Meal Duration and Obesity-Related Indicators among Adolescents: Insights from the EHDLA Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Procedures
2.2.1. Meal Duration (Independent Variable)
2.2.2. Obesity-Related Indicators (Dependent Variables)
2.2.3. Covariates
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abarca-Gómez, L.; Abdeen, Z.A.; Hamid, Z.A.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Acuin, C.; Adams, R.J.; Aekplakorn, W.; Afsana, K.; Aguilar-Salinas, C.A.; et al. Worldwide Trends in Body-Mass Index, Underweight, Overweight, and Obesity from 1975 to 2016: A Pooled Analysis of 2416 Population-Based Measurement Studies in 128·9 Million Children, Adolescents, and Adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Obesity: No European Country Is on Track to Halt Rising Levels by 2025, WHO Warns. BMJ 2022, 377, o1107. [Google Scholar] [CrossRef] [PubMed]
- Lister, N.B.; Baur, L.A.; Felix, J.F.; Hill, A.J.; Marcus, C.; Reinehr, T.; Summerbell, C.; Wabitsch, M. Child and Adolescent Obesity. Nat. Rev. Dis. Primer 2023, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- United States Preventive Services Taskforce. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/document/RecommendationStatementFinal/obesity-in-children-and-adolescents-screening (accessed on 20 June 2024).
- OECD. Health at a Glance 2023: OECD Indicators; OECD: Paris, France, 2023; ISBN 978-92-64-95793-0. [Google Scholar]
- Jebeile, H.; Kelly, A.S.; O’Malley, G.; Baur, L.A. Obesity in Children and Adolescents: Epidemiology, Causes, Assessment, and Management. Lancet Diabetes Endocrinol. 2022, 10, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Hannon, T.S.; Arslanian, S.A. Obesity in Adolescents. N. Engl. J. Med. 2023, 389, 251–261. [Google Scholar] [CrossRef] [PubMed]
- González-Muniesa, P.; Mártinez-González, M.-A.; Hu, F.B.; Després, J.-P.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A.; Martinez, J.A. Obesity. Nat. Rev. Dis. Primer 2017, 3, 17034. [Google Scholar] [CrossRef] [PubMed]
- Adolescent Health and Development. Available online: https://www.who.int/news-room/questions-and-answers/item/adolescent-health-and-development (accessed on 20 June 2024).
- Corkins, M.R.; Daniels, S.R.; de Ferranti, S.D.; Golden, N.H.; Kim, J.H.; Magge, S.N.; Schwarzenberg, S.J. Nutrition in Children and Adolescents. Med. Clin. N. Am. 2016, 100, 1217–1235. [Google Scholar] [CrossRef] [PubMed]
- Alsaker, F.D.; Kroger, J. Self-Concept, Self-Esteem, and Identity. In Handbook of Adolescent Development; Psychology Press: Hove, UK, 2007; ISBN 978-0-203-96986-1. [Google Scholar]
- Webster, D.; Dunne, L.; Hunter, R. Association between Social Networks and Subjective Well-Being in Adolescents: A Systematic Review. Youth Soc. 2021, 53, 175–210. [Google Scholar] [CrossRef]
- Bozzola, E.; Spina, G.; Agostiniani, R.; Barni, S.; Russo, R.; Scarpato, E.; Di Mauro, A.; Di Stefano, A.V.; Caruso, C.; Corsello, G.; et al. The Use of Social Media in Children and Adolescents: Scoping Review on the Potential Risks. Int. J. Environ. Res. Public Health 2022, 19, 9960. [Google Scholar] [CrossRef]
- Jarman, H.K.; Marques, M.D.; McLean, S.A.; Slater, A.; Paxton, S.J. Social Media, Body Satisfaction and Well-Being among Adolescents: A Mediation Model of Appearance-Ideal Internalization and Comparison. Body Image 2021, 36, 139–148. [Google Scholar] [CrossRef]
