Stability and Bioaccessibility of Carotenoids from Sea Buckthorn Pomace Encapsulated in Alginate Hydrogel Beads
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Standards
2.3. Carotenoid Extraction from SBP
2.4. Quantitative Determination of Carotenoids Using Spectrophotometric Analysis
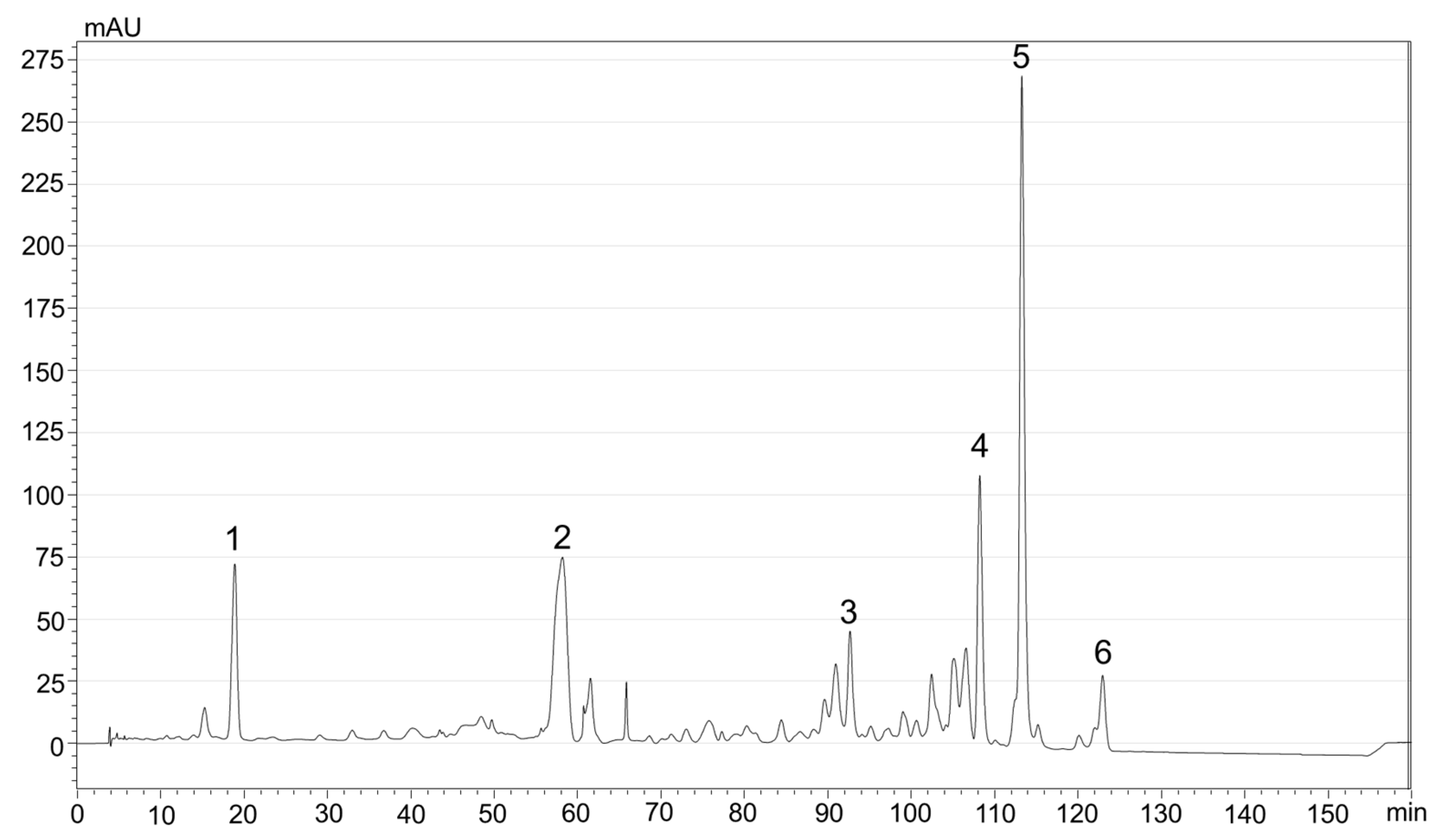
2.5. Identification and Quantification of Carotenoids by HPLC-DAD
2.6. Encapsulation of Carotenoid Extracts in Alginate Hydrogel Beads
2.7. Encapsulation Efficiency
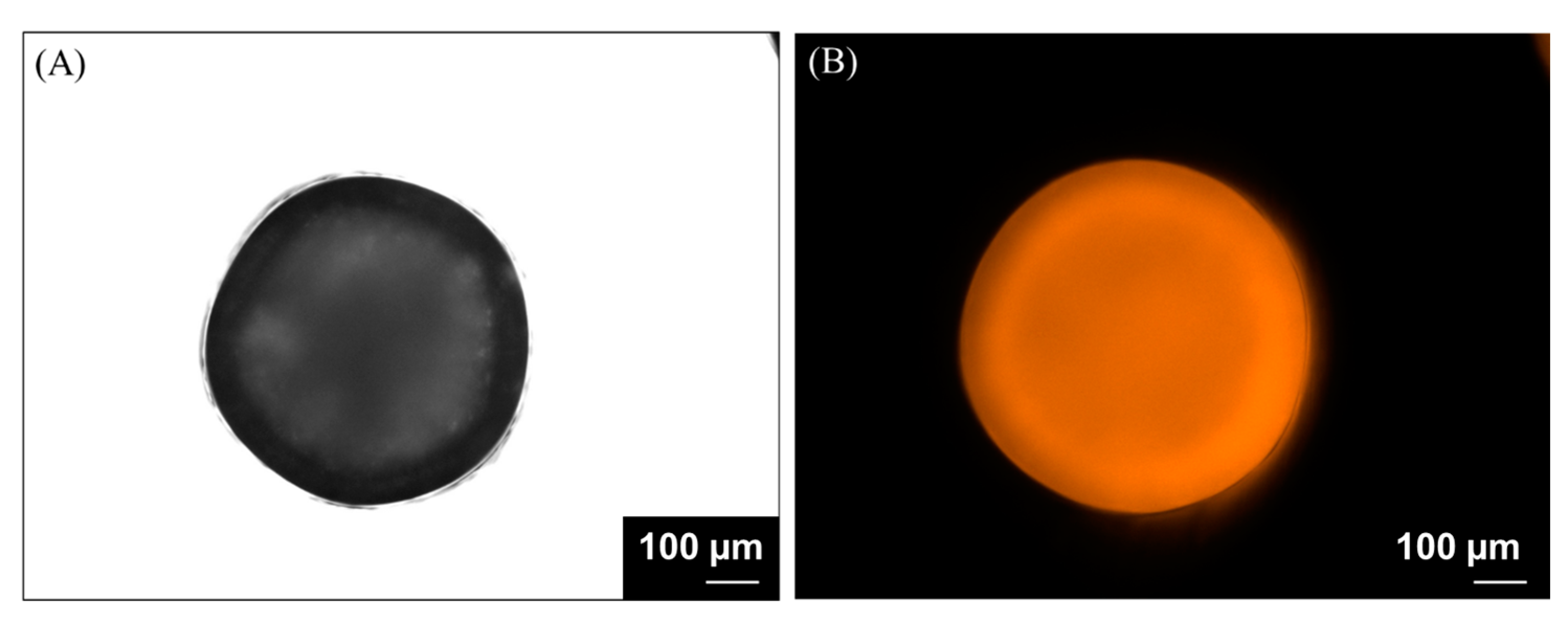
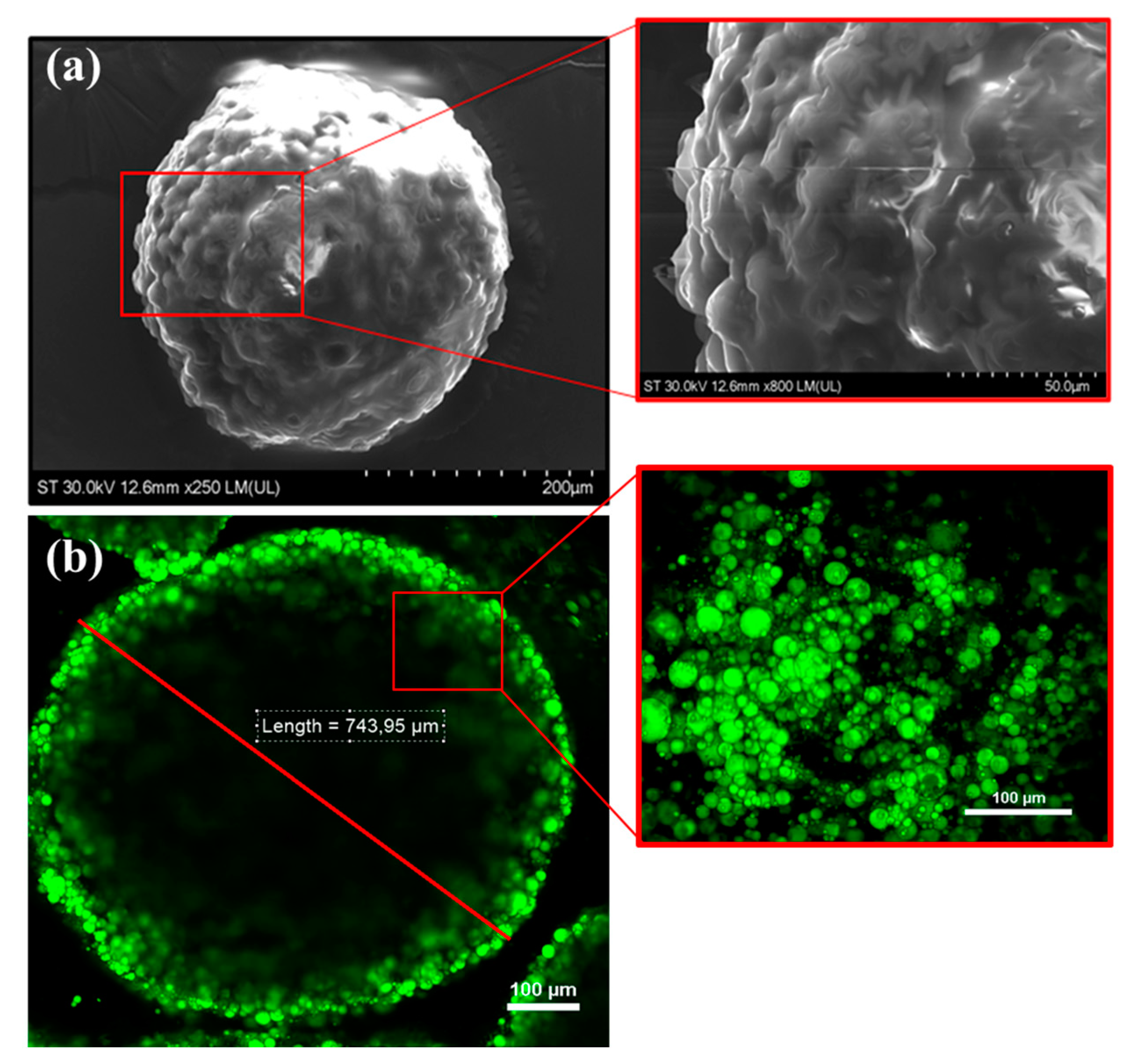
2.8. Characterization of Alginate-Based Hydrogel Beads Containing SBP Extract
2.9. Stability of Carotenoids Encapsulated in Alginate Hydrogel Beads
2.10. Bioaccessibility of Carotenoids from Alginate Hydrogel Beads by In Vitro Digestion
2.11. Statistical Analysis
3. Results and Discussions
3.1. Carotenoids Composition of Sea Buckthorn Pomace
3.2. Encapsulation of Carotenoid Extract
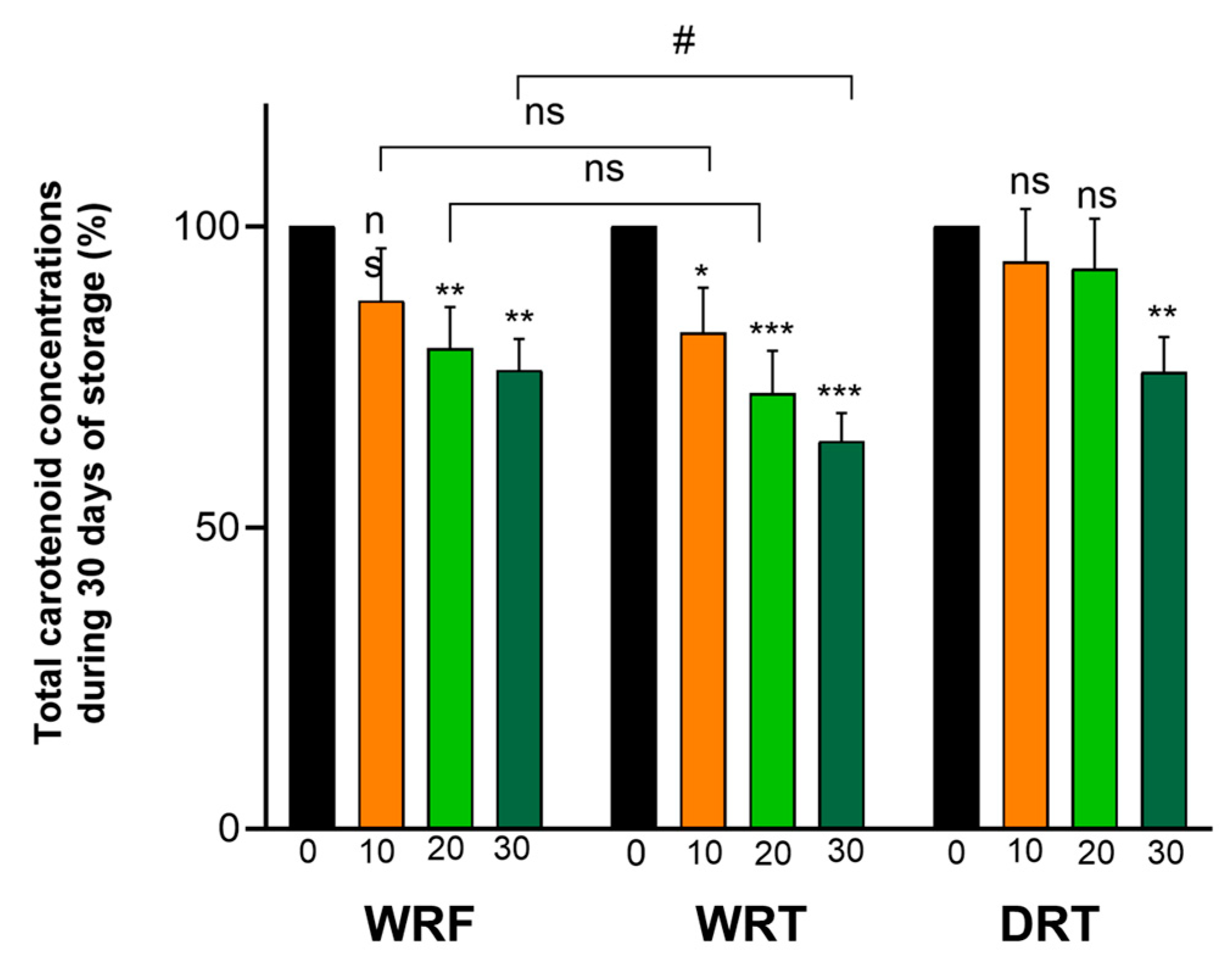
3.3. Stability of Encapsulated Carotenoids from SBP
3.4. Bioaccessibility of Carotenoids from Alginate Hydrogel Beads
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teleszko, M.; Wojdyło, A.; Rudzinska, M.; Oszmianski, J.; Golis, T. Analysis of Lipophilic and Hydrophilic Bioactive Compounds Content in Sea buckthorn (Hippophaë rhamnoides L.) Berries. J. Agric. Food Chem. 2015, 63, 4120–4129. [Google Scholar] [CrossRef] [PubMed]
- Ciesarová, Z.; Murkovic, M.; Cejpek, K.; Kreps, F.; Tobolková, B.; Koplík, R.; Belajová, E.; Kukurová, K.; Daško, Ľ.; Panovská, Z.; et al. Why is sea buckthorn (Hippophae rhamnoides L.) so exceptional? A review. Food Res. Int. 2020, 133, 109170. [Google Scholar] [CrossRef]
- Jasniewska, A.; Diowksz, A. Wide Spectrum of Active Compounds in Sea buckthorn (Hippophae rhamnoides) for Disease Prevention and Food Production. Antioxidants 2021, 10, 1279. [Google Scholar] [CrossRef]
- Olas, B.; Skalski, B.; Ulanowska, K. The Anticancer Activity of Sea buckthorn [Elaeagnus rhamnoides (L.) A. Nelson]. Front. Pharmacol. 2018, 9, 232. [Google Scholar] [CrossRef]
- Wang, K.; Xu, Z.; Liao, X. Bioactive compounds, health benefits and functional food products of sea buckthorn: A review. Crit. Rev. Food Sci. Nutr. 2021, 62, 6761–6782. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in Human Nutrition and Health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; Bonet, M.L.; Borel, P.; Keijer, J.; Landrier, J.-F.; Milisav, I.; Ribot, J.; Riso, P.; Winklhofer-Roob, B.; Sharoni, Y.; et al. Mechanistic aspects of carotenoid health benefits–Where are we now? Nutr. Res. Rev. 2021, 34, 276–302. [Google Scholar] [CrossRef]
