Curcumin: A Golden Approach to Healthy Aging: A Systematic Review of the Evidence
Abstract
1. Introduction
2. Materials and Methods
2.1. Focal Question
2.2. Language
2.3. Literature Search
2.4. Inclusion and Exclusion Criteria
2.5. Data Extraction
2.6. Study Selection
2.7. Quality Assessment
2.8. Registration
3. Results
4. Discussion
4.1. Beneficial Effects of Curcumin and Aging-Related Disorders
4.2. Inflammation
4.3. Oxidative Stress
4.4. Mitochondrial Dysfunction and Apoptosis
4.5. Neurodegenerative Diseases
4.5.1. Cognition
4.5.2. Memory
4.5.3. Alzheimer’s Disease
4.5.4. Parkinson’s Disease
4.6. Fragility
4.7. Sarcopenia
4.8. Depression
4.9. Clinical Trials Performed with Curcumin and Age-Related Disorders
| Reference | Model/Country | Population | Intervention/Comparison | Outcomes | Side Effects |
|---|---|---|---|---|---|
| Sarcopenia | |||||
| [293] | Randomized, placebo-controlled, double-blind clinical trial. India | 30 healthy elderly individuals, 13♂, 17♀, 69.8 ± 5. | Participants received 500 mg/day of Cureit or placebo for 3 months. | ↑ 1.43% in handgrip strength, a considerable increase of 6.08% in weightlifting strength, and a positive impact on the distance covered before feeling tired (↑ 1.15%, along with speed walking (5.51 m)). | No adverse events were observed. |
| Parkinson’s disease | |||||
| [294] | Pilot, randomized, triple-blind, placebo-controlled, add-on trial. Iran | 60 subjects, 45♂, 15♀, 58.2 ± 11.2 y, with idiopathic PD | Subjects received curcumin nanomicelles in capsules 80 mg/day or placebo/9 months. Then, the scores MDS-UPDRS and PDQ-39 were calculated at 3, 6, and 9 months. | Curcumin group did not have a significant improvement in MDS-UPDRS and PDQ-39 scores compared to placebo group. | Nausea, vomiting, and dyspepsia. |
| Frailty | |||||
| [228] | Pilot, 12-week, randomized trial/United States of America | 17 subjects, 8♀, 9♂, 66–94 y, moderately functioning and sedentary, with low-grade systemic inflammation. | 9 subjects were assigned to Curcumin C3 Complex®, receiving 1000 mg/day or placebo. At 0 and at 12 weeks, patients underwent functional testing and lower-limb strength testing. Also, at the beginning of treatment, 4, 8, and 12 weeks, venous blood was collected for safety blood chemistry analyses and biomarkers of inflammation. | Curcumin C3 Complex® group demonstrated large effect sizes in short physical performance battery (d = 0.75), measures of knee extension (d = 0.69), and flexion peak torque (d = 0.82). Furthermore, effects on galectin-3 and IL-6 levels were smaller in curcumin group compared to placebo. | No adverse events were reported. |
| Dementia | |||||
| [295] | Randomized, double-blind, placebo-controlled parallel-group trial. Australia | 60 healthy subjects, 22♂, 38♀, 60–85 y. | Subjects were divided into curcumin group (80 m solid lipid formulation (Longvida® Curcumin-400 mg) or placebo/1 timeday/4 weeks. Participants performed 3 sets of computerized cognitive tasks preceded and followed by an evaluation of state mood. After the first set, a single treatment dose was used, and then the assessment was repeated at 1 h and 3 h after dose administration. | The results showed that 1 h after administration, the curcumin group presented significantly enhanced performance on sustained attention and working memory tasks, compared with placebo. Also, working memory and mood were significantly better during chronic treatment (4 weeks). Furthermore, curcumin significantly reduced total cholesterol and LDL cholesterol levels. | No adverse events were reported. |
| Alzheimer’s disease | |||||
| [296] | 12-week, 2 × 2 factorial, double-blinded, randomized controlled trial. Australia | 29 participants, 12♂, 17♀ (52.3 ± 1.9 y) at high risk of developing diabetes or with impaired fasting glucose | Participants were divided into 4 groups: the placebo; curcumin (2 × 500 mg of curcumin (Meriva®), providing 180 mg of curcumin plus 2 × 1000 mg of corn oil/day); ω3, 2 × 1000 mg of fish oil + placebo; or double active (1000 mg of curcumin (Meriva®) + 21,000 mg of fish oil. | Curcumin reduced triglyceride levels, fasting insulin, atherogenic index and the HOMA2-IR. There were no significant effects on CRP, TC, HDL-c, LDL-c, fasting glycemia, glycated hemoglobin, and body composition (body weight, muscle mass, body mass index, body fat percentage, circumference waist). | No adverse events were observed. |
| [305] | Randomized, double-blind, placebo-controlled for 12 months. Australia. | 160 healthy individuals; 40–90 y, and no significant cerebral vascular disease; no significant cognitive impairments. | They were randomly assigned to treatment groups with BCM-95 ® CG (Biocurcumax TM) capsule 3 x/day (1500 mg/d) or placebo. | No differences were observed between the placebo and treatment groups in changes in cognitive performance. | Gastrointestinal complaints. |
| [297] | Prospective randomized, 4 weeks. United States of America | 19 healthy participants 17♀, 2 ♂ age 40–60 y | The selected population was assigned to placebo interventions of starch × 80 mg/day of curcumin for 4 weeks | There were no significant effects on TC, LDL-c, HDL-c, superoxide dismutase, and glutathione peroxidase; significant reduction in the levels of TG, intercellular adhesion molecule, and plasma amyloid β protein content. Increased NO, myeloperoxidase, catalase activity, and elimination of free radicals. | No adverse events were observed. |
| [306] | Randomized, double-blind, placebo-controlled for 6 months. China. | 34 individuals 29%♂, 71%♀), aged 73.4 ± 8.8 (progressive decline in memory and cognitive function for at least 6 weeks or diagnosed with AD | They presented 3 groups, one consisting of 10 people (control), the second of 8 people (1 g of curcumin), and the third (4 g of curcumin). | There were no significant effects on the lipid profile (LDL-c, HDL-c, TG, and TC) in both groups receiving curcumin. | Constipation, more, diarrhea, and dizziness. |
| Cognition | |||||
| [299] | Randomized, 30-day, double-blind, placebo-controlled, 3-arm pilot study. India. | 18 healthy participants, 12♂ and 6♀, 35–65 y. | Patients were randomized into 3 groups, CGM (500 mg 2×/day for 30 days of curcuma-galactomannoside complex; UC (500 mg 2×/day for 30 days of curcumin with 95% purity) or placebo | CGM: significant ↑↓ in α and β waves, and in the α/β ratio compared to the unformulated curcumin and placebo groups. Furthermore, CGM showed a significant ↓ in audio reaction time (29.8) compared with placebo and 24.6% with UC. Choice-based visual reaction time was also significantly ↓ (36%) in CGM compared to UC and placebo, which yielded 15.36% and 5.2%, respectively. | No adverse events were reported. |
| [300] | Double-blind, placebo-controlled, 12-week trial/Australia. | 79 participants ♀ and ♂healthy, 50–85 y. | Participants were divided into curcumin group (400 mg Longvida© curcumin capsule with 80 mg of curcumin 1×/day/12 weeks) or placebo. | Curcumin group showed better working memory performance at 12 weeks (Serial Threes, Serial Sevens, and performance on a virtual Morris Water Maze) and lower fatigue scores on the POMS at 4 and 12 weeks, and tension, anger, confusion, and total mood disturbance in just 4 weeks. | No adverse events were reported. |
| [301] | 16-week double-blind, randomized placebo-controlled trial/Australia. | 152 older sedentary overweight/obese adults, 50–80 y. | Subjects were divided into 4 groups: fish oil + curcumin placebo, curcumin + fish oil placebo, fish oil + curcumin or placebo. Then, patients ingested 6 capsules/day consisting of 2 fish oil capsules and 400 mg Longvida® Optimised Curcumin containing 80 mg of curcumin, or placebo, 2×/d. Then, an evaluation of Transcranial Doppler ultrasound, blood, glycemia, heart rate, arterial compliance, blood lipids, and C-RP was performed. | Curcumin did not significantly affect the performed parameters alone or in combination with fish oil. | Digestive problems and reflux. |
| [307] | Randomized, double-blind, placebo-controlled pilot clinical trial/USA. | 12 participants 9♂ and 3♀ with chronic schizophrenia, 5–51 y. | Patients were randomized into 2 groups: curcumin (180 mg/d) or placebo. A commercially available surface-controlled water-soluble form of 300 mg curcumin (30% formulation: 90 mg pure curcumin) or matching placebo capsules were provided. | Complementary curcumin treatment showed significant improvement in working memory (Z = 2200, p = 0.028) and reduced IL-6 levels (Z = 2402, p = 0.016) compared to placebo. No significant effect of curcumin on PANSS and Calgary Depression scores was found. | No adverse events were reported. |
| [302] | 18-month, randomized, double-blind, two-group parallel design | 40 adults without dementia, 22♀, 18♂ and 50–90 y. | Subjects were divided into placebo group or Theracurmin group (90 mg of curcumin), 2 ×/d/18 months. Depression Inventory and neuropsychological test battery were applied. | Buschke–Fuld Selective Reminding Test presented a consistent long-term retrieval improvement with curcumin (ES = 0.63, p = 0.002). Curcumin also improved visual memory and attention. | Transient abdominal pain, gastritis, nausea, and heat. |
| [303] | Randomized, 18-month, double-blind, placebo-controlled, parallel-group study. EUA | 40 participants, 51–84 y, without dementia. | They were randomized into 2 groups: Theracurmin group: 90 mg of curcumin, 2 times d 18 months or placebo group. | Curcumin significantly improved long-term recovery of SRT, visual memory, and attention compared with placebo. Assessment of neurodegeneration using PET scans significantly reduce in the amygdala with curcumin. | No adverse events were reported. |
| [304] | 6-week open study/Tehran, Iran. | 111 participants, ♀ and ♂ diagnosed with major depressive disorder | They were divided into standard antidepressant therapy + curcuminoids (1000 mg/d—C3 Complex®) or standard antidepressant therapy alone/6 weeks. | Both groups had a reduction in BDI-II total and subscale scores at the end of the study. Significantly greater ↓ in HADS, anxiety, and depression subscales in the curcuminoids versus control group (p < 0.001). | Gastrointestinal symptoms |
| Study | Question Focus | Allocation Blinding | Double- Blind | Losses (>20%) | Prognostic or Demographic Characteristics | Outcomes | Intention to Treat Analysis | Sample Calculation | Adequate Follow-Up |
|---|---|---|---|---|---|---|---|---|---|
| [293] | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| [294] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| [228] | Yes | Yes | Yes | No | Yes | Yes | No | No | Yes |
| [295] | Yes | Yes | Yes | No | Yes | Yes | No | No | Yes |
| [296] | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| [305] | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Yes |
| [297] | No | No | Yes | No | No | Yes | No | No | Yes |
| [306] | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| [299] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| [300] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| [301] | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| [307] | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| [302] | Yes | Yes | Yes | No | Yes | Yes | No | No | Yes |
| [303] | Yes | No | Yes | No | Yes | Yes | Yes | No | Yes |
| [304] | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes |
5. Bioavailability and Safety
6. Synthesis and Future Research Endeavors
6.1. Advancing Curcumin Therapy: Exploring Formulations and Unraveling Mechanisms for Aging-Related Disorders
6.2. Unveiling Curcumin’s Therapeutic Potential: Insights from Meticulous Clinical Trials and Advanced Neuroimaging Studies in Neurodegenerative Disorders
6.3. Unlocking Synergistic Therapeutic Strategies: Exploring Curcumin Combinations and Molecular Interactions in Disease Management
