A Comprehensive Analysis of Bone Mineral Density Changes across the Lifespan: Insights from National Surveys
Abstract
1. Introduction
2. Materials and Methods
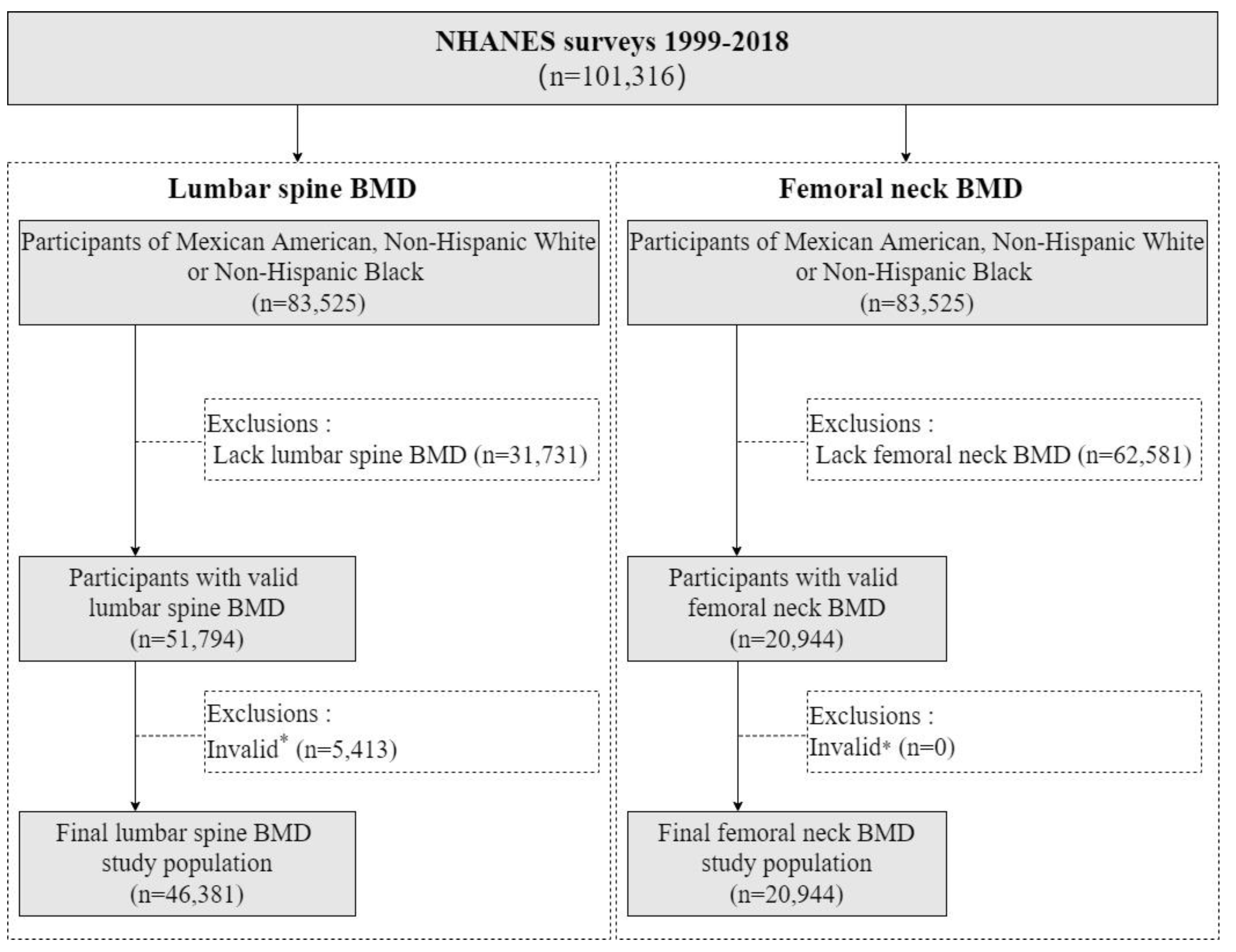
2.1. Study Design and Participants
2.2. Examination of BMD
2.3. Assessment of Sociodemographic Characteristics
2.4. Statistical Analysis
3. Results
3.1. General Characteristics of the Population
3.2. Age-Related Changes in LSBMD Levels and PBMD and Corresponding Ages, Stratified by Sex
3.3. Comparison of LSBMD Levels among Different Ethnic Groups
3.4. Age-Related Changes in FNBMD Levels and PBMD and Corresponding Ages, Stratified by Sex
3.5. Comparison of FNBMD Levels among Different Ethnic Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, H.-W.; Xu, F.-H.; Davies, K.M.; Heaney, R.; Recker, R.R. Differences in bone mineral density, bone mineral content, and bone areal size in fracturing and non-fracturing women, and their interrelationships at the spine and hip. J. Bone Miner. Metab. 2002, 20, 358–366. [Google Scholar] [CrossRef]
- Kanis, J.A.; McCloskey, E.V.; Johansson, H.; Oden, A.; Melton, L.J., 3rd; Khaltaev, N. A reference standard for the description of osteoporosis. Bone 2008, 42, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Vilaca, T.; Eastell, R.; Schini, M. Osteoporosis in men. Lancet Diabetes Endocrinol. 2022, 10, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Warming, L.; Hassager, C.; Christiansen, C. Changes in bone mineral density with age in men and women: A longitudinal study. Osteoporos. Int. 2002, 13, 105–112. [Google Scholar] [CrossRef]
- Karlsson, M.K.; Obrant, K.J.; Nilsson, B.E.; Johnell, O. Changes in bone mineral, lean body mass and fat content as measured by dual energy X-ray absorptiometry: A longitudinal study. Calcif. Tissue Int. 2000, 66, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Bachrach, L.K.; Hastie, T.; Wang, M.-C.; Narasimhan, B.; Marcus, R. Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: A longitudinal study. J. Clin. Endocrinol. Metab. 1999, 84, 4702–4712. [Google Scholar] [CrossRef]
