; Juda, M.; Malm, A.; Lemieszek, M.; Rzeski, W.; Kaczyński, Z. New Biological Activity of the Polysaccharide Fraction from Cantharellus cibarius and Its Structural Characterization. Food Chem. 2018, 268, 355–361. [Google Scholar] [CrossRef]
- Lemieszek, M.K.; Nunes, F.M.; Marques, G.; Rzeski, W. Cantharellus cibarius Branched Mannans Inhibits Colon Cancer Cells Growth by Interfering with Signals Transduction in NF-ĸB Pathway. Int. J. Biol. Macromol. 2019, 134, 770–780. [Google Scholar] [CrossRef]
- Lemieszek, M.K.; Nunes, F.M.; Rzeski, W. Branched Mannans from the Mushroom Cantharellus cibarius Enhance the Anticancer Activity of Natural Killer Cells against Human Cancers of Lung and Colon. Food Funct. 2019, 10, 5816–5826. [Google Scholar] [CrossRef] [PubMed]
- Kolundžić, M.; Stanojković, T.; Radović, J.; Tačić, A.; Dodevska, M.; Milenković, M.; Sisto, F.; Masia, C.; Farronato, G.; Nikolić, V.; et al. Cytotoxic and Antimicrobial Activities of Cantharellus cibarius Fr. (Cantarellaceae). J. Med. Food 2017, 20, 790–796. [Google Scholar] [CrossRef]