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.P.; Bobak, Ł.; Nowicka, P. Anti-Oxidant and Anti-Enzymatic Activities of Sea Buckthorn (Hippophaë rhamnoides L.) Fruits Modulated by Chemical Components. Antioxidants 2019, 8, 618. [Google Scholar] [CrossRef]
- Tudor, C.; Bohn, T.; Iddir, M.; Dulf, F.V.; Focşan, M.; Rugină, D.O.; Pintea, A. Sea Buckthorn Oil as a Valuable Source of Bioaccessible Xanthophylls. Nutrients 2020, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Visan, S.; Soritau, O.; Tatomir, C.; Baldasici, O.; Balacescu, L.; Balacescu, O.; Muntean, P.; Gherasim, C.; Pintea, A. The Bioactive Properties of Carotenoids from Lipophilic Sea buckthorn Extract (Hippophae rhamnoides L.) in Breast Cancer Cell Lines. Molecules 2023, 28, 4486. [Google Scholar] [CrossRef]
- Böhm, V.; Lietz, G.; Olmedilla-Alonso, B.; Phelan, D.; Reboul, E.; Bánati, D.; Borel, P.; Corte-Real, J.; de Lera, A.R.; Desmarchelier, C.; et al. From carotenoid intake to carotenoid blood and tissue concentrations—Implications for dietary intake recommendations. Nutr. Rev. 2021, 79, 544–573. [Google Scholar] [CrossRef] [PubMed]
- Dvořák, P.; Suchý, O.; Straková, E.; Doležalová, J. The effect of a diet supplemented with sea-buckthorn pomace on the colour and viscosity of the egg yolk. Acta Vet. Brno 2017, 86, 303–308. [Google Scholar] [CrossRef]
- Dienaitė, L.; Baranauskienė, R.; Venskutonis, P. Lipophilic extracts isolated from European cranberry bush (Viburnum opulus) and sea buckthorn (Hippophae rhamnoides) berry pomace by supercritical CO2–Promising bioactive ingredients for foods and nutraceuticals. Food Chem. 2021, 348, 129047. [Google Scholar] [CrossRef]
- Bhimjiyani, V.H.; Borugadda, V.B.; Naik, S.; Dalai, A.K. Enrichment of flaxseed (Linum usitatissimum) oil with carotenoids of sea buckthorn pomace via ultrasound-assisted extraction technique. Curr. Res. Food Sci. 2021, 4, 478–488. [Google Scholar] [CrossRef]
- Melendez-Martinez, A.J.; Bohm, V.; Borge, G.I.A.; Cano, M.P.; Fikselova, M.; Gruskiene, R.; Lavelli, V.; Loizzo, M.R.; Mandic, A.I.; Brahm, P.M.; et al. Carotenoids: Considerations for Their Use in Functional Foods, Nutraceuticals, Nutricosmetics, Supplements, Botanicals, and Novel Foods in the Context of Sustainability, Circular Economy, and Climate Change. Annu. Rev. Food Sci. Technol. 2021, 12, 433–460. [Google Scholar] [CrossRef]
- Nour, V.; Panaite, T.D.; Corbu, A.R.; Ropota, M.; Turcu, R.P. Nutritional and Bioactive Compounds in Dried Sea-Buckthorn Pomace. Erwerbs-Obstbau 2021, 63, 91–98. [Google Scholar] [CrossRef]