- Stabouli, S.; Erdine, S.; Suurorg, L.; Jankauskienė, A.; Lurbe, E. Obesity and Eating Disorders in Children and Adolescents: The Bidirectional Link. Nutrients 2021, 13, 4321. [Google Scholar] [CrossRef]
- Ruiz, L.D.; Zuelch, M.L.; Dimitratos, S.M.; Scherr, R.E. Adolescent Obesity: Diet Quality, Psychosocial Health, and Cardiometabolic Risk Factors. Nutrients 2020, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Townley, J.; Northstone, K.; Hinton, E.C.; Hamilton-Shield, J.; Searle, A.; Leary, S. Daily Duration of Eating for Children and Adolescents: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 993. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, A.; le Roux, C.W.; Alexiadou, K.; Tentolouris, N.; Vincent, R.P.; Kyriaki, D.; Perrea, D.; Ghatei, M.A.; Bloom, S.R.; Katsilambros, N. Eating Slowly Increases the Postprandial Response of the Anorexigenic Gut Hormones, Peptide YY and Glucagon-Like Peptide-1. J. Clin. Endocrinol. Metab. 2010, 95, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.M.; Greene, G.W.; Melanson, K.J. Eating Slowly Led to Decreases in Energy Intake within Meals in Healthy Women. J. Am. Diet. Assoc. 2008, 108, 1186–1191. [Google Scholar] [CrossRef]
- Garcidueñas-Fimbres, T.E.; Paz-Graniel, I.; Nishi, S.K.; Salas-Salvadó, J.; Babio, N. Eating Speed, Eating Frequency, and Their Relationships with Diet Quality, Adiposity, and Metabolic Syndrome, or Its Components. Nutrients 2021, 13, 1687. [Google Scholar] [CrossRef] [PubMed]
- Muzenda, T.; Kamkuemah, M.; Battersby, J.; Oni, T. Assessing adolescent diet and physical activity behaviour, knowledge and awareness in low- and middle-income countries: A systematised review of quantitative epidemiological tools. BMC Public Health 2022, 22, 975. [Google Scholar] [CrossRef]
- Hayes, J.F.; Fitzsimmons-Craft, E.E.; Karam, A.M.; Jakubiak, J.; Brown, M.L.; Wilfley, D.E. Disordered Eating Attitudes and Behaviors in Youth with Overweight and Obesity: Implications for Treatment. Curr. Obes. Rep. 2018, 7, 235–246. [Google Scholar] [CrossRef]
- Mihov, Y.; Meyer, A.H.; Kakebeeke, T.H.; Stülb, K.; Arhab, A.; Zysset, A.E.; Leeger-Aschmann, C.S.; Schmutz, E.A.; Kriemler, S.; Jenni, O.G.; et al. Child Eating Behavior Predicts Body Mass Index after 1 Year: Results from the Swiss Preschooler’s Health Study (SPLASHY). Front. Psychol. 2024, 15, 1292939. [Google Scholar] [CrossRef]
- Galhardo, J.; Hunt, L.P.; Lightman, S.L.; Sabin, M.A.; Bergh, C.; Sodersten, P.; Shield, J.P.H. Normalizing Eating Behavior Reduces Body Weight and Improves Gastrointestinal Hormonal Secretion in Obese Adolescents. J. Clin. Endocrinol. Metab. 2012, 97, E193–E201. [Google Scholar] [CrossRef]
- Chen, W.; Liu, X.; Bao, L.; Yang, P.; Zhou, H. Health effects of the time-restricted eating in adults with obesity: A systematic review and meta-analysis. Front. Nutr. 2023, 10, 1079250. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulos, T.; Kokkinos, A.; Liaskos, C.; Tentolouris, N.; Alexiadou, K.; Miras, A.D.; Mourouzis, I.; Perrea, D.; Pantos, C.; Katsilambros, N.; et al. The effect of slow spaced eating on hunger and satiety in overweight and obese patients with type 2 diabetes mellitus. BMJ Open Diabetes Res. Care 2014, 2, e000013. [Google Scholar] [CrossRef] [PubMed]