- Guerin-Laguette, A.; Cummings, N.; Butler, R.C.; Willows, A.; Hesom-Williams, N.; Li, S.; Wang, Y. Lactarius deliciosus and Pinus radiata in New Zealand: Towards the Development of Innovative Gourmet Mushroom Orchards. Mycorrhiza 2014, 24, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, P.; Markiewicz-Żukowska, R.; Gromkowska-Kępka, K.; Naliwajko, S.K.; Moskwa, J.; Bielecka, J.; Grabia, M.; Borawska, M.; Socha, K. Mushrooms as Potential Therapeutic Agents in the Treatment of Cancer: Evaluation of Anti-Glioma Effects of Coprinus comatus, Cantharellus cibarius, Lycoperdon perlatum and Lactarius deliciosus Extracts. Biomed. Pharmacother. 2021, 133, 111090. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Hou, Y.; Hou, W.; Zhu, Y.; Fu, L.; Zhu, H. Structure Elucidation and Anti-Tumor Activities of Water-Soluble Oligosaccharides from Lactarius deliciosus (L. Ex Fr.) Gray. Pharmacogn. Mag. 2015, 11, 716. [Google Scholar] [CrossRef]
- Ding, X.; Hou, Y.; Hou, W. Structure Feature and Antitumor Activity of a Novel Polysaccharide Isolated from Lactarius deliciosus Gray. Carbohydr. Polym. 2012, 89, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Hou, W.; Song, B.; Wang, T.; Wang, F.; Zhong, J.; Hou, Y. Immunostimulant Activity of a Novel Polysaccharide Isolated from Lactarius deliciosus (l. Ex Fr.) Gray. Indian J. Pharm. Sci. 2013, 75, 393. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Simarro, P.; Gómez-Gómez, L.; Ahrazem, O.; Rubio-Moraga, Á. Food and Human Health Applications of Edible Mushroom By-Products. New Biotechnol. 2024, 81, 43–56. [Google Scholar] [CrossRef]
- Kavčič, A.; Mikuš, K.; Debeljak, M.; Teun Van Elteren, J.; Arčon, I.; Kodre, A.; Kump, P.; Karydas, A.G.; Migliori, A.; Czyzycki, M.; et al. Localization, Ligand