- Stanciu, I.; Ungureanu, E.L.; Popa, E.E.; Geicu-Cristea, M.; Draghici, M.; Mitelut, A.C.; Mustatea, G.; Popa, M.E. The Experimental Development of Bread with Enriched Nutritional Properties Using Organic Sea Buckthorn Pomace. Appl. Sci. 2023, 13, 6513. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Zhu, L.; Geng, Z.; Liu, X.; Cheng, Z.; Zhao, M.; Zhang, Q.; Yang, X. Sea Buckthorn Pretreatment, Drying, and Processing of High-Quality Products: Current Status and Trends. Foods 2023, 12, 4255. [Google Scholar] [CrossRef] [PubMed]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors Influencing the Chemical Stability of Carotenoids in Foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef]
- Barba, C.; Frígola, A. Carotenoid encapsulation for food applications. Trends Food Sci. Technol. 2017, 69, 54–63. [Google Scholar] [CrossRef]
- Soukoulis, C.; Bohn, T. A comprehensive overview on the micro- and nano-technological encapsulation advances for enhancing the chemical stability and bioavailability of carotenoids. Crit. Rev. Food Sci. Nutr. 2017, 58, 1–36. [Google Scholar] [CrossRef]
- McClements, D.J. Recent developments in encapsulation and release of functional food ingredients: Delivery by design. Curr. Opin. Food Sci. 2018, 23, 80–84. [Google Scholar] [CrossRef]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocoll. 2018, 88, 146–162. [Google Scholar] [CrossRef]
- Donhowe, E.G.; Flores, F.P.; Kerr, W.L.; Wicker, L.; Kong, F. Characterization and in vitro bioavailability of β-carotene: Effects of microencapsulation method and food matrix. LWT Food Sci. Technol. 2014, 57, 42–48. [Google Scholar] [CrossRef]
- Tolve, R.; Galgano, F.; Caruso, M.C.; Tchuenbou-Magaia, F.L.; Condelli, N.; Favati, F.; Zhang, Z. Encapsulation of health-promoting ingredients: Applications in foodstuffs. Int. J. Food Sci. Nutr. 2016, 67, 888–918. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.S.; Veloso, C.M. Microencapsulation of natural dyes with biopolymers for application in food: A review. Food Hydrocoll. 2021, 112, 106374. [Google Scholar] [CrossRef]
- de Freitas Santos, P.D.; Rubio, F.T.V.; da Silva, M.P.; Pinho, L.S.; Favaro-Trindade, C.S. Microencapsulation of carotenoid-rich materials: A review. Food Res. Int. 2021, 147, 110571. [Google Scholar] [CrossRef] [PubMed]
- Trif, M.; Vodnar, D.C.; Mitrea, L.; Rusu, A.V.; Socol, C.T. Design and Development of Oleoresins Rich in Carotenoids Coated Hydrogel beads. Coatings 2019, 9, 235. [Google Scholar] [CrossRef]