- Monzani, A.; Ricotti, R.; Caputo, M.; Solito, A.; Archero, F.; Bellone, S.; Prodam, F. A Systematic Review of the Association of Skipping Breakfast with Weight and Cardiometabolic Risk Factors in Children and Adolescents. What Should We Better Investigate in the Future? Nutrients 2019, 11, 387. [Google Scholar] [CrossRef] [PubMed]
- Savige, G.; MacFarlane, A.; Ball, K.; Worsley, A.; Crawford, D. Snacking Behaviours of Adolescents and Their Association with Skipping Meals. Int. J. Behav. Nutr. Phys. Act. 2007, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Mambrini, S.P.; Menichetti, F.; Ravella, S.; Pellizzari, M.; De Amicis, R.; Foppiani, A.; Battezzati, A.; Bertoli, S.; Leone, A. Ultra-Processed Food Consumption and Incidence of Obesity and Cardiometabolic Risk Factors in Adults: A Systematic Review of Prospective Studies. Nutrients 2023, 15, 2583. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Jia, M.; Wang, X.; Hong, Y.; Zhao, X.; Zhang, L.; Ru, Y.; Yang, F.; Zhu, S. Associations of Eating Speed with Fat Distribution and Body Shape Vary in Different Age Groups and Obesity Status. Nutr. Metab. 2022, 19, 63. [Google Scholar] [CrossRef] [PubMed]
- Pendergast, F.J.; Livingstone, K.M.; Worsley, A.; McNaughton, S.A. Correlates of Meal Skipping in Young Adults: A Systematic Review. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Smit, H.J.; Kemsley, E.K.; Tapp, H.S.; Henry, C.J.K. Does Prolonged Chewing Reduce Food Intake? Fletcherism Revisited. Appetite 2011, 57, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.; Zhang, L.; Brandon, A.; Wang, Q.; Begg, D.; Qi, Y.; Fu, M.; Kulkarni, R.; Teo, J.; Baldock, P.; et al. Insulin Controls Food Intake and Energy Balance via NPY Neurons. Mol. Metab. 2017, 6, 574–584. [Google Scholar] [CrossRef]
- Fulkerson, J.A.; Larson, N.; Horning, M.; Neumark-Sztainer, D. A Review of Associations Between Family or Shared Meal Frequency and Dietary and Weight Status Outcomes Across the Lifespan. J. Nutr. Educ. Behav. 2014, 46, 2–19. [Google Scholar] [CrossRef]
- Boucsein, A.; Kamstra, K.; Tups, A. Central Signalling Cross-Talk between Insulin and Leptin in Glucose and Energy Homeostasis. J. Neuroendocrinol. 2021, 33, e12944. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.P.; Young, A.J.; Rood, J.C.; Montain, S.J. Independent and Combined Effects of Eating Rate and Energy Density on Energy Intake, Appetite, and Gut Hormones. Obesity 2013, 21, E244–E252. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, A.E.; Agosti, F.; Compri, E.; Giunta, M.; Marazzi, N.; Muller, E.E.; Cella, S.G.; Sartorio, A. Anorexigenic Postprandial Responses of PYY and GLP1 to Slow Ice Cream Consumption: Preservation in Obese Adolescents, but Not in Obese Adults. Eur. J. Endocrinol. 2013, 168, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Rossi, V.; Tagi, V.M.; Baldassarre, P.; Grazi, R.; Taranto, S.; Zuccotti, G. Food Intake and Sleep Disorders in Children and Adolescents with Obesity. Nutrients 2023, 15, 4736. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Yang, K.; Huang, F.; Liu, X.; Li, X.; Luo, Y.; Wu, L.; Guo, X. Association between Self-Reported Eating Speed and Metabolic Syndrome in a Beijing Adult Population: A Cross-Sectional Study. BMC Public Health 2018, 18, 855. [Google Scholar] [CrossRef] [PubMed]