6.4. Fostering Collaboration for Curcumin Translation: Bridging Academia, Industry, Regulation, and Healthcare for Age-Related Disease Management
6.5. Unraveling the Genetic Basis of Curcumin Response: Genome-Wide Association Studies in Aging-Related Disease Management
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AChE | acetylcholinesterase |
| AD | Alzheimer’s disease |
| AMPK | adenosine 5′-monophosphate-activated protein kinase |
| APP | amyloid precursor protein |
| APPsw | APP Swedish Mutant |
| Bax | Bcl-2-associated protein X |
| Bcl-2 | B-cell lymphoma 2 |
| Cdk5 | cyclin-dependent kinase 5 |
| CNTMF | completely natural turmeric matrix formulation |
| COX-2 | cyclooxygenase 2 |
| DM2 | diabetes mellitus type 2 |
| DNA | deoxyribonucleic acid |
| FeONPs-Cur | iron oxide nanoparticles capped with curcumin |
| GSK-3β | glycogen synthase kinase-3β |
| HO-1 | heme oxygenase-1 |
| IL | interleukin |
| JAK/STAT | Janus kinase/signal transducer and activator of transcription |
| JNK | c-Jun N-terminal kinase |
| Keap1 | Kelch-like ECH-associated protein 1 |
| MCT2 | monocarboxylate transporter 2 |
| mTOR | mammalian target of rapamycin |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NDs | neurodegenerative diseases |
| NF-κβ | nuclear factor-kappabeta |
| NO | nitric oxide |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| OS | oxidative stress |
| PD | Parkinson’s disease |
| PI3K | phosphoinositide 3-kinases |
| PI3K/AKT | phosphatidylinositol 3-kinase/protein kinase B |
| PPARγ | peroxisome proliferator-activated receptor gamma |
| RAGE | glycation end products |
| RNS | reactive nitrogen species |
| ROS | oxygen species |
| p- | phosphorylated |
| PPAR-γ | peroxisome proliferator-activated receptor gamma |
| Th17 | T helper 17 |
| TNF | tumor necrosis factor |
| TNF-α | tumor necrosis factor-alpha |
| TLR | Toll-like receptor |
References
- Cai, Y.; Song, W.; Li, J.; Jing, Y.; Liang, C.; Zhang, L.; Zhang, X.; Zhang, W.; Liu, B.; An, Y.; et al. The landscape of aging. Sci. China Life Sci. 2022, 65, 2354–2454. [Google Scholar] [CrossRef] [PubMed]
- Baig, J.; Sawant, N.; Rawat, P.; Reddy, A.P.; Reddy, P.H.; Kshirsagar, S. Abnormal interaction of Rlip with mutant APP/Abeta and phosphorylated tau reduces wild-type Rlip levels and disrupt Rlip function in Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1870, 166858. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Cheng, K.C.; Lin, Y.H.; He, C.X.; Bow, Y.D.; Li, C.Y.; Wu, C.Y.; Wang, H.D.; Sheu, S.J. Prolonged Exposure to High Glucose Induces Premature Senescence Through Oxidative Stress and Autophagy in Retinal Pigment Epithelial Cells. Arch. Immunol. Ther. Exp. 2023, 71, 21. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhu, X.; Wei, L.; Zou, Y.; Qi, X.; Shi, R.; Xu, W.; Wang, X.; Ding, G.; Duan, Y. Aberrant expression of thyroidal hormone receptor α exasperating mitochondrial dysfunction induced sarcopenia in aged mice. Aging 2024, 16, 7141–7152. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A. SIRT7 in the aging process. Cell. Mol. Life Sci. 2022, 79, 297. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.S.; Yarmush, M.L. Current Trends in Anti-Aging Strategies. Annu. Rev. Biomed. Eng. 2023, 25, 363–385. [Google Scholar] [CrossRef]
- Zamboni, G.; Maramotti, R.; Salemme, S.; Tondelli, M.; Adani, G.; Vinceti, G.; Carbone, C.; Filippini, T.; Vinceti, M.; Pagnoni, G.; et al. Age-specific prevalence of the different clinical presentations of AD and FTD in young-onset dementia. J. Neurol. 2024, 271, 4326–4335. [Google Scholar] [CrossRef] [PubMed]
- Guilbaud, E.; Sarosiek, K.A.; Galluzzi, L. Inflammation and mitophagy are mitochondrial checkpoints to aging. Nat. Commun. 2024, 15, 3375. [Google Scholar] [CrossRef]
- Tanaka, M.; Tuka, B.; Vécsei, L. Navigating the Neurobiology of Migraine: From Pathways to Potential Therapies. Cells 2024, 13, 1098. [Google Scholar] [CrossRef]
- Ropert, B.; Gallrein, C.; Schumacher, B. DNA repair deficiencies and neurodegeneration. DNA Repair 2024, 138, 103679. [Google Scholar] [CrossRef] [PubMed]
- McClarty, B.M.; Rodriguez, G.; Dong, H. Class 1 histone deacetylases differentially modulate memory and synaptic genes in a spatial and temporal manner in aged and APP/PS1 mice. Brain Res. 2024, 1837, 148951. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Liu, Y.; Li, J.; Wang, Y.; Ji, P.; Shi, Q.; Han, M.; Xu, H.; Li, W.; Li, W. Ginsenoside Rg1 alleviates chronic inflammation-induced neuronal ferroptosis and cognitive impairments via regulation of AIM2—Nrf2 signaling pathway. J. Ethnopharmacol. 2024, 330, 118205. [Google Scholar] [CrossRef]
- Battaglia, S.; Avenanti, A.; Vécsei, L.; Tanaka, M. Neurodegeneration in Cognitive Impairment and Mood Disorders for Experimental, Clinical and Translational Neuropsychiatry. Biomedicines 2024, 12, 574. [Google Scholar] [CrossRef]
- Cho, J.; Higgason, N.; Rothman, J.; Safford, M.; Pinheiro, L.C. “Should I Prioritize My Cancer or My Diabetes?”: Patient-Perceived Barriers to Co-Managing Cancer and Diabetes Mellitus. J. Cancer Educ. Off. J. Am. Assoc. Cancer Educ. 2024, 39, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Ottosson, F.; Engström, G.; Orho-Melander, M.; Melander, O.; Nilsson, P.M.; Johansson, M. Plasma Metabolome Predicts Aortic Stiffness and Future Risk of Coronary Artery Disease and Mortality After 23 Years of Follow-Up in the General Population. J. Am. Heart Assoc. 2024, 13, e033442. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhuang, Y.; Li, W.; Ma, M.; Lei, F.; Qu, Y.; Li, J.; Luo, H.; Li, C.; Lu, L.; et al. Apoptotic vesicles are required to repair DNA damage and suppress premature cellular senescence. J. Extracell. Vesicles 2024, 13, e12428. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.N.; Lin, M.H.; Tseng, S.H.; Yen, K.H.; Lee, H.F.; Hsiao, F.Y.; Chen, L.K. Protein-enriched soup and weekly exercise improve muscle health: A randomized trial in mid-to-old age with inadequate protein intake. J. Cachexia Sarcopenia Muscle 2024, 4, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
- Bults, M.; van Leersum, C.M.; Olthuis, T.J.J.; Siebrand, E.; Malik, Z.; Liu, L.; Miguel-Cruz, A.; Jukema, J.S.; den Ouden, M.E.M. Acceptance of a Digital Assistant (Anne4Care) for Older Adult Immigrants Living With Dementia: Qualitative Descriptive Study. JMIR Aging 2024, 7, e50219. [Google Scholar] [CrossRef]
- Karimi, H.; Mahdavi, S.; Moghaddam, S.S.; Abbasi-Kangevari, M.; Soleimani, Z.; Esfahani, Z.; Masinaei, M.; Fateh, S.M.; Golestani, A.; Dilmaghani-Marand, A.; et al. Unveiling the lead exposure attributed burden in Iran from 1990 to 2019 through the lens of the Global Burden of Disease study 2019. Sci. Rep. 2024, 14, 8688. [Google Scholar] [CrossRef]
- Laurindo, L.F.; de Carvalho, G.M.; de Oliveira Zanuso, B.; Figueira, M.E.; Direito, R.; de Alvares Goulart, R.; Buglio, D.S.; Barbalho, S.M. Curcumin-Based Nanomedicines in the Treatment of Inflammatory and Immunomodulated Diseases: An Evidence-Based Comprehensive Review. Pharmaceutics 2023, 15, 229. [Google Scholar] [CrossRef] [PubMed]
- Pagotto, G.L.d.O.; Santos, L.M.O.d.; Osman, N.; Lamas, C.B.; Laurindo, L.F.; Pomini, K.T.; Guissoni, L.M.; Lima, E.P.d.; Goulart, R.d.A.; Catharin, V.M.S. Ginkgo biloba: A Leaf of Hope in the Fight against Alzheimer’s Dementia: Clinical Trial Systematic Review. Antioxidants 2024, 13, 651. [Google Scholar] [CrossRef] [PubMed]
- Valotto Neto, L.J.; Reverete de Araujo, M.; Moretti Junior, R.C.; Mendes Machado, N.; Joshi, R.K.; dos Santos Buglio, D.; Barbalho Lamas, C.; Direito, R.; Fornari Laurindo, L.; Tanaka, M. Investigating the Neuroprotective and Cognitive-Enhancing Effects of Bacopa monnieri: A Systematic Review Focused on Inflammation, Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis. Antioxidants 2024, 13, 393. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Gancarz, M.; Kondracka, A.; Rusinek, R.; Oniszczuk, A. Curcumin and Weight Loss: Does It Work? Int. J. Mol. Sci. 2022, 23, 639. [Google Scholar] [CrossRef] [PubMed]
- Razavi, B.M.; Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. A review of therapeutic potentials of turmeric (Curcuma longa) and its active constituent, curcumin, on inflammatory disorders, pain, and their related patents. Phytother. Res. 2021, 35, 6489–6513. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between Gut Microbiota and Curcumin: A New Key of Understanding for the Health Effects of Curcumin. Nutrients 2020, 12, 2499. [Google Scholar] [CrossRef]
- Moskwa, J.; Bronikowska, M.; Socha, K.; Markiewicz-Żukowska, R. Vegetable as a Source of Bioactive Compounds with Photoprotective Properties: Implication in the Aging Process. Nutrients 2023, 15, 3594. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Qu, S. Constituent isoflavones of Puerariae radix as a potential neuroprotector in cognitive impairment: Evidence from preclinical studies. Ageing Res. Rev. 2023, 90, 102040. [Google Scholar] [CrossRef] [PubMed]
- Chainoglou, E.; Hadjipavlou-Litina, D. Curcumin in Health and Diseases: Alzheimer’s Disease and Curcumin Analogues, Derivatives, and Hybrids. Int. J. Mol. Sci. 2020, 21, 1975. [Google Scholar] [CrossRef]
- Budhathoki, R.; Timilsina, A.P.; Regmi, B.P.; Sharma, K.R.; Aryal, N.; Parajuli, N. Metabolome Mining of Curcuma longa L. Using HPLC-MS/MS and Molecular Networking. Metabolites 2023, 13, 898. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, N.A.; Cuciniello, R.; Petillo, G.D.; Piccioni, M.; Filosa, S.; Crispi, S. An Overview of the Enhanced Effects of Curcumin and Chemotherapeutic Agents in Combined Cancer Treatments. Int. J. Mol. Sci. 2023, 24, 12587. [Google Scholar] [CrossRef] [PubMed]
- Zhi, H.W.; Jia, Y.Z.; Bo, H.Q.; Li, H.T.; Zhang, S.S.; Wang, Y.H.; Yang, J.; Hu, M.Z.; Wu, H.Y.; Cui, W.Q.; et al. Curcumin alleviates orofacial allodynia and improves cognitive impairment via regulating hippocampal synaptic plasticity in a mouse model of trigeminal neuralgia. Aging 2023, 15, 4984. [Google Scholar] [CrossRef] [PubMed]
- Marton, L.T.; Barbalho, S.M.; Sloan, K.P.; Sloan, L.A.; Goulart, R.A.; Araújo, A.C.; Bechara, M.D. Curcumin, autoimmune and inflammatory diseases: Going beyond conventional therapy—A systematic review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2140–2157. [Google Scholar] [CrossRef] [PubMed]
- Akaberi, M.; Sahebkar, A.; Emami, S.A. Turmeric and Curcumin: From Traditional to Modern Medicine. Adv. Exp. Med. Biol. 2021, 1291, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.C.; Kamarudin, M.N.A.; Naidu, R. Anticancer Mechanism of Curcumin on Human Glioblastoma. Nutrients 2021, 13, 950. [Google Scholar] [CrossRef] [PubMed]
- Cunha Neto, F.; Marton, L.T.; de Marqui, S.V.; Lima, T.A.; Barbalho, S.M. Curcuminoids from Curcuma longa: New adjuvants for the treatment of crohn’s disease and ulcerative colitis? Crit. Rev. Food Sci. Nutr. 2019, 59, 2136–2143. [Google Scholar] [CrossRef] [PubMed]
- Mazieiro, R.; Frizon, R.R.; Barbalho, S.M.; Goulart, R.A. Is Curcumin a Possibility to Treat Inflammatory Bowel Diseases? J. Med. Food 2018, 21, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Den Hartogh, D.J.; Gabriel, A.; Tsiani, E. Antidiabetic Properties of Curcumin I: Evidence from In Vitro Studies. Nutrients 2020, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Górka, M.; Białoń, N.; Bieczek, D.; Górka, D. Neuroprotective effect of curcumin and its potential use in the treatment of neurodegenerative diseases. Postep. Biochem. 2023, 69, 18–25. [Google Scholar] [CrossRef]
- Jafari-Nozad, A.M.; Jafari, A.; Yousefi, S.; Bakhshi, H.; Farkhondeh, T.; Samarghandian, S. Anti-gout and urate-lowering potentials of curcumin: A review from bench to beside. Curr. Med. Chem. 2023, 31, 3715–3732. [Google Scholar] [CrossRef] [PubMed]