- Rusu, A.V.; Criste, F.L.; Mierliţă, D.; Socol, C.T.; Trif, M. Formulation of Lipoprotein Microencapsulated Beadlets by Ionic Complexes in Algae-Based Carbohydrates. Coatings 2020, 10, 302. [Google Scholar] [CrossRef]
- Roman, D.; Condurache, N.N.; Stănciuc, N.; Andronoiu, D.G.; Aprodu, I.; Enachi, E.; Barbu, V.; Bahrim, G.E.; Stanciu, S.; Râpeanu, G. Advanced Composites Based on Sea Buckthorn Carotenoids for Mayonnaise Enrichment. Polymers 2022, 14, 548. [Google Scholar] [CrossRef]
- Gani, A.; Jan, R.; Ashwar, B.A.; Ashraf, Z.U.; Shah, A.; Gani, A. Encapsulation of saffron and sea buckthorn bioactives: Its utilization for development of low glycemic baked product for growing diabetic population of the world. LWT 2021, 142, 111035. [Google Scholar] [CrossRef]
- Tkacz, K.; Turkiewicz, I.; Nowicka, P.; Wojdyło, A. Microspheres as carriers of sea buckthorn carotenoids and tocols with antidiabetic potential: Effect of biopolymers, cross-linking and storage. Food Biosci. 2024, 59, 103995. [Google Scholar] [CrossRef]
- Čulina, P.; Zorić, Z.; Garofulić, I.E.; Repajić, M.; Dragović-Uzelac, V.; Pedisić, S. Optimization of the Spray-Drying Encapsulation of Sea Buckthorn Berry Oil. Foods 2023, 12, 2448. [Google Scholar] [CrossRef] [PubMed]
- Drozińska, E.; Kanclerz, A.; Kurek, M.A. Microencapsulation of sea buckthorn oil with β-glucan from barley as coating material. Int. J. Biol. Macromol. 2019, 131, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Mihalcea, L.; Turturica, M.; Ghinea, I.O.; Barbu, V.; Ioniţa, E.; Cotârlet, M.; Stanciuc, N. Encapsulation of carotenoids from sea buckthorn extracted by CO2 supercritical fluids method within whey proteins isolates matrices. Innov. Food Sci. Emerg. Technol. 2017, 42, 120–129. [Google Scholar] [CrossRef]
- Roman, D.; Condurache (Lazar), N.N.; Aprodu, I.; Enachi, E.; Barbu, V.; Bahrim, G.E.; Stanciuc, N.; Râpeanu, G. Insights of sea buckthorn extract’s encapsulation by coacervation technique. Inventions 2021, 6, 59. [Google Scholar] [CrossRef]
- Waglewska, E.; Misiaszek, T.; Bazylinska, U. Nanoencapsulation of poorly soluble sea-buckthorn pulp oil in bile salt-origin vesicles: Physicochemical characterization and colloidal stability. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129113. [Google Scholar] [CrossRef]
- Karim, A.; Rehman, A.; Feng, J.; Noreen, A.; Assadpour, E.; Kharazmi, M.S.; Lianfu, Z.; Jafari, S.M. Alginate-based nanocarriers for the delivery and controlled-release of bioactive compounds. Adv. Colloid. Interface Sci. 2022, 307, 102744. [Google Scholar] [CrossRef]