- Garcidueñas-Fimbres, T.E.; Paz-Graniel, I.; Gómez-Martínez, C.; Jurado-Castro, J.M.; Leis, R.; Escribano, J.; Moreno, L.A.; Navas-Carretero, S.; Portoles, O.; Pérez-Vega, K.A.; et al. Associations Between Eating Speed, Diet Quality, Adiposity, and Cardiometabolic Risk Factors. J. Pediatr. 2023, 252, 31–39.e1. [Google Scholar] [CrossRef] [PubMed]
- Teo, P.S.; van Dam, R.M.; Whitton, C.; Tan, L.W.L.; Forde, C.G. Association between Self-Reported Eating Rate, Energy Intake, and Cardiovascular Risk Factors in a Multi-Ethnic Asian Population. Nutrients 2020, 12, 1080. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, R.; Tamakoshi, K.; Yatsuya, H.; Wada, K.; Matsushita, K.; OuYang, P.; Hotta, Y.; Takefuji, S.; Mitsuhashi, H.; Sugiura, K.; et al. Eating Fast Leads to Insulin Resistance: Findings in Middle-Aged Japanese Men and Women. Prev. Med. 2008, 46, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Peñacarrillo, M.L.; Márquez, L.; González, N.; Díaz-Miguel, M.; Valverde, I. Effect of GLP-1 on Lipid Metabolism in Human Adipocytes. Horm. Metab. Res. 2001, 33, 73–77. [Google Scholar] [CrossRef]
- Nagahama, S.; Kurotani, K.; Pham, N.M.; Nanri, A.; Kuwahara, K.; Dan, M.; Nishiwaki, Y.; Mizoue, T. Self-Reported Eating Rate and Metabolic Syndrome in Japanese People: Cross-Sectional Study. BMJ Open 2014, 4, e005241. [Google Scholar] [CrossRef]
- Lee, S.; Ko, B.-J.; Gong, Y.; Han, K.; Lee, A.; Han, B.-D.; Yoon, Y.J.; Park, S.; Kim, J.-H.; Mantzoros, C.S. Self-Reported Eating Speed in Relation to Non-Alcoholic Fatty Liver Disease in Adults. Eur. J. Nutr. 2016, 55, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.L.; Bergh, C.; Södersten, P.; Sabin, M.A.; Hollinghurst, S.; Hunt, L.P.; Shield, J.P.H. Treatment of Childhood Obesity by Retraining Eating Behaviour: Randomised Controlled Trial. BMJ 2010, 340, b5388. [Google Scholar] [CrossRef] [PubMed]
- Ohkuma, T.; Hirakawa, Y.; Nakamura, U.; Kiyohara, Y.; Kitazono, T.; Ninomiya, T. Association between Eating Rate and Obesity: A Systematic Review and Meta-Analysis. Int. J. Obes. 2015, 39, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Wiseman, E.; Pavlou, V.; Lin, S.; Oliveira, M.L.; Varady, K.A. Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab. 2020, 32, 366–378.e3. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, N.; Hu, L.; Li, Z.; Li, R.; Li, C.; Wang, S. Improvement in Chewing Activity Reduces Energy Intake in One Meal and Modulates Plasma Gut Hormone Concentrations in Obese and Lean Young Chinese Men123. Am. J. Clin. Nutr. 2011, 94, 709–716. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, C.; Kok, F.J. Slow Food, Fast Food and the Control of Food Intake. Nat. Rev. Endocrinol. 2010, 6, 290–293. [Google Scholar] [CrossRef] [PubMed]
- López-Gil, J.F. The Eating Healthy and Daily Life Activities (EHDLA) Study. Children 2022, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- De Onis, M. Development of a WHO Growth Reference for School-Aged Children and Adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Lohman, T.G. The Use of Skinfold to Estimate Body Fatness on Children and Youth. J. Phys. Educ. Recreat. Danc. 1987, 58, 98–103. [Google Scholar] [CrossRef]