- Boonla, O.; Kukongviriyapan, U.; Pakdeechote, P.; Kukongviriyapan, V.; Pannangpetch, P.; Prachaney, P.; Greenwald, S.E. Curcumin improves endothelial dysfunction and vascular remodeling in 2K-1C hypertensive rats by raising nitric oxide availability and reducing oxidative stress. Nitric Oxide 2014, 42, 44–53. [Google Scholar] [CrossRef]
- Izadi, M.; Sadri, N.; Abdi, A.; Zadeh, M.M.R.; Jalaei, D.; Ghazimoradi, M.M.; Shouri, S.; Tahmasebi, S. Longevity and anti-aging effects of curcumin supplementation. GeroScience 2024, 46, 2933–2950. [Google Scholar] [CrossRef]
- Xu, J.; Du, P.; Liu, X.; Xu, X.; Ge, Y.; Zhang, C. Curcumin supplementation increases longevity and antioxidant capacity in Caenorhabditis elegans. Front. Pharmacol. 2023, 14, 1195490. [Google Scholar] [CrossRef]
- Kumar, A.; Prakash, A.; Dogra, S. Protective effect of curcumin (Curcuma longa) against D-galactose-induced senescence in mice. J. Asian Nat. Prod. Res. 2011, 13, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, Y.S.; Kim, E.; Kim, Y.; Kim, Y. Curcumin and hesperetin attenuate D-galactose-induced brain senescence in vitro and in vivo. Nutr. Res. Pract. 2020, 14, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Wei, T.T.; Guo, L.; Cao, J.H.; Feng, Y.K.; Guo, S.N.; Liu, G.H.; Ding, Y.; Chai, Y.R. Curcumin protects thymus against D-galactose-induced senescence in mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Open Med. 2009, 3, e123–e130. [Google Scholar] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-inflammatory effects of curcumin in the inflammatory diseases: Status, limitations and countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- de Lima, E.P.; Moretti, R.C., Jr.; Torres Pomini, K.; Laurindo, L.F.; Sloan, K.P.; Sloan, L.A.; Castro, M.V.M.; Baldi, E., Jr.; Ferraz, B.F.R.; de Souza Bastos Mazuqueli Pereira, E.; et al. Glycolipid Metabolic Disorders, Metainflammation, Oxidative Stress, and Cardiovascular Diseases: Unraveling Pathways. Biology 2024, 13, 519. [Google Scholar] [CrossRef]
- Bishayee, A.; Kavalakatt, J.; Sunkara, C.; Johnson, O.; Zinzuwadia, S.S.; Collignon, T.E.; Banerjee, S.; Barbalho, S.M. Litchi (Litchi chinensis Sonn.): A comprehensive and critical review on cancer prevention and intervention. Food Chem. 2024, 457, 140142. [Google Scholar] [CrossRef]
- Direito, R.; Barbalho, S.M.; Sepodes, B.; Figueira, M.E. Plant-Derived Bioactive Compounds: Exploring Neuroprotective, Metabolic, and Hepatoprotective Effects for Health Promotion and Disease Prevention. Pharmaceutics 2024, 16, 577. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Bueno Ottoboni, A.M.M.; Fiorini, A.M.R.; Guiguer, E.L.; Nicolau, C.C.T.; Goulart, R.d.A.; Flato, U.A.P. Grape juice or wine: Which is the best option? Crit. Rev. Food Sci. Nutr. 2020, 60, 3876–3889. [Google Scholar] [CrossRef] [PubMed]
- Bosso, H.; Barbalho, S.M.; de Alvares Goulart, R.; Otoboni, A.M.M.B. Nutrition. Green coffee: Economic relevance and a systematic review of the effects on human health. Crit. Rev. Food Sci. Nutr. 2023, 63, 394–410. [Google Scholar] [CrossRef] [PubMed]
- Laurindo, L.F.; Direito, R.; Bueno Otoboni, A.M.; Goulart, R.A.; Quesada, K.; Barbalho, S.M. Grape processing waste: Effects on inflammatory bowel disease and colorectal cancer. Food Rev. Int. 2024, 40, 336–369. [Google Scholar] [CrossRef]
- Derochette, S.; Franck, T.; Mouithys-Mickalad, A.; Ceusters, J.; Deby-Dupont, G.; Lejeune, J.-P.; Neven, P.; Serteyn, D. Curcumin and resveratrol act by different ways on NADPH oxidase activity and reactive oxygen species produced by equine neutrophils. Chem. Biol. Interact. 2013, 206, 186–193. [Google Scholar] [CrossRef]
- Cordero-Herrera, I.; Martín, M.A.; Goya, L.; Ramos, S. Cocoa flavonoids protect hepatic cells against high-glucose-induced oxidative stress: Relevance of MAPKs. Mol. Nutr. Food Res. 2015, 59, 597–609. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef] [PubMed]
- Pirunkaset, E.; Boonyarat, C.; Maneenet, J.; Khamphukdee, C.; Daodee, S.; Monthakantirat, O.; Awale, S.; Kijjoa, A.; Chulikhit, Y. Effect of Diacetylcurcumin Manganese Complex on Rotenone-Induced Oxidative Stress, Mitochondria Dysfunction, and Inflammation in the SH-SY5Y Parkinson’s Disease Cell Model. Molecules 2024, 29, 957. [Google Scholar] [CrossRef]
- Nair, B.; Adithya, J.K.; Chandrababu, G.; Lakshmi, P.K.; Koshy, J.J.; Manoj, S.V.; Ambiliraj, D.B.; Vinod, B.S.; Sethi, G.; Nath, L.R. Modulation of carcinogenesis with selected GRAS nutraceuticals via Keap1-Nrf2 signaling pathway. Phytother. Res. PTR 2023, 37, 4398–4413. [Google Scholar] [CrossRef]
- Matias, J.N.; Achete, G.; Campanari, G.; Guiguer, É.L.; Araújo, A.C.; Buglio, D.S.; Barbalho, S.M. A systematic review of the antidepressant effects of curcumin: Beyond monoamines theory. Aust. N. Z. J. Psychiatry 2021, 55, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Goulart, R.A.; Barbalho, S.M.; Lima, V.M.; Souza, G.A.; Matias, J.N.; Araújo, A.C.; Rubira, C.J.; Buchaim, R.L.; Buchaim, D.V.; Carvalho, A.C.A.; et al. Effects of the Use of Curcumin on Ulcerative Colitis and Crohn’s Disease: A Systematic Review. J. Med. Food 2021, 24, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Li, K.; Peng, X.-X.; Kan, Y.; Yao, T.-J.; Wang, Z.-Y.; Li, Z.; Liu, H.-Y.; Cai, D. Curcumin derived from medicinal homologous foods: Its main signals in immunoregulation of oxidative stress, inflammation, and apoptosis. Front. Immunol. 2023, 14, 1233652. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, W.; Zhang, Y.; Zeng, Y. Curcumin alleviates imiquimod-induced psoriasis-like inflammation and regulates gut microbiota of mice. Immun. Inflamm. Dis. 2023, 11, e967. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; de Sousa Gonzaga, H.F.; de Souza, G.A.; de Alvares Goulart, R.; de Sousa Gonzaga, M.L.; de Alvarez Rezende, B. Dermatological effects of Curcuma species: A systematic review. Clin. Exp. Dermatol. 2021, 46, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Koboziev, I.; Albracht-Schulte, K.; Mistretta, B.; Scoggin, S.; Yosofvand, M.; Moussa, H.; Zabet-Moghaddam, M.; Ramalingam, L.; Gunaratne, P.H. Curcumin reduces adipose tissue inflammation and alters gut microbiota in diet-induced obese male mice. Mol. Nutr. Food Res. 2021, 65, 2100274. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, Y.; Chen, J.; Tong, C.; Wang, Q.; Piao, Y. Curcumin treatment attenuates cisplatin-induced gastric mucosal inflammation and apoptosis through the NF-κ B and MAPKs signaling pathway. Hum. Human. Exp. Toxicol. 2022, 41, 09603271221128738. [Google Scholar] [CrossRef]
- Rathore, A.S.; Singh, S.S.; Birla, H.; Zahra, W.; Keshri, P.K.; Dilnashin, H.; Singh, R.; Singh, S.; Singh, S.P. Curcumin Modulates p62-Keap1-Nrf2-Mediated Autophagy in Rotenone-Induced Parkinson’s Disease Mouse Models. ACS Chem. Neurosci. 2023, 14, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, W.; Zennadi, R. Keap1-Nrf2 Heterodimer: A Therapeutic Target to Ameliorate Sickle Cell Disease. Antioxidants 2023, 12, 740. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, G.; Akpan, A.; Phelan, M.M.; Wright, H.L. New insights into healthy ageing, inflammageing and frailty using metabolomics. Front. Aging 2024, 5, 1426436. [Google Scholar] [CrossRef]
- Cianciulli, A.; Calvello, R.; Ruggiero, M.; Panaro, M.A. Inflammaging and Brain: Curcumin and Its Beneficial Potential as Regulator of Microglia Activation. Molecules 2022, 27, 341. [Google Scholar] [CrossRef]
- Kujundžić, R.N.; Stepanić, V.; Milković, L.; Gašparović, A.; Tomljanović, M.; Trošelj, K.G. Curcumin and its Potential for Systemic Targeting of Inflamm-Aging and Metabolic Reprogramming in Cancer. Int. J. Mol. Sci. 2019, 20, 1180. [Google Scholar] [CrossRef] [PubMed]
- Devita, M.; Debiasi, G.; Anglani, M.; Ceolin, C.; Mazzonetto, I.; Begliomini, C.; Cauzzo, S.; Raffaelli, C.; Lazzarin, A.; Ravelli, A.; et al. The Role of Cognitive Reserve in Protecting Cerebellar Volumes of Older Adults with mild Cognitive Impairment. Cerebellum 2024, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Ungvari, A.; Patai, R.; Gulej, R.; Yabluchanskiy, A.; Benyo, Z.; Kovacs, I.; Sotonyi, P.; Kirkpartrick, A.C.; Prodan, C.I.; et al. Atherosclerotic burden and cerebral small vessel disease: Exploring the link through microvascular aging and cerebral microhemorrhages. GeroScience 2024, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.F.D.; Bragante, W.R.; Junior, R.C.M.; Laurindo, L.F.; Guiguer, E.L.; Araújo, A.C.; Fiorini, A.M.R.; Nicolau, C.C.T.; Oshiiwa, M.; Lima, E.P.; et al. Effects of Smallanthus sonchifolius Flour on Metabolic Parameters: A Systematic Review. Pharmaceuticals 2024, 17, 658. [Google Scholar] [CrossRef]
- Laurindo, L.F.; Rodrigues, V.D.; Minniti, G.; de Carvalho, A.C.A.; Zutin, T.L.M.; DeLiberto, L.K.; Bishayee, A.; Barbalho, S.M. Pomegranate (Punica granatum L.) phytochemicals target the components of metabolic syndrome. J. Nutr. Biochem. 2024, 131, 109670. [Google Scholar] [CrossRef]
- Wen, R.; Huang, X.; Long, J.; Guo, Y.; Wei, Y.; Lin, P.; Xie, S.; Zhao, Z.; Zhang, L.; Fan, A.Y.; et al. Advances in traditional Chinese herbal medicine and their pharmacodynamic mechanisms in cancer immunoregulation: A narrative review. Transl. Cancer Res. 2024, 13, 1166–1187. [Google Scholar] [CrossRef]
- Minniti, G.; Laurindo, L.F.; Machado, N.M.; Duarte, L.G.; Guiguer, E.L.; Araujo, A.C.; Dias, J.A.; Lamas, C.B.; Nunes, Y.C.; Bechara, M.D.; et al. Mangifera indica L., By-Products, and Mangiferin on Cardio-Metabolic and Other Health Conditions: A Systematic Review. Life 2023, 13, 2270. [Google Scholar] [CrossRef]
- Nunes, Y.C.; Santos, G.O.; Machado, N.M.; Otoboni, A.; Laurindo, L.F.; Bishayee, A.; Fimognari, C.; Bishayee, A.; Barbalho, S.M. Peanut (Arachis hypogaea L.) seeds and by-products in metabolic syndrome and cardiovascular disorders: A systematic review of clinical studies. Phytomedicine 2024, 123, 155170. [Google Scholar] [CrossRef]
- Nishikito, D.F.; Borges, A.C.A.; Laurindo, L.F.; Otoboni, A.; Direito, R.; Goulart, R.A.; Nicolau, C.C.T.; Fiorini, A.M.R.; Sinatora, R.V.; Barbalho, S.M. Anti-Inflammatory, Antioxidant, and Other Health Effects of Dragon Fruit and Potential Delivery Systems for Its Bioactive Compounds. Pharmaceutics 2023, 15, 159. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xin, Q.; Wei, P.; Hua, Y.; Zhang, Y.; Su, Z.; She, G.; Yuan, R. Antioxidant and anti-aging activities of Longan crude and purified polysaccharide (LP-A) in nematode Caenorhabditis elegans. Int. J. Biol. Macromol. 2024, 267, 131634. [Google Scholar] [CrossRef]
- Xu, M.; Wang, W.; Cheng, J.; Qu, H.; Xu, M.; Wang, L. Effects of mitochondrial dysfunction on cellular function: Role in atherosclerosis. Biomed. Pharmacother. 2024, 174, 116587. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Fan, R.; Zhang, Y.; Jia, Z.; Zhang, J.; Pan, H.; Wang, Q. Oxidative stress in the brain-lung crosstalk: Cellular and molecular perspectives. Front. Aging Neurosci. 2024, 16, 1389454. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Monitoring the redox status in multiple sclerosis. Biomedicines 2020, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Stolp, H.B.; Solito, E. Developmental priming of early cerebrovascular ageing: Implications across a lifetime. Int. J. Geriatr. Psychiatry 2024, 39, e6090. [Google Scholar] [CrossRef]
- Hirunsai, M.; Srikuea, R. Differential effects of cholecalciferol and calcitriol on muscle proteolysis and oxidative stress in angiotensin II-induced C2C12 myotube atrophy. Physiol. Rep. 2024, 12, e16011. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, Y.; Lu, S.; Xu, J.; Liu, X.; Yang, D.; Yang, Y.; Hou, L.; Li, N. A crazy trio in Parkinson’s disease: Metabolism alteration, α-synuclein aggregation, and oxidative stress. Mol. Cell. Biochem. 2024. [CrossRef] [PubMed]
- Duan, D.; Li, H.; Chai, S.; Zhang, L.; Fan, T.; Hu, Z.; Feng, Y. The relationship between cardiac oxidative stress, inflammatory cytokine response, cardiac pump function, and prognosis post-myocardial infarction. Sci. Rep. 2024, 14, 8985. [Google Scholar] [CrossRef]