- Hernandez, C.J.; Beaupre, G.S.; Carter, D.R. A theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the development of osteoporosis. Osteoporos. Int. 2003, 14, 843–847. [Google Scholar] [CrossRef]
- Matkovic, V.; Jelic, T.; Wardlaw, G.M.; Ilich, J.Z.; Goel, P.K.; Wright, J.K.; Andon, M.B.; Smith, K.T.; Heaney, R.P. Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis. Inference from a cross-sectional model. J. Clin. Investig. 1994, 93, 799–808. [Google Scholar] [CrossRef]
- Recker, R.R. Bone Gain in Young Adult Women. JAMA 1992, 268, 2403–2408. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Lyle, R.M.; Weaver, C.M.; McCabe, L.D.; McCabe, G.P.; Johnston, C.C.; Teegarden, D. Peak spine and femoral neck bone mass in young women. Bone 2003, 32, 546–553. [Google Scholar] [CrossRef]
- Löfman, O.; Larsson, L.; Toss, G. Bone Mineral Density in Diagnosis of Osteoporosis. J. Clin. Densitom. 2000, 3, 177–186. [Google Scholar] [CrossRef]
- Iki, M.; Kagamimori, S.; Kagawa, Y.; Matsuzaki, T.; Yoneshima, H.; Marumo, F. Bone mineral density of the spine, hip and distal forearm in representative samples of the Japanese female population: Japanese Population-Based Osteoporosis (JPOS) Study. Osteoporos. Int. 2001, 12, 529–537. [Google Scholar] [CrossRef] [PubMed]
- CDC. National Health and Nutrition Examination Survey. 2017. Available online: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed on 15 January 2024).
- CDC. Body Composition Procedures Manual. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/manuals/Body_Composition_Procedures_Manual_2018.pdf (accessed on 15 January 2024).