- Slaughter, M.H.; Lohman, T.G.; Boileau, R.A.; Horswill, C.A.; Stillman, R.J.; Van Loan, M.D.; Bemben, D.A. Skinfold Equations for Estimation of Body Fatness in Children and Youth. Hum. Biol. 1988, 60, 709–723. [Google Scholar]
- Currie, C.; Molcho, M.; Boyce, W.; Holstein, B.; Torsheim, T.; Richter, M. Researching Health Inequalities in Adolescents: The Development of the Health Behaviour in School-Aged Children (HBSC) Family Affluence Scale. Soc. Sci. Med. 2008, 66, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Serra-Majem, L.; Ribas, L.; Ngo, J.; Ortega, R.M.; García, A.; Pérez-Rodrigo, C.; Aranceta, J. Food, Youth and the Mediterranean Diet in Spain. Development of KIDMED, Mediterranean Diet Quality Index in Children and Adolescents. Public Health Nutr. 2004, 7, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, I.T.; Ballart, J.F.; Pastor, G.C.; Jordà, E.B.; Val, V.A. Validation of a short questionnaire on frequency of dietary intake: Reproducibility and validity. Nutr. Hosp. 2008, 23, 242–252. [Google Scholar] [PubMed]
- Saint-Maurice, P.F.; Welk, G.J. Validity and Calibration of the Youth Activity Profile. PLoS ONE 2015, 10, e0143949. [Google Scholar] [CrossRef] [PubMed]
- Adab, P.; Pallan, M.J.; Lancashire, E.R.; Hemming, K.; Frew, E.; Griffin, T.; Barrett, T.; Bhopal, R.; Cade, J.E.; Daley, A.; et al. A Cluster-Randomised Controlled Trial to Assess the Effectiveness and Cost-Effectiveness of a Childhood Obesity Prevention Programme Delivered through Schools, Targeting 6–7 Year Old Children: The WAVES Study Protocol. BMC Public Health 2015, 15, 488. [Google Scholar] [CrossRef] [PubMed]
- Gilic, B.; Malovic, P.; Sunda, M.; Maras, N.; Zenic, N. Adolescents with Higher Cognitive and Affective Domains of Physical Literacy Possess Better Physical Fitness: The Importance of Developing the Concept of Physical Literacy in High Schools. Children 2022, 9, 796. [Google Scholar] [CrossRef] [PubMed]
- Delisle Nyström, C.; Traversy, G.; Barnes, J.D.; Chaput, J.-P.; Longmuir, P.E.; Tremblay, M.S. Associations between Domains of Physical Literacy by Weight Status in 8- to 12-Year-Old Canadian Children. BMC Public Health 2018, 18, 1043. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Ha, S.A.; Seo, J.S.; Sohn, C.M.; Park, H.R.; Kim, K.W. Eating Habits and Eating Behaviors by Family Dinner Frequency in the Lower-Grade Elementary School Students. Nutr. Res. Pract. 2014, 8, 679. [Google Scholar] [CrossRef]
- Fulkerson, J.A.; Neumark-Sztainer, D.; Hannan, P.J.; Story, M. Family Meal Frequency and Weight Status among Adolescents: Cross-Sectional and 5-Year Longitudinal Associations. Obesity 2008, 16, 2529–2534. [Google Scholar] [CrossRef]
- Sen, B. Frequency of Family Dinner and Adolescent Body Weight Status: Evidence from the National Longitudinal Survey of Youth, 1997. Obesity 2006, 14, 2266–2276. [Google Scholar] [CrossRef]
- Valdés, J.; Rodríguez-Artalejo, F.; Aguilar, L.; Jaén-Casquero, M.B.; Royo-Bordonada, M.Á. Frequency of Family Meals and Childhood Overweight: A Systematic Review. Pediatr. Obes. 2013, 8, e1–e13. [Google Scholar] [CrossRef] [PubMed]
- Hammons, A.J.; Fiese, B.H. Is Frequency of Shared Family Meals Related to the Nutritional Health of Children and Adolescents? Pediatrics 2011, 127, e1565–e1574. [Google Scholar] [CrossRef]