- Muhammad, I.; Khan, A.; Mustafa, A.; Elshikh, M.S.; Shen, W. Elucidating the modulatory effect of melatonin on enzyme activity and oxidative stress in wheat: A global meta-analysis. Physiol. Plant. 2024, 176, e14294. [Google Scholar] [CrossRef]
- Ezim, O.E.; Nyeche, J.; Nebeolisa, C.E.; Belonwu, C.D.; Abarikwu, S.O. Ascorbic acid attenuates gasoline-induced testicular toxicity, sperm quality deterioration, and testosterone imbalance in rats. Toxicol. Ind. Health 2024, 40, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Fišar, Z.; Hroudová, J. CoQ(10) and Mitochondrial Dysfunction in Alzheimer’s Disease. Antioxidants 2024, 13, 191. [Google Scholar] [CrossRef] [PubMed]
- Speers, A.B.; Wright, K.M.; Brandes, M.S.; Kedjejian, N.; Matthews, D.G.; Caruso, M.; Harris, C.J.; Koike, S.; Nguyen, T.; Quinn, J.F.; et al. Mode of administration influences plasma levels of active Centella asiatica compounds in 5xFAD mice while markers of neuroinflammation remain unaltered. Front. Neurosci. 2024, 18, 1277626. [Google Scholar] [CrossRef]
- Ferrara, F.; Yan, X.; Pecorelli, A.; Guiotto, A.; Colella, S.; Pasqui, A.; Ivarrson, J.; Lynch, S.; Anderias, S.; Choundhary, H.; et al. Combined exposure to UV and PM affect skin oxinflammatory responses and it is prevented by antioxidant mix topical application: Evidences from clinical study. J. Cosmet. Dermatol. 2024, 8, 2644–2656. [Google Scholar] [CrossRef]
- Novoselova, E.G.; Lunin, S.M.; Khrenov, M.O.; Glushkova, O.V.; Novoselova, T.V.; Parfenyuk, S.B. Pancreas Β-Cells in Type 1 and Type 2 Diabetes: Cell Death, Oxidative Stress and Immune Regulation. Recently Appearing Changes in Diabetes Consequences. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2024, 58, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Uysal, F.; Sukur, G.; Bozdemir, N.; Cinar, O. Antioxidant supplementation may effect DNA methylation patterns, apoptosis, and ROS levels in developing mouse embryos. Histochem. Cell Biol. 2024, 1–10. [Google Scholar] [CrossRef]
- Zia, A.; Farkhondeh, T.; Pourbagher-Shahri, A.M.; Samarghandian, S. The role of curcumin in aging and senescence: Molecular mechanisms. Biomed. Pharmacother. 2021, 134, 111119. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhan, P.; Wang, Q.; Wang, C.; Liu, Y.; Yu, Z.; Zhang, S. Curcumin upregulates the Nrf2 system by repressing inflammatory signaling-mediated Keap1 expression in insulin-resistant conditions. Biochem. Biophys. Res. Commun. 2019, 514, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Scuto, M.C.; Mancuso, C.; Tomasello, B.; Ontario, M.L.; Cavallaro, A.; Frasca, F.; Maiolino, L.; Salinaro, A.T.; Calabrese, E.J.; Calabrese, V. Curcumin, hormesis and the nervous system. Nutrients 2019, 11, 2417. [Google Scholar] [CrossRef]
- Méndez-García, L.A.; Martinez-Castillo, M.; Villegas-Sepúlveda, N.; Orozco, L.; Córdova, E.J. Curcumin induces p53-independent inactivation of Nrf2 during oxidative stress–induced apoptosis. Hum. Human. Exp. Toxicol. 2019, 38, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Azzini, E.; Peña-Corona, S.I.; Hernández-Parra, H.; Chandran, D.; Saleena, L.A.K.; Sawikr, Y.; Peluso, I.; Dhumal, S.; Kumar, M.; Leyva-Gómez, G.; et al. Neuroprotective and anti-inflammatory effects of curcumin in Alzheimer’s disease: Targeting neuroinflammation strategies. Phytother. Res. 2024, 38, 3169–3189. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Liao, H.; Hao, S.; Liu, R.; Huang, H.; Duan, C. Curcumin simultaneously improves mitochondrial dynamics and myocardial cell bioenergy after sepsis via the SIRT1-DRP1/PGC-1α pathway. Heliyon 2024, 10, e28501. [Google Scholar] [CrossRef]
- Osawa, T.; Kato, Y. Protective role of antioxidative food factors in oxidative stress caused by hyperglycemia. Ann. N. Y. Acad. Sci. 2005, 1043, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Hushmandi, K.; Zarrin, V.; Moghadam, E.R.; Hashemi, F.; Makvandi, P.; Samarghandian, S.; Khan, H.; Hashemi, F.; et al. Toward Regulatory Effects of Curcumin on Transforming Growth Factor-Beta Across Different Diseases: A Review. Front. Pharmacol. 2020, 11, 585413. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- Nazarian, M.; Aramjoo, H.; Roshanravan, B.; Samarghandian, S.; Farkhondeh, T. Protective Effects of Curcumin and Nanomicelle Curcumin on Chlorpyrifos-induced Oxidative Damage and Inflammation in the Uterus, Ovary and Brain of Rats. Curr. Pharm. Biotechnol. 2024. [CrossRef]
- Farkhondeh, T.; Zardast, M.; Rajabi, S.; Abdollahi-Karizno, M.; Roshanravan, B.; Havangi, J.; Aschner, M.; Samarghandian, S. Neuroprotective Effects of Curcumin against Chronic ChlorpyrifosInduced Oxidative Damage in Rat Brain Tissue. Curr. Aging Sci. 2024, 17. [Google Scholar] [CrossRef]
- Zhang, M.W.; Sun, X.; Xu, Y.W.; Meng, W.; Tang, Q.; Gao, H.; Liu, L.; Chen, S.H. Curcumin relieves oxaliplatin-induced neuropathic pain via reducing inflammation and activating antioxidant response. Cell Biol. Int. 2024, 6, 872–882. [Google Scholar] [CrossRef]
- Sathyabhama, M.; Priya Dharshini, L.C.; Karthikeyan, A.; Kalaiselvi, S.; Min, T. The credible role of curcumin in oxidative stress-mediated mitochondrial dysfunction in mammals. Biomolecules 2022, 12, 1405. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, J.M.; Zhao, H.; Ao, C.Y.; Ao, L.H.; Ban, J.Q.; Li, J. Mechanism of KAT2A regulation of H3K36ac in manganese-induced oxidative damage to mitochondria in the nervous system and intervention by curcumin. Ecotoxicol. Environ. Saf. 2024, 273, 116155. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Y.; Zou, X.; Zheng, Z.; Zhang, J. Curcumin ameliorates CKD-induced mitochondrial dysfunction and oxidative stress through inhibiting GSK-3β activity. J. Nutr. Biochem. 2020, 83, 108404. [Google Scholar] [CrossRef]
- Saghari, Y.; Movahedi, M.; Tebianian, M.; Entezari, M. The Neuroprotective Effects of Curcumin Nanoparticles on The Cerebral Ischemia-Reperfusion Injury in The Rats-The Roles of The Protein Kinase RNA-Like ER Kinase/Extracellular Signal-Regulated Kinase and Transcription Factor EB proteins. Cell J. 2024, 26, 62–69. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Yang, Y.; Zhang, W.; Luo, L.; Han, F.; Guan, H.; Tao, K.; Hu, D. Curcumin pretreatment protects against hypoxia/reoxgenation injury via improvement of mitochondrial function, destabilization of HIF-1α and activation of Epac1-Akt pathway in rat bone marrow mesenchymal stem cells. Biomed. Pharmacother. 2019, 109, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, S.; Zhu, B.; Shao, S.; Yuan, L. Grape seed procyanidin suppresses inflammation in cigarette smoke-exposed pulmonary arterial hypertension rats by the PPAR-γ/COX-2 pathway. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lim, J.; Oh, J. Taming neuroinflammation in Alzheimer’s disease: The protective role of phytochemicals through the gut-brain axis. Biomed. Pharmacother.=Biomed. Pharmacother. 2024, 178, 117277. [Google Scholar] [CrossRef]
- Chiu, Y.J.; Yang, J.S.; Tsai, F.J.; Chiu, H.Y.; Juan, Y.N.; Lo, Y.H.; Chiang, J.H. Curcumin suppresses cell proliferation and triggers apoptosis in vemurafenib-resistant melanoma cells by downregulating the EGFR signaling pathway. Environ. Toxicol. 2022, 37, 868–879. [Google Scholar] [CrossRef]
- Petiti, J.; Rosso, V.; Lo Iacono, M.; Panuzzo, C.; Calabrese, C.; Signorino, E.; Pironi, L.; Cartellà, A.; Bracco, E.; Pergolizzi, B.; et al. Curcumin induces apoptosis in JAK2-mutated cells by the inhibition of JAK2/STAT and mTORC1 pathways. J. Cell. Mol. Med. 2019, 23, 4349–4357. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.C.; Zhang, Y.F.; Liu, S.S.; Cheng, X.J.; Yang, X.; Cui, X.G.; Zhao, X.R.; Zhao, H.; Hao, M.F.; Li, M.D.; et al. Curcumin alleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt signalling pathways. J. Cell. Mol. Med. 2020, 24, 12355–12367. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, X.; Wang, X.; Liu, B.; Yuan, Y.; Zuo, X. Curcumin attenuates inflammation and cell apoptosis through regulating NF-κB and JAK2/STAT3 signaling pathway against acute kidney injury. Cell Cycle 2020, 19, 1941–1951. [Google Scholar] [CrossRef]
- Souza, P.V.S.d.; Pinto, W.B.V.d.R.; Oliveira, A.S.B. C9orf72-related disorders: Expanding the clinical and genetic spectrum of neurodegenerative diseases. Arq. Neuro-Psiquiatr. 2015, 73, 246–256. [Google Scholar] [CrossRef]
- Bulle Oliveira, A.S.; Batista Pereira, R.D. Amyotrophic Lateral Sclerosis (ALS): Three Letters That Change The People’s Life. Arq. Neuro-Psiquiatr. 2009, 67, 750–782. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Pei, X.; Chen, J.; Wang, L. Identification of cuproptosis-realated key genes and pathways in Parkinson’s disease via bioinformatics analysis. PLoS ONE 2024, 19, e0299898. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Sanchez, V.B.; Xu, P.; Roule, T.; Flores-Mendez, M.; Ciesielski, B.; Yoo, D.; Teshome, H.; Jimenez, T.; Liu, S.; et al. Altered lipid homeostasis is associated with cerebellar neurodegeneration in SNX14 deficiency. JCI Insight 2024, 9, e168594. [Google Scholar] [CrossRef]
- Kovalová, M.; Gottfriedová, N.; Mrázková, E.; Janout, V.; Janoutová, J. Cognitive impairment, neurodegenerative disorders, and olfactory impairment: A literature review. Otolaryngol. Pol. 2024, 78, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bássoli, R.; Audi, D.; Ramalho, B.; Audi, M.; Quesada, K.; Barbalho, S. The Effects of Curcumin on Neurodegenerative Diseases: A Systematic Review. J. Herb. Herbal. Med. 2023, 42, 100771. [Google Scholar] [CrossRef]
- Gunnarsson, L.-G.; Bodin, L. Occupational exposures and neurodegenerative diseases—A systematic literature review and meta-analyses. Int. J. Environ. Res. Public. Health 2019, 16, 337. [Google Scholar] [CrossRef] [PubMed]
- Rekatsina, M.; Paladini, A.; Piroli, A.; Zis, P.; Pergolizzi, J.V.; Varrassi, G. Pathophysiology and therapeutic perspectives of oxidative stress and neurodegenerative diseases: A narrative review. Adv. Ther. 2020, 37, 113–139. [Google Scholar] [CrossRef] [PubMed]
- Katsnelson, A.; De Strooper, B.; Zoghbi, H.Y. Neurodegeneration: From cellular concepts to clinical applications. Sci. Transl. Med. 2016, 8, ps318–ps364. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Mansoori, A.; Sisodiya, J. Current pathologic determinants of complex neurodegenerative diseases: A review. Int. J. Pharm. Technol. 2013, 5, 2607–2621. [Google Scholar]
- Jette, N.; Maxwell, C.J.; Fiest, K.M.; Hogan, D.B. Systematic reviews and meta-analyses of the incidence and prevalence of dementia and its commoner neurodegenerative causes. Can. J. Neurol. Sci. 2016, 43, S1–S2. [Google Scholar] [CrossRef]
- González, H.; Pacheco, R. T-cell-mediated regulation of neuroinflammation involved in neurodegenerative diseases. J. Neuroinflammation 2014, 11, 1–11. [Google Scholar] [CrossRef]
- Tanaka, M.; Chen, C. Towards a mechanistic understanding of depression, anxiety, and their comorbidity: Perspectives from cognitive neuroscience. Front. Behav. Neurosci. 2023, 17, 1268156. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, H.; Ghasemi, F.; Barreto, G.E.; Rafiee, R.; Sathyapalan, T.; Sahebkar, A. Effects of curcumin on mitochondria in neurodegenerative diseases. Biofactors 2020, 46, 5–20. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ohnishi, T.; Nakagawa, R.; Yoshizawa, K. The comparative efficacy and safety of cholinesterase inhibitors in patients with mild-to-moderate Alzheimer’s disease: A Bayesian network meta-analysis. Int. J. Geriatr. Psychiatry 2016, 31, 892–904. [Google Scholar] [CrossRef]
- Mehla, J.; Gupta, P.; Pahuja, M.; Diwan, D.; Diksha, D. Indian medicinal herbs and formulations for Alzheimer’s disease, from traditional knowledge to scientific assessment. Brain Sci. 2020, 10, 964. [Google Scholar] [CrossRef] [PubMed]