- Milivojevic, M.; Popovic, A.; Pajic-Lijakovc, I.; Šoštaric, I.; Kolašinac, S.; Stevanovic, Z.D. Alginate Gel-Based Carriers for Encapsulation of Carotenoids: On Challenges and Applications. Gels 2023, 9, 620. [Google Scholar] [CrossRef]
- Mihalcea, L.; Turturică, M.; Barbu, V.; Ioniţă, E.; Pătraşcu, L.; Cotârleţ, M.; Loredana Dumitraşcu, L.; Aprodu, I.; Râpeanu, G.; Stănciuc, N. Transglutaminase mediated microencapsulation of sea buckthorn supercritical CO2 extract in whey protein isolate and valorization in highly value added food products. Food Chem. 2018, 262, 30–38. [Google Scholar] [CrossRef]
- de Campo, C.; Queiroz Assis, R.; Marques da Silva, M.; Haas Costa, T.M.; Paese, K.; Stanisçuaski Guterres, S.; de Oliveira Rios, A.; Hickmann Flôres, S. Incorporation of zeaxanthin nanoparticles in yogurt: Influence on physicochemical properties, carotenoid stability and sensory analysis. Food Chem. 2019, 301, 125230. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Hao, M.; Li, Y.; Zhang, J.; Ding, Y.; Lyu, F. Fortification of yogurt with bioactive functional foods and ingredients and associated challenges—A review. Trends Food Sci. Technol. 2022, 129, 558–580. [Google Scholar] [CrossRef]
- Gherasim, E.C.; Bunea, A.; Rugină, D.; Pintea, A. Green extraction of carotenoids from sea buckthorn pomace the effect of solvent and extraction method. ProEnvironment 2023, 16, 41–50. [Google Scholar]
- Britton, G. UV/Visible Spectroscopy. In Carotenoids Volume 1B: Spectroscopy; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 1995; pp. 13–63. [Google Scholar]
- Pop, O.L.; Dulf, F.V.; Cuibus, L.; Castro-Giráldez, M.; Fito, P.J.; Vodnar, D.C.; Coman, C.; Socaciu, C.; Suharoschi, R. Characterization of a Sea Buckthorn Extract and Its Effect on Free and Encapsulated Lactobacillus casei. Int. J. Mol. Sci. 2017, 18, 2513. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Giuffrida, D.; Pintea, A.; Dugo, P.; Torre, G.; Pop, R.M.; Mondello, L. Determination of carotenoids and their esters in fruits of sea buckthorn (Hippophae rhamnoides L.) by HPLC-DAD-APCI-MS. Phytochem. Anal. 2011, 23, 267–273. [Google Scholar] [CrossRef]
- Pop, R.M.; Weesepoel, Y.; Socaciu, C.; Pintea, A.; Vincken, J.P.; Gruppen, H. Carotenoid composition of berries and leaves from six Romanian sea buckthorn (Hippophae rhamnoides L.) varieties. Food Chem. 2014, 147, 1–9. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Klimantakis, K.; Kalaitzis, P.; Biliaderis, C.G.; Mourtzinos, I. Enrichment of sunflower oil with tomato carotenoids and its encapsulation in Ca-alginate beads: Preparation, characterization and chemical stability upon in vitro digestion. Food Hydrocoll. 2024, 151, 109855. [Google Scholar] [CrossRef]