- Pearce, A.L.; Neuwald, N.V.; Evans, J.S.; Romano, O.; Rolls, B.J.; Keller, K.L. Child Eating Behaviors Are Consistently Linked to Intake across Meals That Vary in Portion Size. Appetite 2024, 196, 107258. [Google Scholar] [CrossRef] [PubMed]
- Slyper, A.H.; Kopfer, K.; Huang, W.-M.; Re’em, Y. Increased Hunger and Speed of Eating in Obese Children and Adolescents. J. Pediatr. Endocrinol. Metab. 2014, 27, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.S.; Elsworth, R.; Perry, R.; Hamilton-Shield, J.P.; Kinnear, F.; Hinton, E.C. The Feasibility, Acceptability, and Benefit of Interventions That Target Eating Speed in the Clinical Treatment of Children and Adolescents with Overweight or Obesity: A Systematic Review and Meta-Analysis. Appetite 2022, 168, 105780. [Google Scholar] [CrossRef] [PubMed]
- Miquel-Kergoat, S.; Azais-Braesco, V.; Burton-Freeman, B.; Hetherington, M.M. Effects of Chewing on Appetite, Food Intake and Gut Hormones: A Systematic Review and Meta-Analysis. Physiol. Behav. 2015, 151, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Wansink, B. From Mindless Eating to Mindlessly Eating Better. Physiol. Behav. 2010, 100, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Baradia, R.; Ghosh, J. Impact of Mindful Eating among Adolescent. Int. J. Sci. Res. (IJSR) 2020, 10, 11–15. [Google Scholar]
- Hendrickson, K.L.; Rasmussen, E.B. Mindful Eating Reduces Impulsive Food Choice in Adolescents and Adults. Health Psychol. 2017, 36, 226–235. [Google Scholar] [CrossRef]
- Mantzios, M.; Wilson, J.C. Mindfulness, Eating Behaviours, and Obesity: A Review and Reflection on Current Findings. Curr. Obes. Rep. 2015, 4, 141–146. [Google Scholar] [CrossRef]
- Mattes, R.D. Snacking: A Cause for Concern. Physiol. Behav. 2018, 193, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.; Slavin, J. Snacking for a Cause: Nutritional Insufficiencies and Excesses of U.S. Children, a Critical Review of Food Consumption Patterns and Macronutrient and Micronutrient Intake of U.S. Children. Nutrients 2014, 6, 4750–4759. [Google Scholar] [CrossRef] [PubMed]
- Emily, H. Food Marketing|UConn Rudd Center for Food Policy and Health. 2020. Available online: https://uconnruddcenter.org/wp-content/uploads/sites/2909/2022/11/Rudd-Targeted-Marketing-Report-2022.pdf (accessed on 13 July 2024).
- Kumar, S.; Croghan, I.T.; Biggs, B.K.; Croghan, K.; Prissel, R.; Fuehrer, D.; Donelan-Dunlap, B.; Sood, A. Fami-ly-Based Mindful Eating Intervention in Adolescents with Obesity: A Pilot Randomized Clinical Trial. Children 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, G.A.; Cook, L.; Spruijt-Metz, D.; Black, D.S. Mindfulness-Based Interventions for Obesity-Related Eating Behaviours: A Literature Review. Obes. Rev. 2014, 15, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Feunekes, G.I.J.; de Graaf, C.; van Staveren, W.A. Social Facilitation of Food Intake Is Mediated by Meal Duration. Physiol. Behav. 1995, 58, 551–558. [Google Scholar] [CrossRef]
- Ministerio de Sanidad—Sanidad En Datos—Informe Anual Del Sistema Nacional de Salud. Available online: https://www.sanidad.gob.es/estadEstudios/estadisticas/sisInfSanSNS/tablasEstadisticas/InfAnSNS.htm (accessed on 20 June 2024).