Environment, Bioavailability and Toxicity of Mercury in Boletus Spp. and Scutiger pes-caprae Mushrooms. Ecotoxicol. Environ. Saf. 2019, 184, 109623. [Google Scholar] [CrossRef]
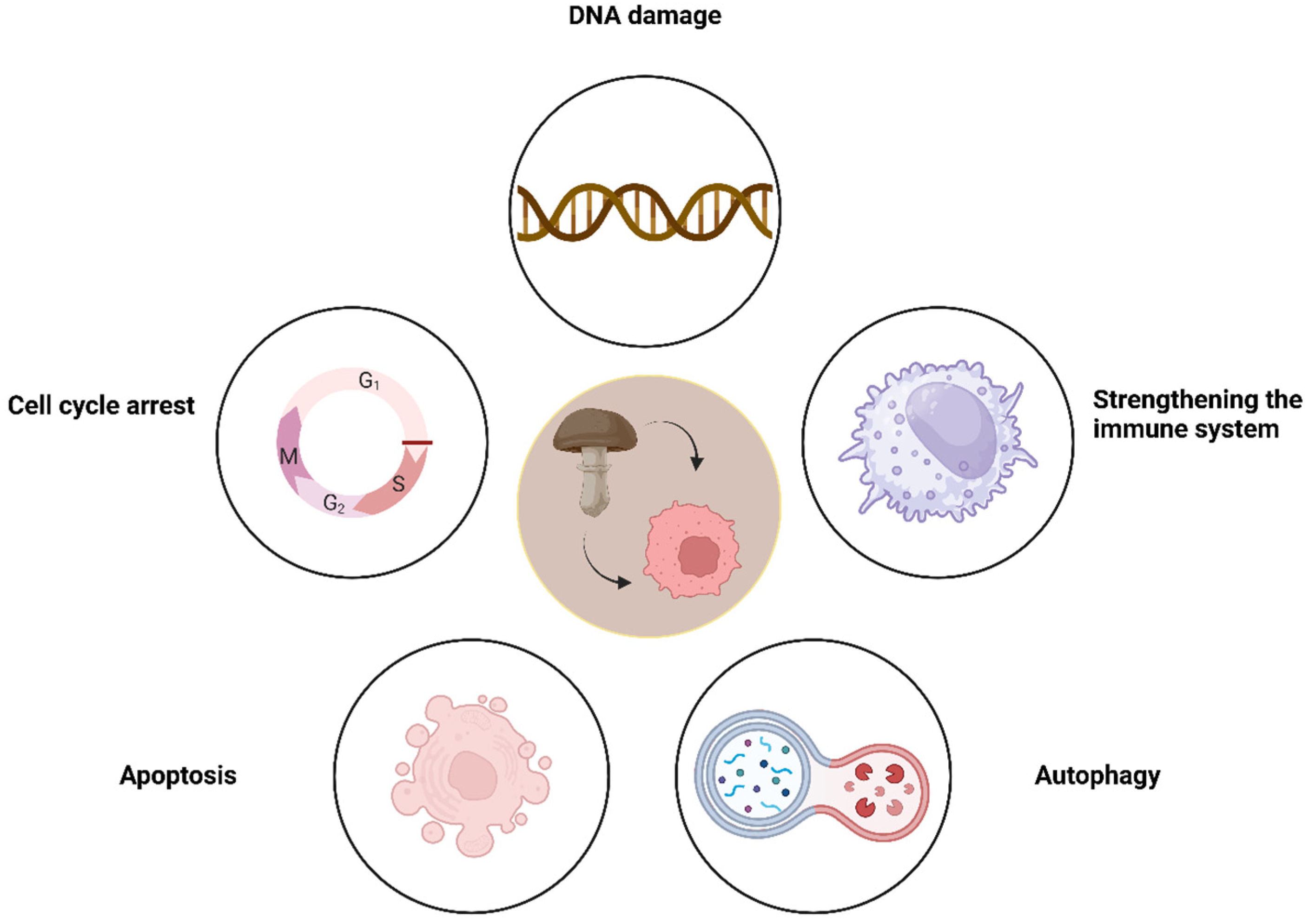



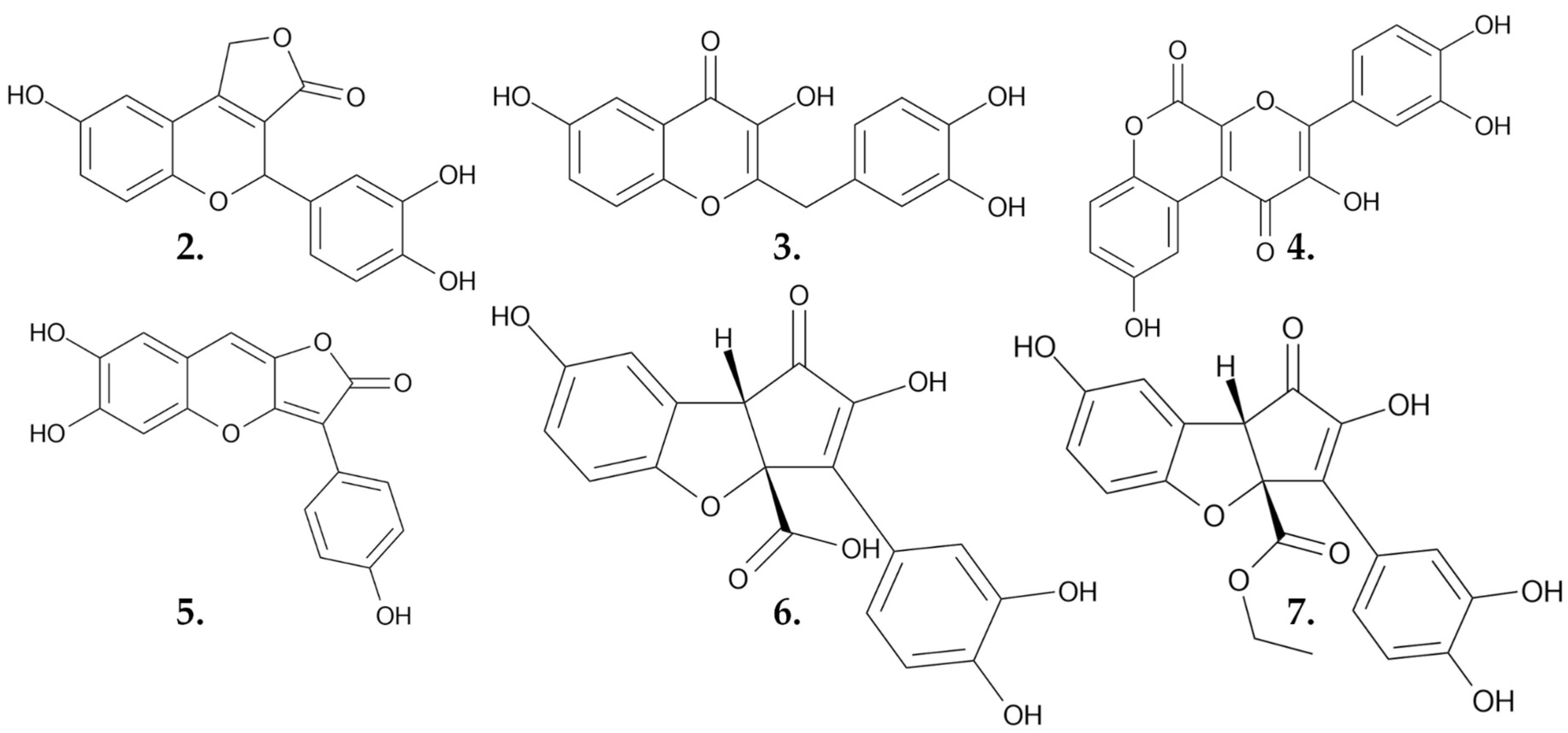

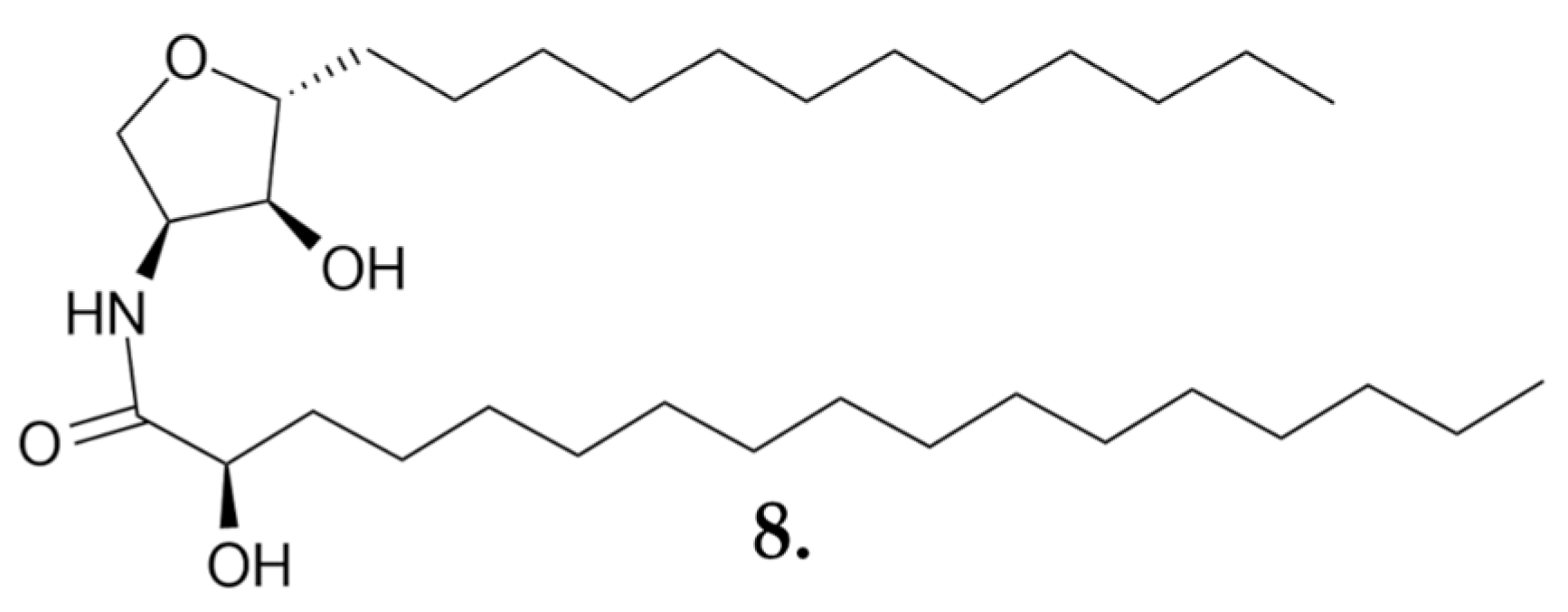
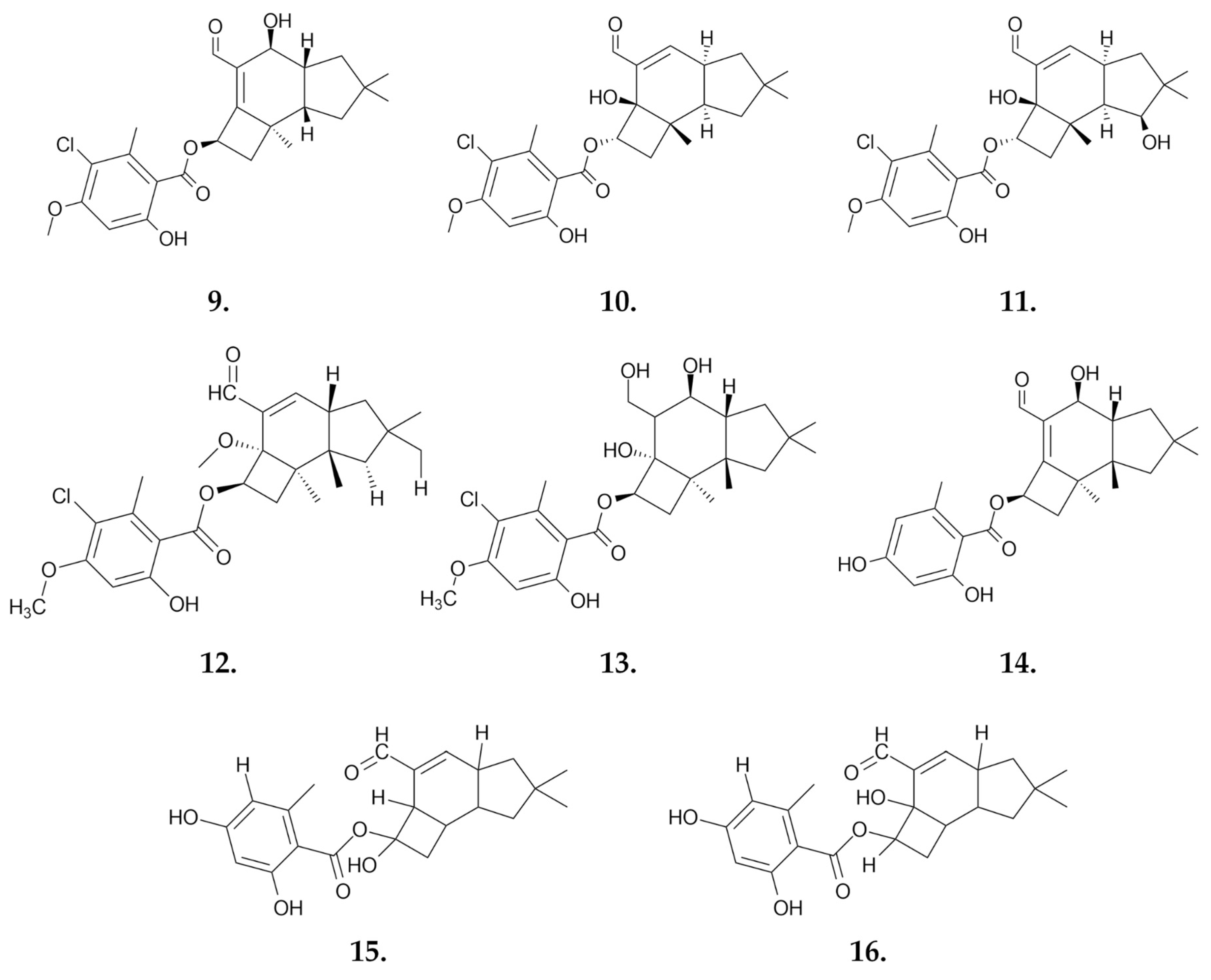

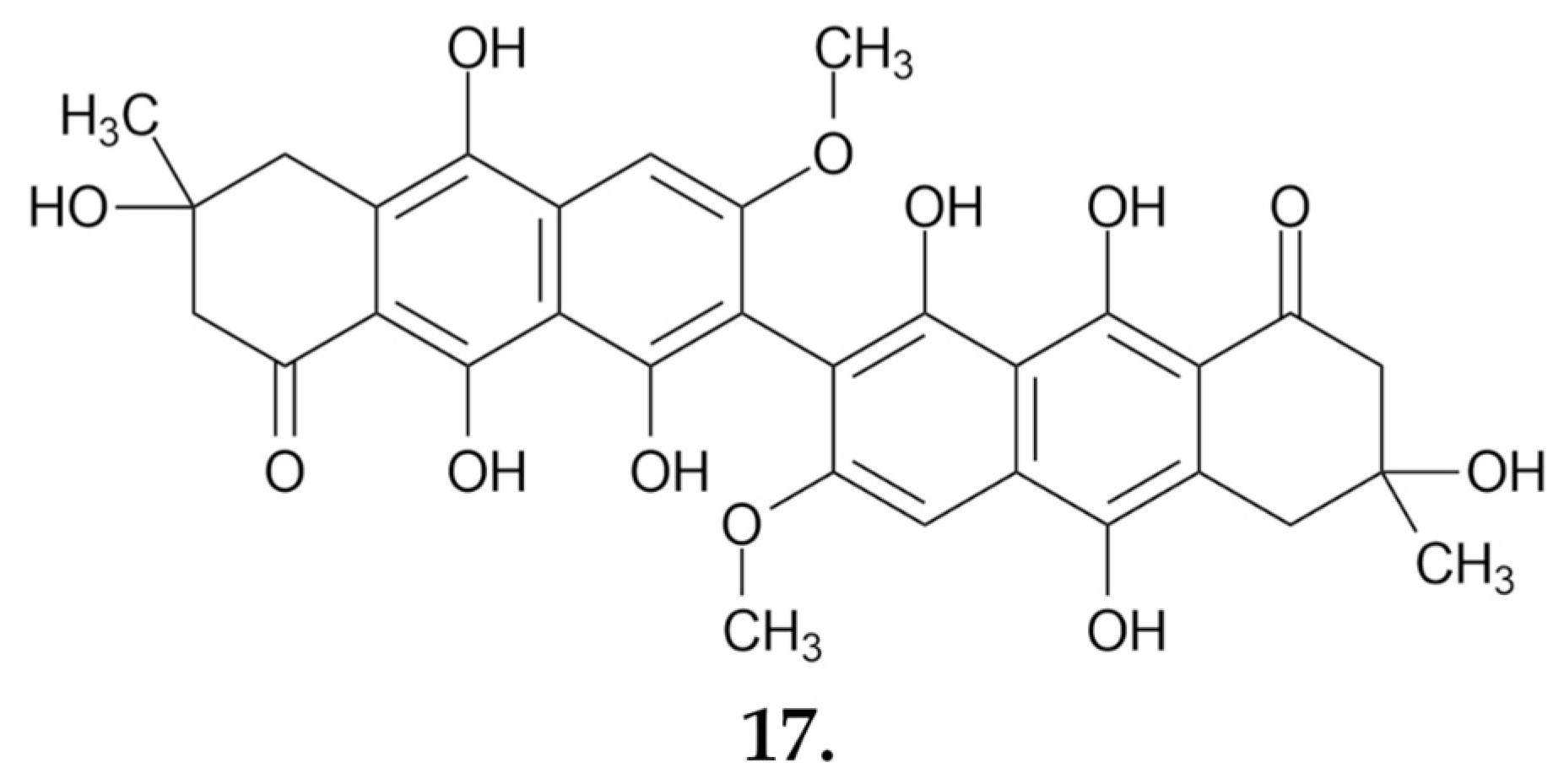


| Compound | IC50 Values [µM], 72 h |
|---|---|
| Suillusol A (1) | 35.60 |
| Suillusol B (2) | 10.85 |
| Suillusol C (3) | 32.62 |
| Suillusol D (4) | 63.68 |
| Suillusinoic acid (5) | 56.78 |
| Ethyl suillusinoate (6) | 61.89 |
| Compound/Cell Line | 4-O-Methylarmillaridin | 5′-Methoxy-6′-Chloroarmillane | Dehydroarmillylorsellinate | 6′-Chloro-13-Hydroxydihydro-Melleolide |
|---|---|---|---|---|
| MCF-7 | 6.7 | 26.5 | 8.0 | >100 |
| Jurkat | 4.1 | 13.3 | 16.9 | 46.6 |
| HeLa | 18.8 | 35.8 | 15.2 | >100 |
| K-562 | 20.6 | 25.0 | 5.0 | >100 |