- Nguyen, T.V.; Maynard, L.M.; Towne, B.; Roche, A.F.; Wisemandle, W.; Li, J.; Guo, S.S.; Chumlea, W.C.; Siervogel, R.M. Sex Differences in Bone Mass Acquisition During Growth. J. Clin. Densitom. 2001, 4, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Bachrach, L.K.; Gordon, C.M.; Section On, E. Bone Densitometry in Children and Adolescents. Pediatrics 2016, 138, e20162398. [Google Scholar] [CrossRef]
- Looker, A.C.; Melton, L.J., 3rd; Borrud, L.G.; Shepherd, J.A. Lumbar spine bone mineral density in US adults: Demographic patterns and relationship with femur neck skeletal status. Osteoporos. Int. 2012, 23, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Orwoll, E.S.; Oviatt, S.K.; Mann, T. The impact of osteophytic and vascular calcifications on vertebral mineral density measurements in men. J. Clin. Endocrinol. Metab. 1990, 70, 1202–1207. [Google Scholar] [CrossRef]
- Liu, G.; Peacock, M.; Eilam, O.; Dorulla, G.; Braunstein, E.; Johnston, C.C. Effect of osteoarthritis in the lumbar spine and hip on bone mineral density and diagnosis of osteoporosis in elderly men and women. Osteoporos. Int. 1997, 7, 564–569. [Google Scholar] [CrossRef]
- Muraki, S.; Yamamoto, S.; Ishibashi, H.; Horiuchi, T.; Hosoi, T.; Orimo, H.; Nakamura, K. Impact of degenerative spinal diseases on bone mineral density of the lumbar spine in elderly women. Osteoporos. Int. 2004, 15, 724–728. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Jang, S.; Ryu, O.H. Age-Related Changes in the Prevalence of Osteoporosis according to Gender and Skeletal Site: The Korea National Health and Nutrition Examination Survey 2008–2010. Endocrinol. Metab. 2013, 28, 180–191. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Cheng, X.; Lu, Y.; Li, N.; Gong, Y.; Pei, Y.; Li, C. The effect of age on the changes in bone mineral density and osteoporosis detection rates in Han Chinese men over the age of 50. Aging Male 2014, 17, 166–173. [Google Scholar] [CrossRef]
- Piroska, M.; Tarnoki, D.L.; Szabo, H.; Jokkel, Z.; Meszaros, S.; Horvath, C.; Tarnoki, A.D. Strong Genetic Effects on Bone Mineral Density in Multiple Locations with Two Different Techniques: Results from a Cross-Sectional Twin Study. Medicina 2021, 57, 248. [Google Scholar] [CrossRef] [PubMed]
- Zhai, G.; Andrew, T.; Kato, B.S.; Blake, G.M.; Spector, T.D. Genetic and environmental determinants on bone loss in postmenopausal Caucasian women: A 14-year longitudinal twin study. Osteoporos. Int. 2009, 20, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Slemenda, C.W.; Christian, J.C.; Williams, C.J.; Norton, J.A.; Johnston, C.C., Jr. Genetic determinants of bone mass in adult women: A reevaluation of the twin model and the potential importance of gene interaction on heritability estimates. J. Bone Miner. Res. 1991, 6, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Hopper, J.L.; Green, R.M.; Nowson, C.A.; Young, D.; Sherwin, A.J.; Kaymakci, B.; Larkins, R.G.; Wark, J.D. Genetic, common environment, and individual specific components of variance for bone mineral density in 10- to 26-year-old females: A twin study. Am. J. Epidemiol. 1998, 147, 17–29. [Google Scholar] [CrossRef]
- Elhakeem, A.; Heron, J.; Tobias, J.H.; Lawlor, D.A. Physical Activity Throughout Adolescence and Peak Hip Strength in Young Adults. JAMA Netw. Open 2020, 3, e2013463. [Google Scholar] [CrossRef]
- Whitfield, G.P.; Kohrt, W.M.; Pettee Gabriel, K.K.; Rahbar, M.H.; Kohl, H.W., 3rd. Bone mineral density across a range of physical activity volumes: NHANES 2007–2010. Med. Sci. Sports Exerc. 2015, 47, 326–334. [Google Scholar] [CrossRef]
- Zhang, J.; Parsons, C.; Fuggle, N.; Ward, K.A.; Cooper, C.; Dennison, E. Is Regular Weight-Bearing Physical Activity Throughout the Lifecourse Associated with Better Bone Health in Late Adulthood? Calcif. Tissue Int. 2022, 111, 279–287. [Google Scholar] [CrossRef]