- De Bont, J.; Díaz, Y.; Casas, M.; García-Gil, M.; Vrijheid, M.; Duarte-Salles, T. Time Trends and Sociodemographic Factors Associated With Overweight and Obesity in Children and Adolescents in Spain. JAMA Netw. Open 2020, 3, e201171. [Google Scholar] [CrossRef]
| Variable | Meal Duration Status | |||
|---|---|---|---|---|
| Short (≤70 min) | Moderate (71 to 95 min) | Long (≥96 min) | ||
| Participants | n (%) | 287 (38.0) | 235 (31.1) | 233 (30.9) |
| Age (years) | Median (IQR) | 14.0 (2.0) | 14.0 (2.0) | 14.0 (2.0) |
| Sex | Boys (%) | 130 (45.3) | 120 (51.1) | 91 (39.1) |
| Girls (%) | 157 (54.7) | 115 (48.9) | 142 (60.9) | |
| FAS-III (score) | Median (IQR) | 8.0 (2.0) | 8.0 (2.0) | 9.0 (3.0) b |
| YAP-S physical activity (score) | Median (IQR) | 2.6 (0.9) | 2.6 (0.9) | 2.7 (1.0) a |
| YAP-S sedentary behaviors (score) | Median (IQR) | 2.6 (0.8) | 2.6 (0.8) | 2.4 (0.6) a,b |
| Overall sleep duration (minutes) | Median (IQR) | 497.1 (75.0) | 497.1 (62.1) | 497.1 (68.6) |
| KIDMED (score) | Median (IQR) | 7.0 (3.0) | 7.0 (3.0) | 7.0 (4.0) a,b |
| Energy intake (kcal) | Median (IQR) | 2523.9 (1485.8) | 2575.1 (1344.6) | 2712.6 (1544.8) |
| Breakfast duration (minutes) | Median (IQR) | 5.0 (5.0) | 10.0 (10.0) a | 15.0 (5.0) a,b |
| Lunch duration (minutes) | Median (IQR) | 15.0 (0.0) | 30.0 (0.0) a | 45.0 (15.0) a,b |
| Dinner duration (minutes) | Median (IQR) | 15.0 (5.0) | 30.0 (0.0) a | 45.0 (15.0) a,b |
| Morning snack duration (minutes) | Median (IQR) | 10.0 (5.0) | 10.0 (5.0) a | 15.0 (20.0) a,b |
| Afternoon snack duration (minutes) | Median (IQR) | 5.0 (5.0) | 10.0 (5.0) a | 15.0 (5.0) a,b |
| Global meal duration (minutes) | Median (IQR) | 55.0 (15.0) | 85.0 (10.0) a | 120.0 (35.0) a,b |
| BMI (z score) † | Median (IQR) | 0.0 (1.7) | 0.1 (2.2) | –0.3 (2.0) a,b |
| WC (cm) | Median (IQR) | 71.6 (13.2) | 71.0 (14.0) | 69.5 (11.4) a,b |