- Savic Gajic, I.M.; Savic, I.M.; Gajic, D.G.; Dosic, A. Ultrasound-Assisted Extraction of Carotenoids from Orange Peel Using Olive Oil and Its Encapsulation in Ca-Alginate Beads. Biomolecules 2021, 11, 225. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; McClements, D.J. Encapsulation of β-carotene in alginate-based hydrogel beads: Impact on physicochemical stability and bioaccessibility. Food Hydrocoll. 2016, 61, 1–10. [Google Scholar] [CrossRef]
- Mellado-Ortega, E.; Hornero-Méndez, D. Lutein esterification in wheat flour increases the carotenoid retention and is induced by storage temperatures. Foods 2017, 6, 111. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, M.; Huang, Y.; Wang, Y.; Luo, F.; Liao, L. Stability of carotenoids and carotenoid esters in pumpkin (Cucurbita maxima) slices during hot air drying. Food Chem. 2022, 367, 130710. [Google Scholar] [CrossRef]
- Durante, M.; Lenucci, M.S.; Laddomada, B.; Mita, G.; Caretto, S. Effects of Sodium Alginate Bead Encapsulation on the Storage Stability of Durum Wheat (Triticum durum Desf.) Bran Oil Extracted by Supercritical CO2. J. Agric. Food Chem. 2012, 60, 10689–10695. [Google Scholar] [CrossRef]
- Liu, W.; Wang, J.; McClements, D.J.; Zou, L. Encapsulation of β-carotene-loaded oil droplets in caseinate/alginate microparticles: Enhancement of carotenoid stability and bioaccessibility. J. Funct. Foods 2018, 40, 527–535. [Google Scholar] [CrossRef]
- Han, J.; Zhang, Z.; Shang, W.; Yan, J.; McClements, D.J.; Xiao, H.; Wu, H.; Zhu, B. Modulation of physicochemical stability and bioaccessibility of β-carotene using alginate beads and emulsion stabilized by scallop (Patinopecten yessoensis) gonad protein isolates. Food Res. Int. 2020, 129, 108875. [Google Scholar] [CrossRef]
- Voo, W.P.; Lee, B.B.; Idris, A.; Islam, A.; Tey, B.T.; Chan, E.S. Production of ultra-high concentration calcium alginate beads with prolonged dissolution profile. RSC Adv. 2015, 5, 36687–36695. [Google Scholar] [CrossRef]
- Vulić, J.; Šeregelj, V.; Kalušević, A.; Lević, S.; Nedović, V.; Tumbas Šaponjac, V.; Čanadanović-Brunet, J.; Ćetković, G. Bioavailability and Bioactivity of Encapsulated Phenolics and Carotenoids Isolated from Red Pepper Waste. Molecules 2019, 24, 2837. [Google Scholar] [CrossRef]