- Daly, R.M.; Ahlborg, H.G.; Ringsberg, K.; Gardsell, P.; Sernbo, I.; Karlsson, M.K. Association between changes in habitual physical activity and changes in bone density, muscle strength, and functional performance in elderly men and women. J. Am. Geriatr. Soc. 2008, 56, 2252–2260. [Google Scholar] [CrossRef]
- Lee, J.H.; Ha, A.W.; Kim, W.K.; Kim, S.H. The Combined Effects of Milk Intake and Physical Activity on Bone Mineral Density in Korean Adolescents. Nutrients 2021, 713. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, N.; Wang, Q.; Feng, J.; Sun, D.; Zhang, Q.; Huang, J.; Wen, Q.; Hu, R.; Wang, L.; et al. The Prevalence of Osteoporosis in China, a Nationwide, Multicenter DXA Survey. J. Bone Miner. Res. 2019, 34, 1789–1797. [Google Scholar] [CrossRef]
- Yang, J.; Sun, J.; Luo, F.; Sun, Q.; Zhao, L.; Su, N.; Du, X.; Huang, H.; Shen, Y.; Chen, L. Peak BMD assessment in a Chinese infantry recruit group. Int. J. Sports Med. 2011, 32, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.-H.; Choi, J.-S.; Shin, M.-H.; Kweon, S.-S.; Park, K.-S.; Lee, Y.-H.; Nam, H.-S.; Jeong, S.-K.; Im, J.-S. Prevalence of osteoporosis and reference data for lumbar spine and hip bone mineral density in a Korean population. J. Bone Miner. Metab. 2008, 26, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Marwaha, R.K.; Tandon, N.; Shivaprasad, C.; Kanwar, R.; Mani, K.; Aggarwal, R.; Bhadra, K.; Singh, S.; Sharma, B.; Tripathi, R.P. Peak Bone Mineral Density of Physically Active Healthy Indian Men with Adequate Nutrition and No Known Current Constraints to Bone Mineralization. J. Clin. Densitom. 2009, 12, 314–321. [Google Scholar] [CrossRef]
- Nguyen, H.G.; Pham, M.T.; Ho-Pham, L.T.; Nguyen, T.V. Lean mass and peak bone mineral density. Osteoporos. Sarcopenia 2020, 6, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, E.; Rosengren, B.E.; Karlsson, M.K. Does peak bone mass correlate with peak bone strength? Cross-sectional normative dual energy X-ray absorptiometry data in 1052 men aged 18–28 years. BMC Musculoskelet. Disord. 2019, 20, 404. [Google Scholar] [CrossRef]
- Kroger, H.; Heikkinen, J.; Laitinen, K.; Kotaniemi, A. Dual-energy X-ray absorptiometry in normal women: A cross-sectional study of 717 Finnish volunteers. Osteoporos. Int. 1992, 2, 135–140. [Google Scholar] [CrossRef]
- Looker, A.C.; Melton, L.J., 3rd; Borrud, L.G.; Shepherd, J.A. Changes in femur neck bone density in US adults between 1988–1994 and 2005–2008: Demographic patterns and possible determinants. Osteoporos. Int. 2012, 23, 771–780. [Google Scholar] [CrossRef]
- Lloyd, T.; Eggli, D.F. Measurement of Bone Mineral Content and Bone Density in Healthy Twelve-Year-Old White Females. J. Nucl. Med. 1992, 33, 1143–1145. [Google Scholar]
- Melton, L.J., 3rd; Khosla, S.; Atkinson, E.J.; Oconnor, M.K.; Ofallon, W.M.; Riggs, B.L. Cross-sectional versus longitudinal evaluation of bone loss in men and women. Osteoporos. Int. 2000, 11, 592–599. [Google Scholar] [CrossRef]




| Characteristics | Participants, No. (Weighted %) | |||||
|---|---|---|---|---|---|---|
| Male | Female | |||||
| Mexican American | Non-Hispanic Whites | Non-Hispanic Blacks | Mexican American | Non-Hispanic Whites | Non-Hispanic Blacks | |
| Lumbar spine BMD analysis (N = 46,381) # | 6509 (12.2) | 10,869 (74.9) | 6782 (13.0) | 5850 (10.4) | 10,077 (75.5) | 6294 (14.1) |
| Age (years) | ||||||
| 8–12 (childhood) | 1255 (12.9) | 1256 (8.2) | 1252 (11.9) | 1099 (14.1) | 1078 (7.1) | 1143 (10.3) |
| 13–18 (adolescence) | 1623 (14.2) | 1614 (10.5) | 1706 (14.7) | 1295 (14.3) | 1293 (9.3) | 1274 (11.4) |