| IC50 Values [µM], 48 h Exposures | |||||
|---|---|---|---|---|---|
| Compound | MCF-7 | SMMC-7221 | A-549 | HL-60 | SW480 |
| Terreumol A (19) | 4.0 | 11.3 | 4.2 | 16.1 | 14.2 |
| Terreumol C | 5.6 | 24.7 | 16.4 | 24.6 | >40 |
| Terreumol D | 17.8 | 14.9 | 6.5 | 21 | 21.1 |
| Order | Species | |
|---|---|---|
 | Boletales | Leccinum aurantiacum |
| Boletales | Leccinum griseum | |
| Boletales | Leccinum versipelle | |
| Boletales | Suillus variegatus | |
| Boletales | Suillus bovinus | |
| Boletales | Suillus grevillei | |
| Boletales | Imleria badia | |
| Boletales | Xerocomus subtomentosus | |
| Boletales | Xerocomellus chrysenteron | |
| Agaricales | Cortinarius caperatus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roszczenko, P.; Szewczyk-Roszczenko, O.K.; Gornowicz, A.; Iwańska, I.A.; Bielawski, K.; Wujec, M.; Bielawska, A. The Anticancer Potential of Edible Mushrooms: A Review of Selected Species from Roztocze, Poland. Nutrients 2024, 16, 2849. https://doi.org/10.3390/nu16172849
Roszczenko P, Szewczyk-Roszczenko OK, Gornowicz A, Iwańska IA, Bielawski K, Wujec M, Bielawska A. The Anticancer Potential of Edible Mushrooms: A Review of Selected Species from Roztocze, Poland. Nutrients. 2024; 16(17):2849. https://doi.org/10.3390/nu16172849
Chicago/Turabian StyleRoszczenko, Piotr, Olga Klaudia Szewczyk-Roszczenko, Agnieszka Gornowicz, Iga Anna Iwańska, Krzysztof Bielawski, Monika Wujec, and Anna Bielawska. 2024. "The Anticancer Potential of Edible Mushrooms: A Review of Selected Species from Roztocze, Poland" Nutrients 16, no. 17: 2849. https://doi.org/10.3390/nu16172849
APA StyleRoszczenko, P., Szewczyk-Roszczenko, O. K., Gornowicz, A., Iwańska, I. A., Bielawski, K., Wujec, M., & Bielawska, A. (2024). The Anticancer Potential of Edible Mushrooms: A Review of Selected Species from Roztocze, Poland. Nutrients, 16(17), 2849. https://doi.org/10.3390/nu16172849