| Skinfold triceps (mm) | Median (IQR) | 15.0 (9.0) | 15.0 (10.0) | 14.5 (10.0) |
| Skinfold calf (mm) | Median (IQR) | 15.0 (9.2) | 15.5 (11.0) | 15.0 (10.0) |
| Body fat (%) ‡ | Median (IQR) | 23.4 (11.6) | 24.6 (12.8) | 22.8 (11.8) |
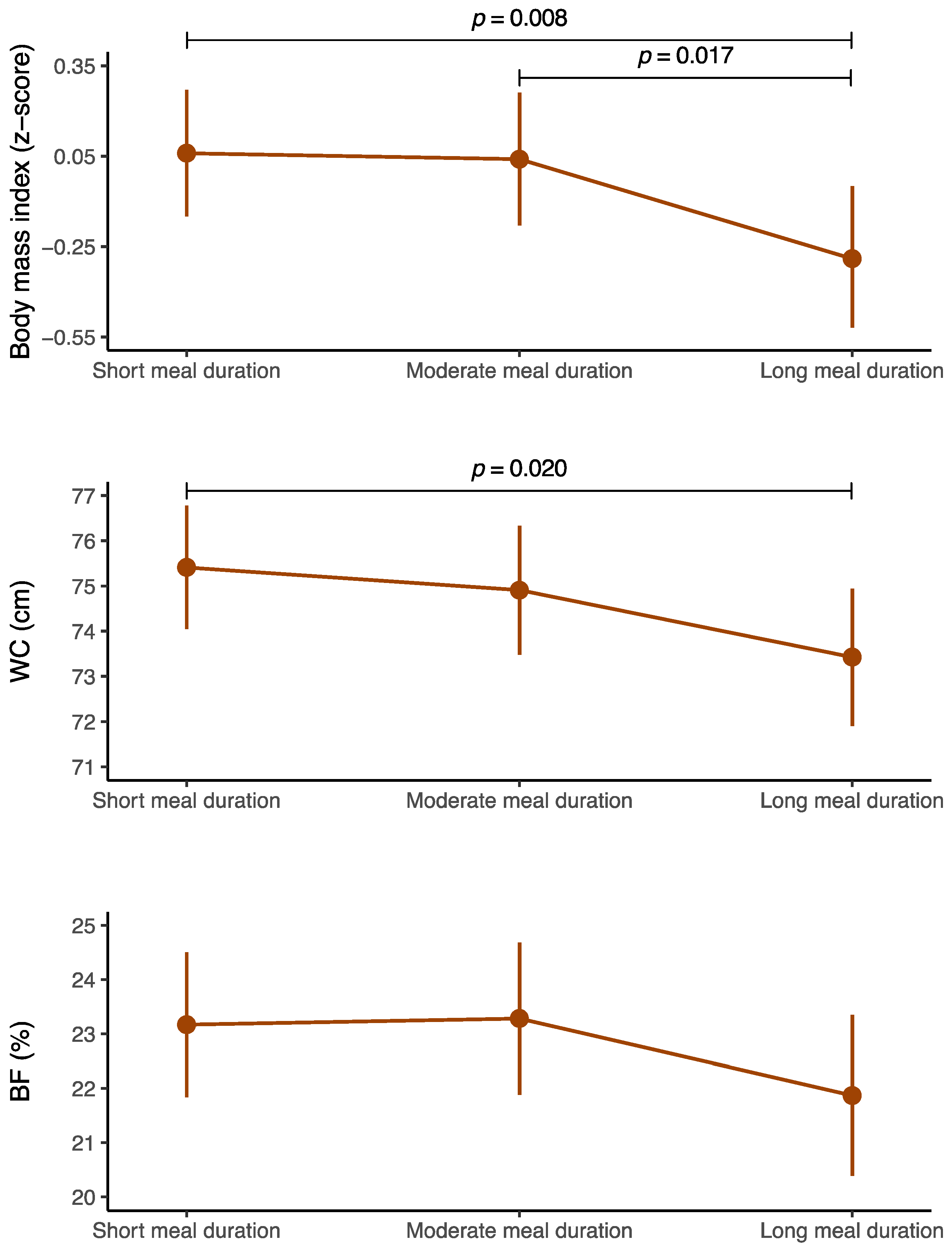
| Predictor | B | SE | LLCI | ULCI | p Value | |
|---|---|---|---|---|---|---|
| BMI (z score) † | Short meal duration (≤70 min) | Reference | ||||
| Moderate meal duration (71 to 95 min) | –0.021 | 0.129 | –0.274 | 0.232 | 0.872 | |
| Long meal duration (≥96 min) | –0.347 | 0.131 | –0.604 | –0.090 | 0.008 | |
| WC (cm) | Short meal duration (≤70 min) | Reference | ||||
| Moderate meal duration (71 to 95 min) | –0.504 | 0.845 | –2.164 | 1.155 | 0.551 | |
| Long meal duration (≥96 min) | –1.987 | 0.854 | –3.664 | –0.310 | 0.020 | |
| Body fat (%) ‡ | Short meal duration (≤70 min) | Reference | ||||
| Moderate meal duration (71 to 95 min) | 0.114 | 0.825 | –1.505 | 1.733 | 0.890 | |
| Long meal duration (≥96 min) | –1.300 | 0.834 | –2.937 | 0.336 | 0.119 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-López, M.F.; López-Gil, J.F. Meal Duration and Obesity-Related Indicators among Adolescents: Insights from the EHDLA Study. Nutrients 2024, 16, 2769. https://doi.org/10.3390/nu16162769
Martínez-López MF, López-Gil JF. Meal Duration and Obesity-Related Indicators among Adolescents: Insights from the EHDLA Study. Nutrients. 2024; 16(16):2769. https://doi.org/10.3390/nu16162769
Chicago/Turabian StyleMartínez-López, Mayra Fernanda, and José Francisco López-Gil. 2024. "Meal Duration and Obesity-Related Indicators among Adolescents: Insights from the EHDLA Study" Nutrients 16, no. 16: 2769. https://doi.org/10.3390/nu16162769
APA StyleMartínez-López, M. F., & López-Gil, J. F. (2024). Meal Duration and Obesity-Related Indicators among Adolescents: Insights from the EHDLA Study. Nutrients, 16(16), 2769. https://doi.org/10.3390/nu16162769