| 19–44 (young adulthood) | 2144 (53.9) | 3844 (43.4) | 2106 (45.7) | 1892 (49.1) | 3472 (40.1) | 1972 (44.2) |
| 45–64 (midlife) | 1040 (16.4) | 2445 (29.0) | 1232 (22.9) | 1079 (18.5) | 2491 (31.3) | 1405 (27.3) |
| 65–85 (late life) | 447 (2.6) | 1710 (8.9) | 486 (4.8) | 485 (4.0) | 1743 (12.2) | 500 (6.8) |
| Education level * | ||||||
| <College degree | 3250 (92.2) | 5775 (67.7) | 3161 (84.6) | 3155 (90.9) | 5662 (68.2) | 3159 (80.9) |
| ≥College degree | 242 (7.8) | 2210 (32.3) | 559 (15.4) | 247 (9.1) | 2077 (31.8) | 691 (19.1) |
| Ratio of family income to poverty (mean ± SD) | 1.79 ± 1.32 | 2.92 ± 1.64 | 2.14 ± 1.54 | 1.78 ± 1.36 | 2.81 ± 1.64 | 2.02 ± 1.51 |
| Femoral neck BMD analysis (N = 20,944) # | 2581 (10.5) | 5479 (77.8) | 2788 (11.7) | 2393 (8.7) | 5119 (78.6) | 2584 (12.7) |
| Age (years) | ||||||
| 8–12 (childhood) | 431 (10.4) | 397 (5.4) | 330 (8.0) | 422 (12.1) | 372 (4.8) | 356 (8.1) |
| 13–18 (adolescence) | 442 (9.8) | 507 (7.3) | 458 (10.6) | 414 (11.1) | 445 (6.6) | 421 (9.6) |
| 19–44 (young adulthood) | 795 (47.1) | 1393 (29.8) | 669 (35.0) | 685 (40.0) | 1327 (28.6) | 627 (33.8) |
| 45–64 (midlife) | 644 (25.9) | 1581 (38.2) | 835 (34.6) | 599 (27.5) | 1519 (37.4) | 777 (35.1) |
| 65–85 (late life) | 269 (6.8) | 1601 (19.3) | 496 (11.8) | 273 (9.4) | 1456 (22.6) | 403 (13.3) |
| Education level * | ||||||
| <College degree | 1500 (91.4) | 3246 (67.2) | 1619 (84.6) | 1369 (90.3) | 3132 (68.6) | 1443 (81.4) |
| ≥College degree | 125 (8.6) | 1252 (32.8) | 295 (15.4) | 124 (9.7) | 1118 (31.4) | 305 (18.6) |
| Ratio of family income to poverty (mean ± SD) | 1.84 ± 1.36 | 2.96 ± 1.63 | 2.34 ± 1.55 | 1.84 ± 1.38 | 2.86 ± 1.63 | 2.17 ± 1.51 |
| Sex and Bone Sites | PBMD, Weighted Mean ± SD, g/cm2 | Corresponding Age (Weighted No. *), Mean, Years | ||||
|---|---|---|---|---|---|---|
| Mexican American | Non-Hispanic Whites | Non-Hispanic Blacks | Mexican American | Non-Hispanic Whites | Non-Hispanic Blacks | |
| Lumbar spine | ||||||
| Male | 0.985 ± 0.098 a | 1.055 ± 0.119 b | 1.132 ± 0.146 c | 22 (n = 238,323) | 23 (n = 1,117,690) | 24 (n = 179,315) |
| Female Ⅰ # | 1.016 ± 0.120 a | 1.059 ± 0.115 ab | 1.095 ± 0.131 b | 20 (n = 202,365) | 21 (n = 885,651) | 20 (n = 231,616) |
| Female II # | 1.013 ± 0.105 a | 1.078 ± 0.131 b | 1.140 ± 0.154 c | 36 (n = 182,449) | 36 (n = 999,886) | 37 (n = 200,151) |
| Psex | 0.105 | 0.122 | 0.698 | |||
| Femoral neck | ||||||
| Male | 0.967 ± 0.099 a | 0.957 ± 0.111 a | 1.037 ± 0.199 b | 21 (n = 89,648) | 20 (n = 361,998) | 21 (n = 75,213) |
| Female | 0.881 ± 0.134 a | 0.893 ± 0.111 a | 1.000 ± 0.152 b | 19 (n = 53,746) | 19 (n = 203,033) | 20 (n = 66,661) |
| Psex | <0.001 | <0.001 | <0.001 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Huang, G.; Hou, D.; Cheng, Y.; Zhang, T.; Liang, Y.; Liu, J. A Comprehensive Analysis of Bone Mineral Density Changes across the Lifespan: Insights from National Surveys. Nutrients 2024, 16, 2804. https://doi.org/10.3390/nu16162804
Li T, Huang G, Hou D, Cheng Y, Zhang T, Liang Y, Liu J. A Comprehensive Analysis of Bone Mineral Density Changes across the Lifespan: Insights from National Surveys. Nutrients. 2024; 16(16):2804. https://doi.org/10.3390/nu16162804
Chicago/Turabian StyleLi, Tao, Guimin Huang, Dongqing Hou, Yijing Cheng, Tong Zhang, Yajun Liang, and Junting Liu. 2024. "A Comprehensive Analysis of Bone Mineral Density Changes across the Lifespan: Insights from National Surveys" Nutrients 16, no. 16: 2804. https://doi.org/10.3390/nu16162804
APA StyleLi, T., Huang, G., Hou, D., Cheng, Y., Zhang, T., Liang, Y., & Liu, J. (2024). A Comprehensive Analysis of Bone Mineral Density Changes across the Lifespan: Insights from National Surveys. Nutrients, 16(16), 2804. https://doi.org/10.3390/nu16162804