- Lemmens, L.; Colle, I.; Van Buggenhout, S.; Palmero, P.; Van Loey, A.; Hendrickx, M. Carotenoid bioaccessibility in fruit-and vegetable-based food products as affected by product (micro)structural characteristics and the presence of lipids: A review. Trends Food Sci. Technol. 2014, 38, 125–135. [Google Scholar] [CrossRef]
- Corte-Real, J.; Bohn, T. Interaction of divalent minerals with liposoluble nutrients and phytochemicals during digestion and influences on their bioavailability—A review. Food Chem. 2018, 252, 285–293. [Google Scholar] [CrossRef]
- Wen, X.; Hempel, J.; Schweiggert, R.M.; Wang, Y.; Ni, Y.; Carle, R. Screening of critical factors influencing the efficient hydrolysis of zeaxanthin dipalmitate in an adapted in vitro- digestion model. Food Chem. 2018, 257, 36–43. [Google Scholar] [CrossRef]
- Goñi, I.; Serrano, J.; Saura-Calixto, F. Bioaccessibility of β-Carotene, Lutein, and Lycopene from Fruits and Vegetables. J. Agric. Food Chem. 2006, 54, 5382–5387. [Google Scholar] [CrossRef] [PubMed]

| Sample | Days of Storage | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | 25 | 30 | ||
| Zea | WRF | 0.39 ± 0.02 | 0.33 ± 0.02 ** | 0.32 ± 0.01 *** | 0.3 ± 0.01 **** | 0.29 ± 0.02 **** | 0.26 ± 0.02 **** | 0.18 ± 0.01 **** |
| WRT | 0.38 ± 0.04 | 0.29 ± 0.03 ** | 0.29 ± 0.03 ** | 0.26 ± 0.02 *** | 0.23 ± 0.02 **** | 0.24 ± 0.01 **** | 0.18 ± 0.01 **** | |
| DRT | 1.99 ± 0.08 | 1.77 ± 0.08 ** | 1.56 ± 0.06 **** | 1.57 ± 0.05 **** | 1.56 ± 0.06 **** | 1.43 ± 0.03 **** | 1.01 ± 0.04 **** | |
| ZMP | WRF | 0.54 ± 0.04 | 0.51 ± 0.03 ns | 0.49 ± 0.04 ns | 0.47 ± 0.02 ns | 0.44 ± 0.03 * | 0.43 ± 0.04 ** | 0.43 ± 0.03 ** |
| WRT | 0.53 ± 0.05 | 0.44 ± 0.05 * | 0.43 ± 0.04 * | 0.42 ± 0.03 * | 0.38 ± 0.02 ** | 0.37 ± 0.03 *** | 0.35 ± 0.03 *** | |
| DRT | 3.11 ± 0.09 | 2.87 ± 0.06 *** | 2.84 ± 0.08 *** | 2.77 ± 0.07 **** | 2.72 ± 0.05 **** | 2.7 ± 0.05 **** | 2.21 ± 0.06 **** | |
| ZDP | WRF | 1.5 ± 0.06 | 1.39 ± 0.04 * | 1.32 ± 0.05 *** | 1.31 ± 0.04 *** | 1.2 ± 0.03 **** | 1.19 ± 0.01 **** | 1.16 ± 0.02 **** |
| WRT | 1.4 ± 0.04 | 1.19 ± 0.04 **** | 1.19 ± 0.03 **** | 1.15 ± 0.03 **** | 1.05 ± 0.01 **** | 1 ± 0.02 **** | 0.95 ± 0.01 **** | |
| DRT | 8.01 ± 0.09 | 8.45 ± 0.09 **** | 7.76 ± 0.07 ** | 7.67 ± 0.08 *** | 7.57 ± 0.06 **** | 7.43 ± 0.05 **** | 6.4 ± 0.03 **** | |
| β-carotene | WRF | 0.98 ± 0.07 | 0.90 ± 0.05 ns | 0.89 ± 0.06 ns | 0.88 ± 0.06 ns | 0.81 ± 0.05 ** | 0.80 ± 0.04 ** | 0.64 ± 0.04 **** |
| WRT | 0.96 ± 0.08 | 0.79 ± 0.06 * | 0.76 ± 0.07 ** | 0.74 ± 0.06 ** | 0.68 ± 0.04 *** | 0.66 ± 0.03 *** | 0.61 ± 0.03 **** | |
| DRT | 5.49 ± 0.09 | 5.22 ± 0.09 ** | 4.93 ± 0.07 **** | 4.97 ± 0.08 **** | 4.97 ± 0.07 **** | 4.69 ± 0.05 **** | 4.00 ± 0.04 **** | |