- Popa-Wagner, A.; Dumitrascu, D.I.; Capitanescu, B.; Petcu, E.B.; Surugiu, R.; Fang, W.-H.; Dumbrava, D.-A. Dietary habits, lifestyle factors and neurodegenerative diseases. Neural Regen. Res. 2020, 15, 394–400. [Google Scholar] [CrossRef]
- Fasihi, M.; Samimi-Badabi, M.; Robat-Jazi, B.; Bitarafan, S.; Moghadasi, A.N.; Mansouri, F.; Yekaninejad, M.S.; Izad, M.; Saboor-Yaraghi, A.A. Immunoregulatory Effects of the Active Form of Vitamin D (Calcitriol), Individually and in Combination with Curcumin, on Peripheral Blood Mononuclear Cells (PBMCs) of Multiple Sclerosis (MS) Patients. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2024, 23. [Google Scholar] [CrossRef]
- Chae, J.; Choi, Y.; Hong, J.; Kim, N.; Kim, J.; Lee, H.Y.; Choi, J. Anticancer and Antibacterial Properties of Curcumin-Loaded Mannosylated Solid Lipid Nanoparticles for the Treatment of Lung Diseases. ACS Appl. Bio. Mater. 2024, 7, 2175–2185. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Tang, X.; Deng, P.; Hui, H.; Chen, B.; An, J.; Zhang, G.; Shi, K.; Wang, J.; He, Y.; et al. Interleukin-4 from curcumin-activated OECs emerges as a central modulator for increasing M2 polarization of microglia/macrophage in OEC anti-inflammatory activity for functional repair of spinal cord injury. Cell Commun. Signal. CCS 2024, 22, 162. [Google Scholar] [CrossRef] [PubMed]
- Harada, C.N.; Natelson Love, M.C.; Triebel, K.L. Normal cognitive aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef]
- Bliss, E.S.; Wong, R.H.; Howe, P.R.; Mills, D.E. Benefits of exercise training on cerebrovascular and cognitive function in ageing. J. Cereb. Blood Flow. Metab. 2021, 41, 447–470. [Google Scholar] [CrossRef]
- Ahmad, M. Protective effects of curcumin against lithium-pilocarpine induced status epilepticus, cognitive dysfunction and oxidative stress in young rats. Saudi J. Biol. Sci. 2013, 20, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.R.; Franks, S.F. Efficacy of curcumin for age-associated cognitive decline: A narrative review of preclinical and clinical studies. Geroscience 2018, 40, 73–95. [Google Scholar] [CrossRef]
- Cho, J.A.; Park, S.H.; Cho, J.; Kim, J.O.; Yoon, J.H.; Park, E. Exercise and Curcumin in Combination Improves Cognitive Function and Attenuates ER Stress in Diabetic Rats. Nutrients 2020, 12, 1309. [Google Scholar] [CrossRef] [PubMed]
- Kodali, M.; Hattiangady, B.; Shetty, G.A.; Bates, A.; Shuai, B.; Shetty, A.K. Curcumin treatment leads to better cognitive and mood function in a model of Gulf War Illness with enhanced neurogenesis, and alleviation of inflammation and mitochondrial dysfunction in the hippocampus. Brain Behav. Immun. 2018, 69, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Grimm, A.; Eckert, A. Brain aging and neurodegeneration: From a mitochondrial point of view. J. Neurochem. 2017, 143, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.T.; Dong, S.Q.; Wang, S.S.; Chen, M.; Li, C.F.; Geng, D.; Zhu, J.X.; Liu, Q.; Cheng, J. Curcumin attenuates cognitive impairment by enhancing autophagy in chemotherapy. Neurobiol. Dis. 2020, 136, 104715. [Google Scholar] [CrossRef] [PubMed]
- Rueda, N.; Vidal, V.; García-Cerro, S.; Puente, A.; Campa, V.; Lantigua, S.; Narcís, O.; Bartesaghi, R.; Martínez-Cué, C. Prenatal, but not Postnatal, Curcumin Administration Rescues Neuromorphological and Cognitive Alterations in Ts65Dn Down Syndrome Mice. J. Nutr. 2020, 150, 2478–2489. [Google Scholar] [CrossRef]
- Noorafshan, A.; Abdollahifar, M.A.; Karbalay-Doust, S.; Asadi-Golshan, R.; Rashidian-Rashidabadi, A. Protective effects of curcumin and sertraline on the behavioral changes in chronic variable stress-induced rats. Exp. Neurobiol. 2013, 22, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Contarini, G.; Sut, S.; Dall’Acqua, S.; Confortin, F.; Pagetta, A.; Giusti, P.; Zusso, M. Curcumin Prevents Acute Neuroinflammation and Long-Term Memory Impairment Induced by Systemic Lipopolysaccharide in Mice. Front. Pharmacol. 2018, 9, 183. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Tests for learning and memory in rodent regulatory studies. Curr. Res. Toxicol. 2024, 6, 100151. [Google Scholar] [CrossRef] [PubMed]
- Lang, B.; Kahnau, P.; Hohlbaum, K.; Mieske, P.; Andresen, N.P.; Boon, M.N.; Thöne-Reineke, C.; Lewejohann, L.; Diederich, K. Challenges and advanced concepts for the assessment of learning and memory function in mice. Front. Behav. Neurosci. 2023, 17, 1230082. [Google Scholar] [CrossRef] [PubMed]
- Denninger, J.K.; Smith, B.M.; Kirby, E.D. Novel Object Recognition and Object Location Behavioral Testing in Mice on a Budget. J. Vis. Exp. 2018, 141. [Google Scholar] [CrossRef]
- Battaglia, S.; Avenanti, A.; Vécsei, L.; Tanaka, M. Neural correlates and molecular mechanisms of memory and learning. Int. J. Mol. Sci. 2024, 25, 2724. [Google Scholar] [CrossRef] [PubMed]
- Changlek, S.; Rana, M.N.; Phyu, M.P.; Karim, N.; Majima, H.J.; Tangpong, J. Curcumin Suppresses Lead-Induced Inflammation and Memory Loss in Mouse Model and In Silico Molecular Docking. Foods 2022, 11, 856. [Google Scholar] [CrossRef]
- Lu, W.T.; Sun, S.Q.; Li, Y.; Xu, S.Y.; Gan, S.W.; Xu, J.; Qiu, G.P.; Zhuo, F.; Huang, S.Q.; Jiang, X.L.; et al. Curcumin Ameliorates Memory Deficits by Enhancing Lactate Content and MCT2 Expression in APP/PS1 Transgenic Mouse Model of Alzheimer’s Disease. Anat. Rec. 2019, 302, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Sarlak, Z.; Oryan, S.; Moghaddasi, M. Interaction between the antioxidant activity of curcumin and cholinergic system on memory retention in adult male Wistar rats. Iran. J. Basic. Med. Sci. 2015, 18, 398–403. [Google Scholar] [PubMed]
- Ikram, M.; Saeed, K.; Khan, A.; Muhammad, T.; Khan, M.S.; Jo, M.G.; Rehman, S.U.; Kim, M.O. Natural Dietary Supplementation of Curcumin Protects Mice Brains against Ethanol-Induced Oxidative Stress-Mediated Neurodegeneration and Memory Impairment via Nrf2/TLR4/RAGE Signaling. Nutrients 2019, 11, 1082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fang, Y.; Xu, Y.; Lian, Y.; Xie, N.; Wu, T.; Zhang, H.; Sun, L.; Zhang, R.; Wang, Z. Curcumin Improves Amyloid β-Peptide (1–42) Induced Spatial Memory Deficits through BDNF-ERK Signaling Pathway. PLoS ONE 2015, 10, e0131525. [Google Scholar] [CrossRef]
- Gouras, G.K.; Olsson, T.T.; Hansson, O. β-Amyloid peptides and amyloid plaques in Alzheimer’s disease. Neurotherapeutics 2015, 12, 3–11. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef]
- Metaxas, A.; Kempf, S.J. Neurofibrillary tangles in Alzheimer’s disease: Elucidation of the molecular mechanism by immunohistochemistry and tau protein phospho-proteomics. Neural Regen. Res. 2016, 11, 1579–1581. [Google Scholar] [CrossRef] [PubMed]
- Minter, M.R.; Taylor, J.M.; Crack, P.J. The contribution of neuroinflammation to amyloid toxicity in Alzheimer’s disease. J. Neurochem. 2016, 136, 457–474. [Google Scholar] [CrossRef]
- Trejo-Lopez, J.A.; Yachnis, A.T.; Prokop, S. Neuropathology of Alzheimer’s Disease. Neurotherapeutics 2022, 19, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Domingues, C.; da Cruz, E.; Silva, O.A.B.; Henriques, A.G. Impact of Cytokines and Chemokines on Alzheimer’s Disease Neuropathological Hallmarks. Curr. Alzheimer Res. 2017, 14, 870–882. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghraiybah, N.F.; Wang, J.; Alkhalifa, A.E.; Roberts, A.B.; Raj, R.; Yang, E.; Kaddoumi, A. Glial Cell-Mediated Neuroinflammation in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 10572. [Google Scholar] [CrossRef] [PubMed]
- Twarowski, B.; Herbet, M. Inflammatory Processes in Alzheimer’s Disease-Pathomechanism, Diagnosis and Treatment: A Review. Int. J. Mol. Sci. 2023, 24, 6518. [Google Scholar] [CrossRef] [PubMed]
- González-Reyes, R.E.; Nava-Mesa, M.O.; Vargas-Sánchez, K.; Ariza-Salamanca, D.; Mora-Muñoz, L. Involvement of Astrocytes in Alzheimer’s Disease from a Neuroinflammatory and Oxidative Stress Perspective. Front. Mol. Neurosci. 2017, 10, 427. [Google Scholar] [CrossRef]
- Ono, K.; Hasegawa, K.; Naiki, H.; Yamada, M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J. Neurosci. Res. 2004, 75, 742–750. [Google Scholar] [CrossRef]
- Lim, G.P.; Chu, T.; Yang, F.; Beech, W.; Frautschy, S.A.; Cole, G.M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001, 21, 8370–8377. [Google Scholar] [CrossRef]
- Hoppe, J.B.; Coradini, K.; Frozza, R.L.; Oliveira, C.M.; Meneghetti, A.B.; Bernardi, A.; Pires, E.S.; Beck, R.C.; Salbego, C.G. Free and nanoencapsulated curcumin suppress β-amyloid-induced cognitive impairments in rats: Involvement of BDNF and Akt/GSK-3β signaling pathway. Neurobiol. Learn. Mem. 2013, 106, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alloza, M.; Borrelli, L.A.; Rozkalne, A.; Hyman, B.T.; Bacskai, B.J. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 2007, 102, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Goozee, K.G.; Shah, T.M.; Sohrabi, H.R.; Rainey-Smith, S.R.; Brown, B.; Verdile, G.; Martins, R.N. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br. J. Nutr. 2016, 115, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Agarwal, S.; Seth, B.; Yadav, A.; Nair, S.; Bhatnagar, P.; Karmakar, M.; Kumari, M.; Chauhan, L.K.; Patel, D.K.; et al. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/β-catenin pathway. ACS Nano 2014, 8, 76–103. [Google Scholar] [CrossRef]
- Martos, D.; Lőrinczi, B.; Szatmári, I.; Vécsei, L.; Tanaka, M. The Impact of C-3 Side Chain Modifications on Kynurenic Acid: A Behavioral Analysis of Its Analogs in the Motor Domain. Int. J. Mol. Sci. 2024, 25, 3394. [Google Scholar] [CrossRef]
- Tanaka, M.; Bohár, Z.; Vécsei, L. Are kynurenines accomplices or principal villains in dementia? Maintenance of kynurenine metabolism. Molecules 2020, 25, 564. [Google Scholar] [CrossRef]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef]
- Pan, R.; Qiu, S.; Lu, D.X.; Dong, J. Curcumin improves learning and memory ability and its neuroprotective mechanism in mice. Chin. Med. J. 2008, 121, 832–839. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, Q.Y.; Li, H.Y.; Zhou, X.; Liu, Y.; Zhang, H. Curcumin ameliorates cognitive deficits heavy ion irradiation-induced learning and memory deficits through enhancing of Nrf2 antioxidant signaling pathways. Pharmacol. Biochem. Behav. 2014, 126, 181–186. [Google Scholar] [CrossRef]
- Hacioglu, C.; Kar, F.; Kar, E.; Kara, Y.; Kanbak, G. Effects of Curcumin and Boric Acid Against Neurodegenerative Damage Induced by Amyloid Beta (1–42). Biol. Trace Elem. Res. 2021, 199, 3793–3800. [Google Scholar] [CrossRef]
- Ahlijanian, M.K.; Barrezueta, N.X.; Williams, R.D.; Jakowski, A.; Kowsz, K.P.; McCarthy, S.; Coskran, T.; Carlo, A.; Seymour, P.A.; Burkhardt, J.E.; et al. Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc. Natl. Acad. Sci. USA 2000, 97, 2910–2915. [Google Scholar] [CrossRef]
- Chow, H.M.; Guo, D.; Zhou, J.C.; Zhang, G.Y.; Li, H.F.; Herrup, K.; Zhang, J. CDK5 activator protein p25 preferentially binds and activates GSK3β. Proc. Natl. Acad. Sci. USA 2014, 111, E4887–E4895. [Google Scholar] [CrossRef]
- Alamro, A.A.; Alsulami, E.A.; Almutlaq, M.; Alghamedi, A.; Alokail, M.; Haq, S.H. Therapeutic Potential of Vitamin D and Curcumin in an. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520924311. [Google Scholar] [CrossRef]
- Das, T.K.; Jana, P.; Chakrabarti, S.K.; Abdul Hamid, M.R.W. Curcumin Downregulates GSK3 and Cdk5 in Scopolamine-Induced Alzheimer’s Disease Rats Abrogating Aβ. J. Alzheimers Dis. Rep. 2019, 3, 257–267. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Li, S.; Wang, X.; Liu, B.; Fu, Q.; Ma, S. Curcumin attenuates glutamate neurotoxicity in the hippocampus by suppression of ER stress-associated TXNIP/NLRP3 inflammasome activation in a manner dependent on AMPK. Toxicol. Appl. Pharmacol. 2015, 286, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, N.; Lin, L. Curcumin Suppresses Apoptosis and Inflammation in Hypoxia/Reperfusion-Exposed Neurons via Wnt Signaling Pathway. Med. Sci. Monit. 2020, 26, e920445. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lee, W.K.; Kim, H.S.; Seo, J.A.; Kim, D.H.; Han, H.C.; Min, B.H. Clusterin overexpression protects against western diet-induced obesity and NAFLD. Sci. Rep. 2020, 10, 17484. [Google Scholar] [CrossRef]
- Shao, S.; Ye, X.; Su, W.; Wang, Y. Curcumin alleviates Alzheimer’s disease by inhibiting inflammatory response, oxidative stress and activating the AMPK pathway. J. Chem. Neuroanat. 2023, 134, 102363. [Google Scholar] [CrossRef] [PubMed]
- Yavarpour-Bali, H.; Ghasemi-Kasman, M.; Pirzadeh, M. Curcumin-loaded nanoparticles: A novel therapeutic strategy in treatment of central nervous system disorders. Int. J. Nanomed. 2019, 14, 4449–4460. [Google Scholar] [CrossRef]
- Panzarini, E.; Mariano, S.; Tacconi, S.; Carata, E.; Tata, A.M.; Dini, L. Novel Therapeutic Delivery of Nanocurcumin in Central Nervous System Related Disorders. Nanomaterials 2020, 11, 2. [Google Scholar] [CrossRef]
- Ruan, Y.; Xiong, Y.; Fang, W.; Yu, Q.; Mai, Y.; Cao, Z.; Wang, K.; Lei, M.; Xu, J.; Liu, Y.; et al. Highly sensitive Curcumin-conjugated nanotheranostic platform for detecting amyloid-beta plaques by magnetic resonance imaging and reversing cognitive deficits of Alzheimer’s disease via NLRP3-inhibition. J. Nanobiotechnol. 2022, 20, 322. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.; Yeung, C.F.; Ho, S.W.; Chow, S.F.; Chow, A.H.; Baum, L. Highly stabilized curcumin nanoparticles tested in an in vitro blood-brain barrier model and in Alzheimer’s disease Tg2576 mice. AAPS J. 2013, 15, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-Y.; Chun, Y.-S.; Kim, J.-K.; Lee, J.-O.; Ku, S.-K.; Shim, S.-M. Curcumin attenuates sarcopenia in chronic forced exercise executed aged mice by regulating muscle degradation and protein synthesis with antioxidant and anti-inflammatory effects. J. Agric. Food Chem. 2021, 69, 6214–6228. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.B.; O’Callaghan, J.P. Biomarkers of Parkinson’s disease: Present and future. Metabolism 2015, 64, S40–S46. [Google Scholar] [CrossRef] [PubMed]
- Cabreira, V.; Massano, J. Doença de Parkinson: Revisão clínica e atualização [Parkinson’s disease: Clinical review and update]. Acta Med. Port. 2019, 32, 661–670. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Revolutionizing our understanding of Parkinson’s disease: Dr. Heinz Reichmann’s pioneering research and future research direction. J. Neural. Transm. 2024. [Google Scholar] [CrossRef]
- Buglio, D.S.; Marton, L.T.; Laurindo, L.F.; Guiguer, E.L.; Araújo, A.C.; Buchaim, R.L.; Goulart, R.A.; Rubira, C.J.; Barbalho, S.M. The Role of Resveratrol in Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review. J. Med. Food 2022, 25, 797–806. [Google Scholar] [CrossRef]
- de Oliveira Zanuso, B.; de Oliveira Dos Santos, A.R.; Miola, V.F.B.; Guissoni Campos, L.M.; Spilla, C.S.G.; Barbalho, S.M. Panax ginseng and aging related disorders: A systematic review. Exp. Gerontol. 2022, 161, 111731. [Google Scholar] [CrossRef] [PubMed]
- Solleiro-Villavicencio, H.; Rivas-Arancibia, S. Effect of chronic oxidative stress on neuroinflammatory response mediated by CD4+ T cells in neurodegenerative diseases. Front. Cell. Neurosci. 2018, 12, 114. [Google Scholar] [CrossRef]
- Puspita, L.; Chung, S.Y.; Shim, J.-w. Oxidative stress and cellular pathologies in Parkinson’s disease. Mol. Brain 2017, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Martinez, B.; Peplow, P.V. Neuroprotection by immunomodulatory agents in animal models of Parkinson’s disease. Neural Regen. Res. 2018, 13, 1493–1506. [Google Scholar] [PubMed]
- Achete de Souza, G.; de Marqui, S.V.; Matias, J.N.; Guiguer, E.L.; Barbalho, S.M. Effects of Ginkgo biloba on Diseases Related to Oxidative Stress. Planta Medica 2020, 86, 376–386. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Direito, R.; Laurindo, L.F.; Marton, L.T.; Guiguer, E.L.; Goulart, R.A.; Tofano, R.J.; Carvalho, A.C.A.; Flato, U.A.P.; Capelluppi Tofano, V.A.; et al. Ginkgo biloba in the Aging Process: A Narrative Review. Antioxidants 2022, 11, 525. [Google Scholar] [CrossRef] [PubMed]
- Dhouib, I.B.; Annabi, A.; Doghri, R.; Rejeb, I.; Dallagi, Y.; Bdiri, Y.; Lasram, M.M.; Elgaaied, A.; Marrakchi, R.; Fazaa, S. Neuroprotective effects of curcumin against acetamiprid-induced neurotoxicity and oxidative stress in the developing male rat cerebellum: Biochemical, histological, and behavioral changes. Environ. Sci. Pollut. Res. 2017, 24, 27515–27524. [Google Scholar] [CrossRef]
- Mamun, A.A.; Shao, C.; Geng, P.; Wang, S.; Xiao, J. Polyphenols Targeting NF-κB Pathway in Neurological Disorders: What We Know So Far? Int. J. Biol. Sci. 2024, 20, 1332–1355. [Google Scholar] [CrossRef]
- Reglodi, D.; Renaud, J.; Tamas, A.; Tizabi, Y.; Socías, S.B.; Del-Bel, E.; Raisman-Vozari, R. Novel tactics for neuroprotection in Parkinson’s disease: Role of antibiotics, polyphenols and neuropeptides. Prog. Progress. Neurobiol. 2017, 155, 120–148. [Google Scholar] [CrossRef]
- Bhat, A.; Mahalakshmi, A.M.; Ray, B.; Tuladhar, S.; Hediyal, T.A.; Manthiannem, E.; Padamati, J.; Chandra, R.; Chidambaram, S.B.; Sakharkar, M.K. Benefits of curcumin in brain disorders. BioFactors 2019, 45, 666–689. [Google Scholar] [CrossRef]
- Sharma, N.; Nehru, B.J.I. Curcumin affords neuroprotection and inhibits α-synuclein aggregation in lipopolysaccharide-induced Parkinson’s disease model. Inflammopharmacology 2018, 26, 349–360. [Google Scholar] [CrossRef]
- Khadrawy, Y.A.; Hosny, E.N.; Eldein Mohamed, H.S. Assessment of the neuroprotective effect of green synthesized iron oxide nanoparticles capped with curcumin against a rat model of Parkinson’s disease. Iran. J. Basic. Med. Sci. 2024, 27, 81–89. [Google Scholar] [CrossRef]
- Bandeen-Roche, K.; Seplaki, C.L.; Huang, J.; Buta, B.; Kalyani, R.R.; Varadhan, R.; Xue, Q.-L.; Walston, J.D.; Kasper, J.D. Frailty in older adults: A nationally representative profile in the United States. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2015, 70, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Proietti, M.; Cesari, M. Frailty: What is it? In Frailty and Cardiovascular Diseases; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2020; pp. 1–7. [Google Scholar]
- Cesari, M.; Calvani, R.; Marzetti, E. Frailty in older persons. Clin. Geriatr. Med. 2017, 33, 293–303. [Google Scholar] [CrossRef]
- Asavamongkolkul, A.; Adulkasem, N.; Chotiyarnwong, P.; Vanitcharoenkul, E.; Chandhanayingyong, C.; Laohaprasitiporn, P.; Soparat, K.; Unnanuntana, A. Prevalence of osteoporosis, sarcopenia, and high falls risk in healthy community-dwelling Thai older adults: A nationwide cross-sectional study. JBMR Plus 2024, 8, ziad020. [Google Scholar] [CrossRef] [PubMed]
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty: Implications for clinical practice and public health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Bandeen-Roche, K.; Xue, Q.-L.; Ferrucci, L.; Walston, J.; Guralnik, J.M.; Chaves, P.; Zeger, S.L.; Fried, L.P. Phenotype of frailty: Characterization in the women’s health and aging studies. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 262–266. [Google Scholar] [CrossRef]
- Xue, Q.-L.; Walston, J.D.; Fried, L.P.; Beamer, B.A. Prediction of risk of falling, physical disability, and frailty by rate of decline in grip strength: The women’s health and aging study. Arch. Intern. Med. 2011, 171, 1119–1121. [Google Scholar] [CrossRef] [PubMed]
- Ensrud, K.E.; Ewing, S.K.; Taylor, B.C.; Fink, H.A.; Stone, K.L.; Cauley, J.A.; Tracy, J.K.; Hochberg, M.C.; Rodondi, N.; Cawthon, P.M. Frailty and risk of falls, fracture, and mortality in older women: The study of osteoporotic fractures. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 744–751. [Google Scholar] [CrossRef]
- Makary, M.A.; Segev, D.L.; Pronovost, P.J.; Syin, D.; Bandeen-Roche, K.; Patel, P.; Takenaga, R.; Devgan, L.; Holzmueller, C.G.; Tian, J. Frailty as a predictor of surgical outcomes in older patients. J. Am. Coll. Surg. 2010, 210, 901–908. [Google Scholar] [CrossRef] [PubMed]
- McAdams-DeMarco, M.A.; Suresh, S.; Law, A.; Salter, M.L.; Gimenez, L.F.; Jaar, B.G.; Walston, J.D.; Segev, D.L. Frailty and falls among adult patients undergoing chronic hemodialysis: A prospective cohort study. BMC Nephrol. 2013, 14, 224. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, P.M.; Marshall, L.M.; Michael, Y.; Dam, T.T.; Ensrud, K.E.; Barrett-Connor, E.; Orwoll, E.S.; Group, O.F.i.M.R. Frailty in older men: Prevalence, progression, and relationship with mortality. J. Am. Geriatr. Soc. 2007, 55, 1216–1223. [Google Scholar] [CrossRef]
- Collard, R.M.; Boter, H.; Schoevers, R.A.; Oude Voshaar, R.C. Prevalence of frailty in community-dwelling older persons: A systematic review. J. Am. Geriatr. Soc. 2012, 60, 1487–1492. [Google Scholar] [CrossRef]
- Santos-Eggimann, B.; Cuénoud, P.; Spagnoli, J.; Junod, J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2009, 64, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, S.; Uehara, M.; Tokida, R.; Nishimura, H.; Sakai, N.; Horiuchi, H.; Kato, H.; Takahashi, J. Male-female disparity in clinical features and significance of mild vertebral fractures in community-dwelling residents aged 50 and over. Sci. Rep. 2024, 14, 5602. [Google Scholar] [CrossRef] [PubMed]
- Nicol, L.M.; Rowlands, D.S.; Fazakerly, R.; Kellett, J. Curcumin supplementation likely attenuates delayed onset muscle soreness (DOMS). Eur. J. Appl. Physiol. 2015, 115, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Drobnic, F.; Riera, J.; Appendino, G.; Togni, S.; Franceschi, F.; Valle, X.; Pons, A.; Tur, J. Reduction of delayed onset muscle soreness by a novel curcumin delivery system (Meriva®): A randomised, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2014, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Perna, S.; Alalwan, T.A.; Al-Thawadi, S.; Negro, M.; Parimbelli, M.; Cerullo, G.; Gasparri, C.; Guerriero, F.; Infantino, V.; Diana, M. Evidence-based role of nutrients and antioxidants for chronic pain management in musculoskeletal frailty and sarcopenia in aging. Geriatrics 2020, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Mankowski, R.T.; Sibille, K.T.; Leeuwenburgh, C.; Lin, Y.; Hsu, F.C.; Qiu, P.; Sandesara, B.; Anton, S.D. Effects of Curcumin C3 Complex® on Physical Function in Moderately Functioning Older Adults with Low-Grade Inflammation—A Pilot Trial. J. Frailty Aging 2023, 12, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Saud Gany, S.L.; Chin, K.-Y.; Tan, J.K.; Aminuddin, A.; Makpol, S. Curcumin as a therapeutic agent for sarcopenia. Nutrients 2023, 15, 2526. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Morley, J.E.; Abbatecola, A.M.; Argiles, J.M.; Baracos, V.; Bauer, J.; Bhasin, S.; Cederholm, T.; Coats, A.J.S.; Cummings, S.R.; Evans, W.J. Sarcopenia with limited mobility: An international consensus. J. Am. Med. Dir. Assoc. 2011, 12, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Mellen, R.H.; Girotto, O.S.; Marques, E.B.; Laurindo, L.F.; Grippa, P.C.; Mendes, C.G.; Garcia, L.N.H.; Bechara, M.D.; Barbalho, S.M.; Sinatora, R.V.; et al. Insights into Pathogenesis, Nutritional and Drug Approach in Sarcopenia: A Systematic Review. Biomedicines 2023, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Pescinini-Salzedas, L.M.; Minniti, G.; Laurindo, L.F.; Barbalho, S.M.; Vargas Sinatora, R.; Sloan, L.A.; Haber, R.S.A.; Araújo, A.C.; Quesada, K.; et al. Organokines, Sarcopenia, and Metabolic Repercussions: The Vicious Cycle and the Interplay with Exercise. Int. J. Mol. Sci. 2022, 23, 13452. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Flato, U.A.P.; Tofano, R.J.; Goulart, R.A.; Guiguer, E.L.; Detregiachi, C.R.P.; Buchaim, D.V.; Araújo, A.C.; Buchaim, R.L.; Reina, F.T.R.; et al. Physical Exercise and Myokines: Relationships with Sarcopenia and Cardiovascular Complications. Int. J. Mol. Sci. 2020, 21, 3607. [Google Scholar] [CrossRef]