| γ-carotene | WRF | 0.25 ± 0.03 | 0.21 ± 0.02 * | 0.20 ± 0.01 ** | 0.18 ± 0.02 *** | 0.19 ± 0.01 *** | 0.17 ± 0.01 *** | 0.18 ± 0.01 *** |
| WRT | 0.24 ± 0.03 | 0.18 ± 0.02 ** | 0.18 ± 0.02 ** | 0.17 ± 0.01 ** | 0.15 ± 0.02 *** | 0.14 ± 0.01 **** | 0.13 ± 0.01 **** | |
| DRT | 1.25 ± 0.05 | 1.26 ± 0.05 ns | 1.12 ± 0.03 ** | 1.09 ± 0.03 *** | 1.1 ± 0.03 *** | 1.06 ± 0.01 **** | 0.88 ± 0.01 **** | |
| Lycopene | WRF | 0.24 ± 0.02 | 0.22 ± 0.02 ns | 0.21 ± 0.01 ns | 0.21 ± 0.02 ns | 0.20 ± 0.01 * | 0.18 ± 0.01 ** | 0.15 ± 0.01 *** |
| WRT | 0.19 ± 0.03 | 0.18 ± 0.02 ns | 0.17 ± 0.02 ns | 0.16 ± 0.02 ns | 0.15 ± 0.01 ns | 0.14 ± 0.01 * | 0.12 ± 0.01 *** | |
| DRT | 1.21 ± 0.03 | 1.22 ± 0.03 ns | 1.13 ± 0.02 ** | 1.13 ± 0.02 ** | 1.08 ± 0.01 **** | 1.04 ± 0.01 **** | 0.84 ± 0.01 **** | |
| Carotenoid | Hydrated Capsules (WET) | Dehydrated Capsules (DHD) | ||
|---|---|---|---|---|
| Control | With Yogurt | Control | With Yogurt | |
| Zeaxanthin | 6.31 ± 2.4 | 4.73 ± 2.1 | 28.67 ± 3.2 | 41.64 ± 3.9 |
| Zeaxanthin myristate-palmitate | 64.26 ± 4.3 | 50.87 ± 3.9 | 42.1 ± 4.1 | 49.13 ± 3.7 |
| Zeaxanthin dipalmitate | 66.05 ± 4.1 | 46.85 ± 2.3 | 39.58 ± 3.8 | 45.93 ± 5.3 |
| β-carotene | 51.42 ± 3.6 | 36.24 ± 2.2 | 41.48 ± 3.9 | 43.66 ± 2.7 |
| γ-carotene | 51.38 ± 3.9 | 36.47 ± 2.3 | 40.85 ± 2.5 | 38.83 ± 1.2 |
| Lycopene | 11.08 ± 1.9 | 6.98 ± 0.7 | 29.58 ± 3.7 | 24.12 ± 1.3 |
| Total Bioaccessibility | 42.1 ± 4.6 | 29.5 ± 3.9 | 40.8 ± 4 | 40.7 ± 3.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gherasim, C.E.; Focşan, M.; Ciont, C.; Bunea, A.; Rugină, D.; Pintea, A. Stability and Bioaccessibility of Carotenoids from Sea Buckthorn Pomace Encapsulated in Alginate Hydrogel Beads. Nutrients 2024, 16, 2726. https://doi.org/10.3390/nu16162726
Gherasim CE, Focşan M, Ciont C, Bunea A, Rugină D, Pintea A. Stability and Bioaccessibility of Carotenoids from Sea Buckthorn Pomace Encapsulated in Alginate Hydrogel Beads. Nutrients. 2024; 16(16):2726. https://doi.org/10.3390/nu16162726
Chicago/Turabian StyleGherasim, Cristina Elena, Monica Focşan, Călina Ciont, Andrea Bunea, Dumitriţa Rugină, and Adela Pintea. 2024. "Stability and Bioaccessibility of Carotenoids from Sea Buckthorn Pomace Encapsulated in Alginate Hydrogel Beads" Nutrients 16, no. 16: 2726. https://doi.org/10.3390/nu16162726
APA StyleGherasim, C. E., Focşan, M., Ciont, C., Bunea, A., Rugină, D., & Pintea, A. (2024). Stability and Bioaccessibility of Carotenoids from Sea Buckthorn Pomace Encapsulated in Alginate Hydrogel Beads. Nutrients, 16(16), 2726. https://doi.org/10.3390/nu16162726