- Park, J.; Park, S. Association of Handgrip Strength and Cardiovascular Disease Risk Among Middle-Aged Postmenopausal Women: An Analysis of the Korea National Health and Nutrition Examination Survey 2014-2019. Vasc. Health Risk Manag. 2024, 20, 183–194. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Prado Neto, E.V.; De Alvares Goulart, R.; Bechara, M.D.; Baisi Chagas, E.F.; Audi, M.; Guissoni Campos, L.M.; Landgraf Guiger, E.; Buchaim, R.L.; Buchaim, D.V.; et al. Myokines: A descriptive review. J. Sports Med. Phys. Fit. 2020, 60, 1583–1590. [Google Scholar] [CrossRef]
- Ferri, E.; Marzetti, E.; Calvani, R.; Picca, A.; Cesari, M.; Arosio, B. Role of age-related mitochondrial dysfunction in sarcopenia. Int. J. Mol. Sci. 2020, 21, 5236. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Ni, W.; Bai, Y.; Yuan, X.; Zhang, Y.; Zhang, H.; Sun, Y.; Xu, J. Cross-sectional and longitudinal associations of apolipoprotein A1 and B with glycosylated hemoglobin in Chinese adults. Sci. Rep. 2022, 12, 2751. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Parise, A.; Meschi, T. Accounting gut microbiota as the mediator of beneficial effects of dietary (poly) phenols on skeletal muscle in aging. Nutrients 2023, 15, 2367. [Google Scholar] [CrossRef]
- Goates, S.; Du, K.; Arensberg, M.; Gaillard, T.; Guralnik, J.; Pereira, S.L. Economic impact of hospitalizations in US adults with sarcopenia. J. Frailty Aging 2019, 8, 93–99. [Google Scholar] [CrossRef]
- Dalle, S.; Rossmeislova, L.; Koppo, K. The role of inflammation in age-related sarcopenia. Front. Physiol. 2017, 8, 311540. [Google Scholar] [CrossRef]
- Bian, A.-L.; Hu, H.-Y.; Rong, Y.-D.; Wang, J.; Wang, J.-X.; Zhou, X.-Z. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur. J. Med. Res. 2017, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Schaap, L.A.; Pluijm, S.M.; Deeg, D.J.; Visser, M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am. J. Med. 2006, 119, 526.e9–526.e17. [Google Scholar] [CrossRef]
- Baylis, D.; Bartlett, D.B.; Syddall, H.E.; Ntani, G.; Gale, C.R.; Cooper, C.; Lord, J.M.; Sayer, A.A. Immune-endocrine biomarkers as predictors of frailty and mortality: A 10-year longitudinal study in community-dwelling older people. Age 2013, 35, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer. Res. 2003, 23, 363–398. [Google Scholar]
- Gorza, L.; Germinario, E.; Tibaudo, L.; Vitadello, M.; Tusa, C.; Guerra, I.; Bondì, M.; Salmaso, S.; Caliceti, P.; Vitiello, L. Chronic systemic curcumin administration antagonizes murine sarcopenia and presarcopenia. Int. J. Mol. Sci. 2021, 22, 11789. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, C.; Dai, C.; Zhang, Y.; Wang, K.; Gao, Z.; Chen, X.; Yang, X.; Sun, H.; Yao, X.; et al. Nutritional Strategies for Muscle Atrophy: Current Evidence and Underlying Mechanisms. Mol. Nutr. Food Res. 2024, 68, e2300347. [Google Scholar] [CrossRef]
- Das, S.; Preethi, B.; Kushwaha, S.; Shrivastava, R. Therapeutic strategies to modulate gut microbial health: Approaches for sarcopenia management. Histol. Histopathol. 2024, 18730. [Google Scholar] [CrossRef]
- Kawamori, T.; Lubet, R.; Steele, V.E.; Kelloff, G.J.; Kaskey, R.B.; Rao, C.V.; Reddy, B.S. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 1999, 59, 597–601. [Google Scholar] [PubMed]
- Asai, A.; Miyazawa, T. Occurrence of orally administered curcuminoid as glucuronide and glucuronide/sulfate conjugates in rat plasma. Life Sci. 2000, 67, 2785–2793. [Google Scholar] [CrossRef]
- Öner-İyidoğan, Y.; Tanrıkulu-Küçük, S.; Seyithanoğlu, M.; Koçak, H.; Doğru-Abbasoğlu, S.; Aydin, A.F.; Beyhan-Özdaş, Ş.; Yapişlar, H.; Koçak-Toker, N. Effect of curcumin on hepatic heme oxygenase 1 expression in high fat diet fed rats: Is there a triangular relationship? Can. J. Physiol. Pharmacol. 2014, 92, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Receno, C.N.; Liang, C.; Korol, D.L.; Atalay, M.; Heffernan, K.S.; Brutsaert, T.D.; DeRuisseau, K.C. Effects of Prolonged Dietary Curcumin Exposure on Skeletal Muscle Biochemical and Functional Responses of Aged Male Rats. Int. J. Mol. Sci. 2019, 5, 1178. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.J.; Yang, I.H.; Lin, Y.W.; Lin, J.N.; Wu, C.C.; Chiang, C.Y.; Lai, K.H.; Lin, F.H. Curcumin-Loaded Hydrophobic Surface-Modified Hydroxyapatite as an Antioxidant for Sarcopenia Prevention. Antioxid. 2021, 10, 616. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Yang, J.M.; Wu, Y.; Li, H.; Zhong, Y.B.; Luo, Y.; Xie, R.L. Curcumin-activated Wnt5a pathway mediates Ca(2+) channel opening to affect myoblast differentiation and skeletal muscle regeneration. J. Cachexia Sarcopenia Muscle 2024. [Google Scholar] [CrossRef]
- Sani, A.; Hasegawa, K.; Yamaguchi, Y.; Panichayupakaranant, P.; Pengjam, Y. Inhibitory effects of curcuminoids on dexamethasone-induced muscle atrophy in differentiation of C2C12 cells. Phytomed. Plus 2021, 1, 100012. [Google Scholar] [CrossRef]
- Sivertsen, H.; Bjørkløf, G.H.; Engedal, K.; Selbæk, G.; Helvik, A.S. Depression and Quality of Life in Older Persons: A Review. Dement. Geriatr. Cogn. Disord. 2015, 40, 311–339. [Google Scholar] [CrossRef]
- Michaud, C.M.; Murray, C.J.L.; Bloom, B.R. Burden of Disease—Implications for Future Research. JAMA 2001, 285, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- He, Z.F.; Tan, W.Y.; Ma, H.; Shuai, Y.; Shan, Z.; Zhai, J.; Qiu, Y.; Zeng, H.; Chen, X.L.; Wang, S.B.; et al. Prevalence and factors associated with depression and anxiety among older adults: A large-scale cross-sectional study in China. J. Affect. Disord. 2024, 346, 135–143. [Google Scholar] [CrossRef]
- Ferrari, A.J.; Somerville, A.J.; Baxter, A.J.; Norman, R.; Patten, S.B.; Vos, T.; Whiteford, H.A. Global variation in the prevalence and incidence of major depressive disorder: A systematic review of the epidemiological literature. Psychol. Med. 2013, 43, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Wegmann, E.; Yang, H.; Montag, C. Social Networks Use Disorder and Associations With Depression and Anxiety Symptoms: A Systematic Review of Recent Research in China. Front. Psychol. 2020, 11, 211. [Google Scholar] [CrossRef] [PubMed]
- Monroe, S.M.; Harkness, K.L. Major Depression and Its Recurrences: Life Course Matters. Annu. Rev. Clin. Psychol. 2022, 18, 329–357. [Google Scholar] [CrossRef]
- Bai, Y.; Cai, Y.; Chang, D.; Li, D.; Huo, X.; Zhu, T. Immunotherapy for depression: Recent insights and future targets. Pharmacol. Ther. 2024, 257, 108624. [Google Scholar] [CrossRef]
- Alexopoulos, G.S. Depression in the elderly. Lancet 2005, 365, 1961–1970. [Google Scholar] [CrossRef]
- Giovannini, S.; Onder, G.; van der Roest, H.G.; Topinkova, E.; Gindin, J.; Cipriani, M.C.; Denkinger, M.D.; Bernabei, R.; Liperoti, R. Use of antidepressant medications among older adults in European long-term care facilities: A cross-sectional analysis from the SHELTER study. BMC Geriatr. 2020, 20, 310. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.; Tam, W.W.; Zhang, M.W.; Ho, C.S.; Husain, S.F.; McIntyre, R.S.; Ho, R.C. IL-1β, IL-6, TNF- α and CRP in Elderly Patients with Depression or Alzheimer’s disease: Systematic Review and Meta-Analysis. Sci. Rep. 2018, 8, 12050. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ho, R.C.; Mak, A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: A meta-analysis and meta-regression. J. Affect. Disord. 2012, 139, 230–239. [Google Scholar] [CrossRef]
- Haapakoski, R.; Mathieu, J.; Ebmeier, K.P.; Alenius, H.; Kivimäki, M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun. 2015, 49, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Hannestad, J.; DellaGioia, N.; Bloch, M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: A meta-analysis. Neuropsychopharmacology 2011, 36, 2452–2459. [Google Scholar] [CrossRef] [PubMed]
- Kiraly, D.D.; Horn, S.R.; Van Dam, N.T.; Costi, S.; Schwartz, J.; Kim-Schulze, S.; Patel, M.; Hodes, G.E.; Russo, S.J.; Merad, M.; et al. Altered peripheral immune profiles in treatment-resistant depression: Response to ketamine and prediction of treatment outcome. Transl. Psychiatry 2017, 7, e1065. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Guo, Y.J.; Han, W.X.; Yang, M.Q.; Wen, L.P.; Wang, K.Y.; Jiang, P. Curcumin relieves depressive-like behaviors via inhibition of the NLRP3 inflammasome and kynurenine pathway in rats suffering from chronic unpredictable mild stress. Int. Immunopharmacol. 2019, 67, 138–144. [Google Scholar] [CrossRef]
- Yu, J.J.; Pei, L.B.; Zhang, Y.; Wen, Z.Y.; Yang, J.L. Chronic Supplementation of Curcumin Enhances the Efficacy of Antidepressants in Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. J. Clin. Psychopharmacol. 2015, 35, 406–410. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Z.; Gao, Z.; Xie, K.; Zhang, Q.; Jiang, H.; Pang, Q. Effects of curcumin on learning and memory deficits, BDNF, and ERK protein expression in rats exposed to chronic unpredictable stress. Behav. Brain Res. 2014, 271, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, K.; Sun, N.; Jin, X.L.; Kase, Y.; Takeda, S.; Maruyama, W.; Tabira, T. Saikokaryukotsuboreito, a herbal medicine, prevents chronic stress-induced dysfunction of glucocorticoid negative feedback system in rat brain. Pharmacol. Biochem. Behav. 2007, 86, 55–61. [Google Scholar] [CrossRef]
- Huang, Z.; Zhong, X.M.; Li, Z.Y.; Feng, C.R.; Pan, A.J.; Mao, Q.Q. Curcumin reverses corticosterone-induced depressive-like behavior and decrease in brain BDNF levels in rats. Neurosci. Lett. 2011, 493, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.M.; Pülschen, D.; Thome, J. The role of oxidative stress in depressive disorders. Curr. Pharm. Des. 2012, 18, 5890–5899. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.R.; Thakare, V.N.; Patil, S.R. Protective effect of curcumin on experimentally induced inflammation, hepatotoxicity and cardiotoxicity in rats: Evidence of its antioxidant property. Exp. Toxicol. Pathol. 2011, 63, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.Y.; Chen, W.F.; Zhou, B.; Yang, L.; Liu, Z.L. Inhibition of lipid peroxidation and protein oxidation in rat liver mitochondria by curcumin and its analogues. Biochim. Biophys. Acta 2006, 1760, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Al-Rubaei, Z.M.; Mohammad, T.U.; Ali, L.K. Effects of local curcumin on oxidative stress and total antioxidant capacity in vivo study. Pak. J. Biol. Sci. 2014, 17, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Gilhotra, N.; Dhingra, D. GABAergic and nitriergic modulation by curcumin for its antianxiety-like activity in mice. Brain Res. 2010, 1352, 167–175. [Google Scholar] [CrossRef]
- Głombik, K.; Stachowicz, A.; Trojan, E.; Ślusarczyk, J.; Suski, M.; Chamera, K.; Kotarska, K.; Olszanecki, R.; Basta-Kaim, A. Mitochondrial proteomics investigation of frontal cortex in an animal model of depression: Focus on chronic antidepressant drugs treatment. Pharmacol. Rep. 2018, 70, 322–330. [Google Scholar] [CrossRef]
- Wang, R.; Xu, Y.; Wu, H.L.; Li, Y.B.; Li, Y.H.; Guo, J.B.; Li, X.J. The antidepressant effects of curcumin in the forced swimming test involve 5-HT1 and 5-HT2 receptors. Eur. J. Pharmacol. 2008, 578, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.K.; Bhutani, M.K.; Bishnoi, M. Antidepressant activity of curcumin: Involvement of serotonin and dopamine system. Psychopharmacology 2008, 201, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, M.K.; Bishnoi, M.; Kulkarni, S.K. Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical changes. Pharmacol. Biochem. Behav. 2009, 92, 39–43. [Google Scholar] [CrossRef]
- Xu, Y.; Ku, B.S.; Yao, H.Y.; Lin, Y.H.; Ma, X.; Zhang, Y.H.; Li, X.J. Antidepressant effects of curcumin in the forced swim test and olfactory bulbectomy models of depression in rats. Pharmacol. Biochem. Behav. 2005, 82, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Lu, C.W.; Wang, C.C.; Wang, Y.C.; Wang, S.J. Curcumin inhibits glutamate release in nerve terminals from rat prefrontal cortex: Possible relevance to its antidepressant mechanism. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.; Boles, R.G. Comment on treatment of psychiatric illness in patients with mitochondrial disease. Psychosomatics 2011, 52, 497–498. [Google Scholar] [CrossRef]
- Sequeira, A.; Rollins, B.; Magnan, C.; van Oven, M.; Baldi, P.; Myers, R.M.; Barchas, J.D.; Schatzberg, A.F.; Watson, S.J.; Akil, H.; et al. Mitochondrial mutations in subjects with psychiatric disorders. PLoS ONE 2015, 10, e0127280. [Google Scholar] [CrossRef]
- Wang, X.; Muhammad, I.; Sun, X.; Han, M.; Hamid, S.; Zhang, X. Protective role of curcumin in ameliorating AFB(1)-induced apoptosis via mitochondrial pathway in liver cells. Mol. Biol. Rep. 2018, 45, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Tian, X.; Guo, Y.; Duan, W.; Bu, H.; Li, C. Activation of nuclear factor erythroid 2-related factor 2 cytoprotective signaling by curcumin protect primary spinal cord astrocytes against oxidative toxicity. Biol. Pharm. Bull. 2011, 34, 1194–1197. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Zhang, J. Curcumin in antidepressant treatments: An overview of potential mechanisms, pre-clinical/clinical trials and ongoing challenges. Basic. Clin. Pharmacol. Toxicol. 2020, 127, 243–253. [Google Scholar] [CrossRef]
- Varma, K.; Amalraj, A.; Divya, C.; Gopi, S. The Efficacy of the Novel Bioavailable Curcumin (Cureit) in the Management of Sarcopenia in Healthy Elderly Subjects: A Randomized, Placebo-Controlled, Double-Blind Clinical Study. J. Med. Food 2021, 24, 40–49. [Google Scholar] [CrossRef]
- Ghodsi, H.; Rahimi, H.R.; Aghili, S.M.; Saberi, A.; Shoeibi, A. Evaluation of curcumin as add-on therapy in patients with Parkinson’s disease: A pilot randomized, triple-blind, placebo-controlled trial. Clin. Neurol. Neurosurg. 2022, 218, 107300. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.H.; Pipingas, A.; Scholey, A.B. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol. 2015, 29, 642–651. [Google Scholar] [CrossRef]
- Thota, R.N.; Acharya, S.H.; Garg, M.L. Curcumin and/or omega-3 polyunsaturated fatty acids supplementation reduces insulin resistance and blood lipids in individuals with high risk of type 2 diabetes: A randomised controlled trial. Lipids Health Dis. 2019, 18, 31. [Google Scholar] [CrossRef]
- DiSilvestro, R.A.; Joseph, E.; Zhao, S.; Bomser, J. Diverse effects of a low dose supplement of lipidated curcumin in healthy middle aged people. Nutr. J. 2012, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Baum, L.; Cheung, S.K.; Mok, V.C.; Lam, L.C.; Leung, V.P.; Hui, E.; Ng, C.C.; Chow, M.; Ho, P.C.; Lam, S.; et al. Curcumin effects on blood lipid profile in a 6-month human study. Pharmacol. Res. 2007, 56, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.; Das, S.S.; Kannan, R.; Swick, A.G.; Matthewman, C.; Maliakel, B.; Ittiyavirah, S.P.; Krishnakumar, I.M. The effects of oral administration of curcumin-galactomannan complex on brain waves are consistent with brain penetration: A randomized, double-blinded, placebo-controlled pilot study. Nutr. Neurosci. 2022, 25, 1240–1249. [Google Scholar] [CrossRef]
- Cox, K.H.M.; White, D.J.; Pipingas, A.; Poorun, K.; Scholey, A. Further Evidence of Benefits to Mood and Working Memory from Lipidated Curcumin in Healthy Older People: A 12-Week, Double-Blind, Placebo-Controlled, Partial Replication Study. Nutrients 2020, 12, 1678. [Google Scholar] [CrossRef]
- Kuszewski, J.C.; Wong, R.H.X.; Wood, L.G.; Howe, P.R.C. Effects of fish oil and curcumin supplementation on cerebrovascular function in older adults: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Small, G.W.; Siddarth, P.; Li, Z.; Miller, K.J.; Ercoli, L.; Emerson, N.D.; Martinez, J.; Wong, K.P.; Liu, J.; Merrill, D.A.; et al. Memory and Brain Amyloid and Tau Effects of a Bioavailable Form of Curcumin in Non-Demented Adults: A Double-Blind, Placebo-Controlled 18-Month Trial. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2018, 26, 266–277. [Google Scholar] [CrossRef]
- Ross, S.M. Curcuma longa (Theracumin®): A Bioavailable Form of Curcumin and Its Cognitive Benefits. Holist. Nurs. Pr. Pract. 2018, 32, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Badeli, R.; Karami, G.R.; Sahebkar, A. Investigation of the efficacy of adjunctive therapy with bioavailability-boosted curcuminoids in major depressive disorder. Phytother. Res. 2015, 29, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Rainey-Smith, S.R.; Brown, B.M.; Sohrabi, H.R.; Shah, T.; Goozee, K.G.; Gupta, V.B.; Martins, R.N. Curcumin and cognition: A randomised, placebo-controlled, double-blind study of community-dwelling older adults. Br. J. Nutr. 2016, 115, 2106–2113. [Google Scholar] [CrossRef]
- Yaikwawong, M.; Jansarikit, L.; Jirawatnotai, S.; Chuengsamarn, S. The Effect of Curcumin on Reducing Atherogenic Risks in Obese Patients with Type 2 Diabetes: A Randomized Controlled Trial. Nutrients 2024, 16, 2441. [Google Scholar] [CrossRef]
- Kucukgoncu, S.; Guloksuz, S.; Tek, C. Effects of Curcumin on Cognitive Functioning and Inflammatory State in Schizophrenia: A Double-Blind, Placebo-Controlled Pilot Trial. J. Clin. Psychopharmacol. 2019, 39, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Harsha, C.; Banik, K.; Vikkurthi, R.; Sailo, B.L.; Bordoloi, D.; Gupta, S.C.; Aggarwal, B.B. Is curcumin bioavailability a problem in humans: Lessons from clinical trials. Expert. Opin. Drug Metab. Toxicol. 2019, 15, 705–733. [Google Scholar] [CrossRef] [PubMed]
- Vashisht, M.; Rani, P.; Onteru, S.K.; Singh, D. Curcumin Encapsulated in Milk Exosomes Resists Human Digestion and Possesses Enhanced Intestinal Permeability in Vitro. Appl. Biochem. Biotechnol. 2017, 183, 993–1007. [Google Scholar] [CrossRef]
- Marton, L.T.; Pescinini, E.S.L.M.; Camargo, M.E.C.; Barbalho, S.M.; Haber, J.; Sinatora, R.V.; Detregiachi, C.R.P.; Girio, R.J.S.; Buchaim, D.V.; Cincotto Dos Santos Bueno, P. The Effects of Curcumin on Diabetes Mellitus: A Systematic Review. Front. Endocrinol. 2021, 12, 669448. [Google Scholar] [CrossRef]
- Akuri, M.C.; Barbalho, S.M.; Val, R.M.; Guiguer, E.L. Reflections about Osteoarthritis and Curcuma longa. Pharmacogn. Rev. 2017, 11, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Gopi, S.; Jacob, J.; Varma, K.; Jude, S.; Amalraj, A.; Arundhathy, C.A.; George, R.; Sreeraj, T.R.; Divya, C.; Kunnumakkara, A.B.; et al. Comparative Oral Absorption of Curcumin in a Natural Turmeric Matrix with Two Other Curcumin Formulations: An Open-label Parallel-arm Study. Phytother. Res. 2017, 31, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Kocher, A.; Bohnert, L.; Schiborr, C.; Frank, J. Highly bioavailable micellar curcuminoids accumulate in blood, are safe and do not reduce blood lipids and inflammation markers in moderately hyperlipidemic individuals. Mol. Nutr. Food Res. 2016, 60, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Yang, T.; Yang, K.; Yu, G.; Li, J.; Xiang, W.; Chen, H. Efficacy and Safety of Curcumin and Curcuma longa Extract in the Treatment of Arthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trial. Front. Immunol. 2022, 13, 891822. [Google Scholar] [CrossRef] [PubMed]
- Shep, D.; Khanwelkar, C.; Gade, P.; Karad, S. Safety and efficacy of curcumin versus diclofenac in knee osteoarthritis: A randomized open-label parallel-arm study. Trials 2019, 20, 214. [Google Scholar] [CrossRef] [PubMed]
- Pivari, F.; Mingione, A.; Piazzini, G.; Ceccarani, C.; Ottaviano, E.; Brasacchio, C.; Dei Cas, M.; Vischi, M.; Cozzolino, M.G.; Fogagnolo, P.; et al. Curcumin Supplementation (Meriva®) Modulates Inflammation, Lipid Peroxidation and Gut Microbiota Composition in Chronic Kidney Disease. Nutrients 2022, 14, 231. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendía, L.E.; Sahebkar, A. Efficacy and Safety of Phytosomal Curcumin in Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Drug Res. 2017, 67, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vécsei, L. A Decade of Dedication: Pioneering Perspectives on Neurological Diseases and Mental Illnesses. Biomedicines 2024, 12, 1083. [Google Scholar] [CrossRef]
- Tanaka, M.; Battaglia, S.; Giménez-Llort, L.; Chen, C.; Hepsomali, P.; Avenanti, A.; Vécsei, L. Innovation at the intersection: Emerging translational research in neurology and psychiatry. Cells 2024, 13, 790. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. From Lab to Life: Exploring Cutting-Edge Models for Neurological and Psychiatric Disorders. Biomedicines 2024, 12, 613. [Google Scholar] [CrossRef]
- Tajti, J.; Szok, D.; Csáti, A.; Szabó, Á.; Tanaka, M.; Vécsei, L. Exploring novel therapeutic targets in the common pathogenic factors in migraine and neuropathic pain. Int. J. Mol. Sci. 2023, 24, 4114. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Török, N.; Tóth, F.; Szabó, Á.; Vécsei, L. Co-players in chronic pain: Neuroinflammation and the tryptophan-kynurenine metabolic pathway. Biomedicines 2021, 9, 897. [Google Scholar] [CrossRef]
- Battaglia, S.; Schmidt, A.; Hassel, S.; Tanaka, M. Case reports in neuroimaging and stimulation. Front. Psychiatry 2023, 14, 1264669. [Google Scholar] [CrossRef] [PubMed]
- Balogh, L.; Tanaka, M.; Török, N.; Vécsei, L.; Taguchi, S. Crosstalk between existential phenomenological psychotherapy and neurological sciences in mood and anxiety disorders. Biomedicines 2021, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Jászberényi, M.; Thurzó, B.; Bagosi, Z.; Vécsei, L.; Tanaka, M. The Orexin/Hypocretin System, the Peptidergic Regulator of Vigilance, Orchestrates Adaptation to Stress. Biomedicines 2024, 12, 448. [Google Scholar] [CrossRef]
- Tanaka, M.; Diano, M.; Battaglia, S. Insights into structural and functional organization of the brain: Evidence from neuroimaging and non-invasive brain stimulation techniques. Front. Psychiatry 2023, 14, 1225755. [Google Scholar] [CrossRef]
- Liloia, D.; Zamfira, D.A.; Tanaka, M.; Manuello, J.; Crocetta, A.; Keller, R.; Cozzolino, M.; Duca, S.; Cauda, F.; Costa, T. Disentangling the role of gray matter volume and concentration in autism spectrum disorder: A meta-analytic investigation of 25 years of voxel-based morphometry research. Neurosci. Biobehav. Rev. 2024, 164, 105791. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Körtési, T.; Szok, D.; Tajti, J.; Vécsei, L. From CGRP to PACAP, VIP, and beyond: Unraveling the next chapters in migraine treatment. Cells 2023, 12, 2649. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Vécsei, L. Preclinical modeling in depression and anxiety: Current challenges and future research directions. Adv. Clin. Exp. Med. 2023, 32, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Polyák, H.; Galla, Z.; Nánási, N.; Cseh, E.K.; Rajda, C.; Veres, G.; Spekker, E.; Szabó, Á.; Klivényi, P.; Tanaka, M. The tryptophan-kynurenine metabolic system is suppressed in cuprizone-induced model of demyelination simulating progressive multiple sclerosis. Biomedicines 2023, 11, 945. [Google Scholar] [CrossRef]
- Török, N.; Maszlag-Török, R.; Molnár, K.; Szolnoki, Z.; Somogyvári, F.; Boda, K.; Tanaka, M.; Klivényi, P.; Vécsei, L. Single Nucleotide Polymorphisms of Indoleamine 2, 3-Dioxygenase1 Influenced the Age Onset of Parkinson’s Disease. Front. Biosci. 2022, 27, 265. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabó, Á.; Vécsei, L.; Giménez-Llort, L. Emerging Translational Research in Neurological and Psychiatric Diseases: From In Vitro to In Vivo Models. Int. J. Mol. Sci. 2023, 24, 15739. [Google Scholar] [CrossRef] [PubMed]

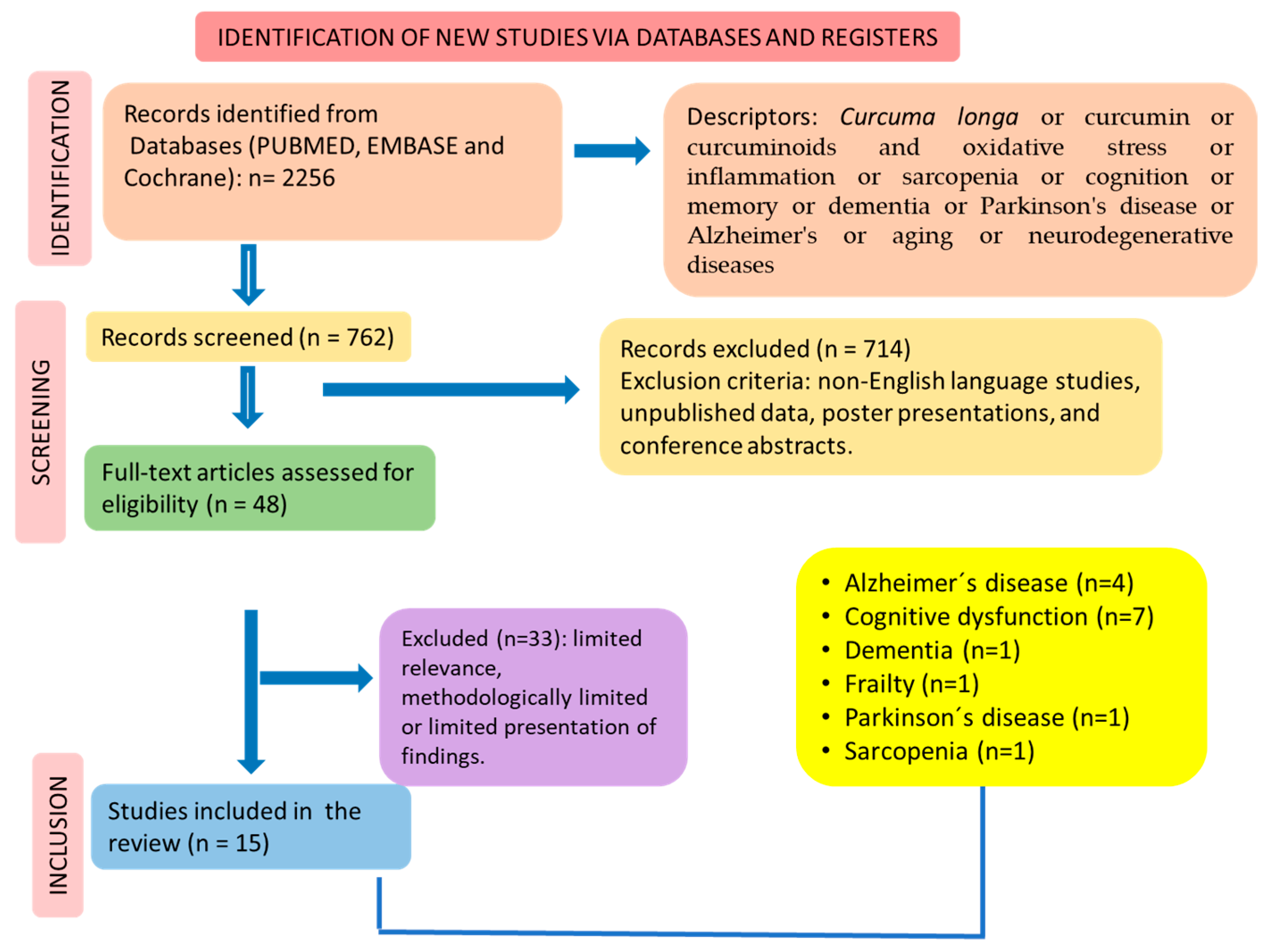
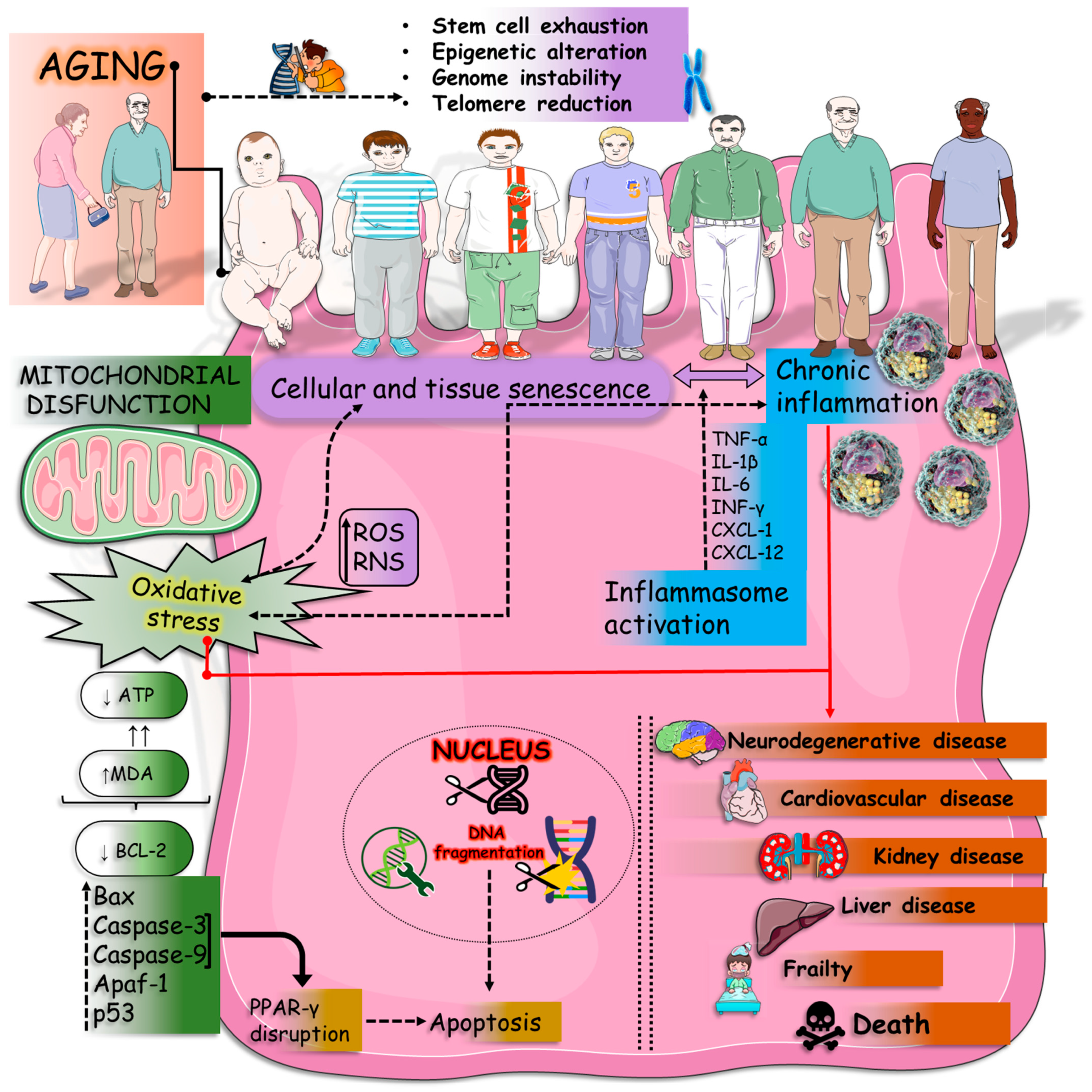
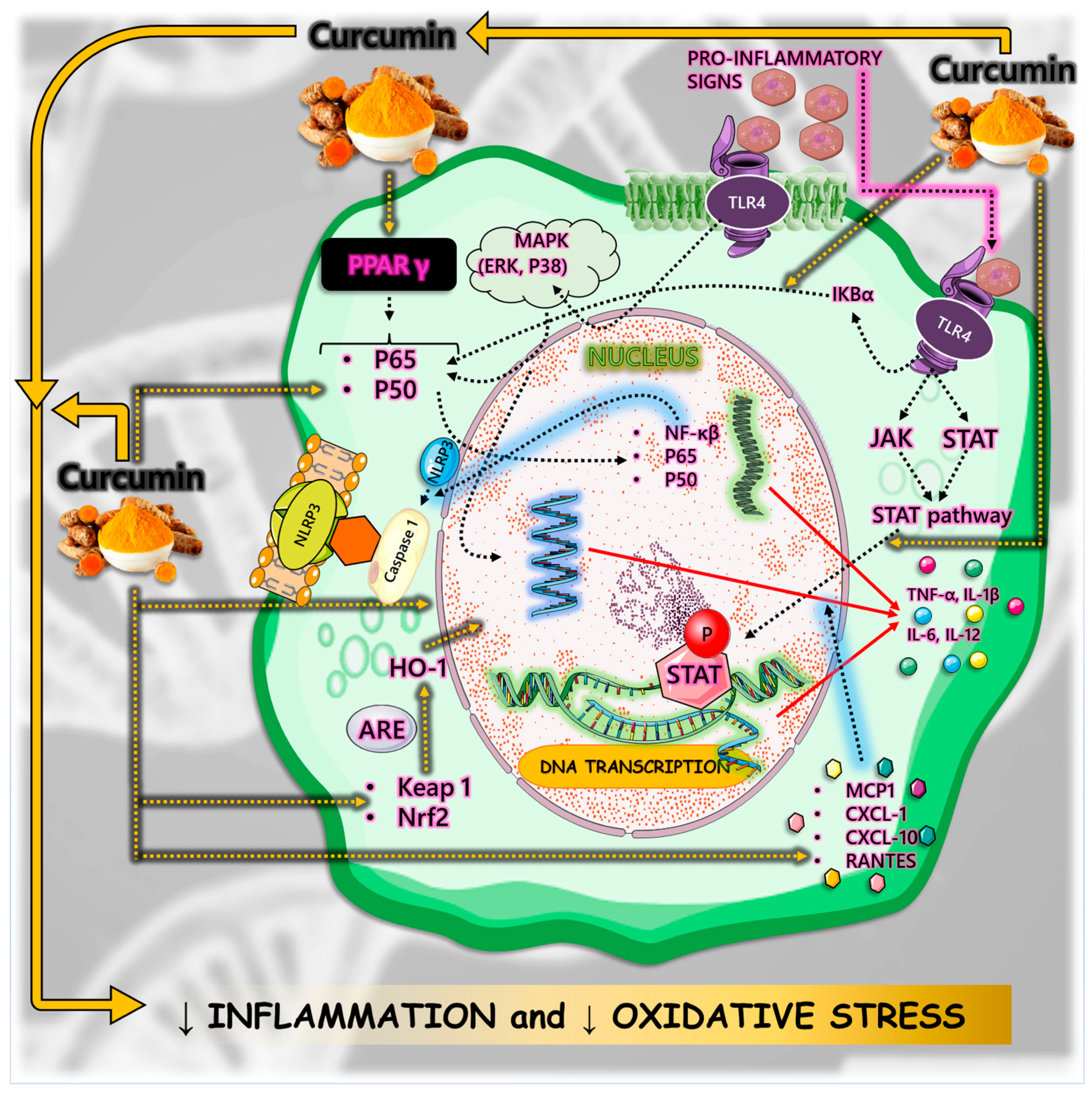
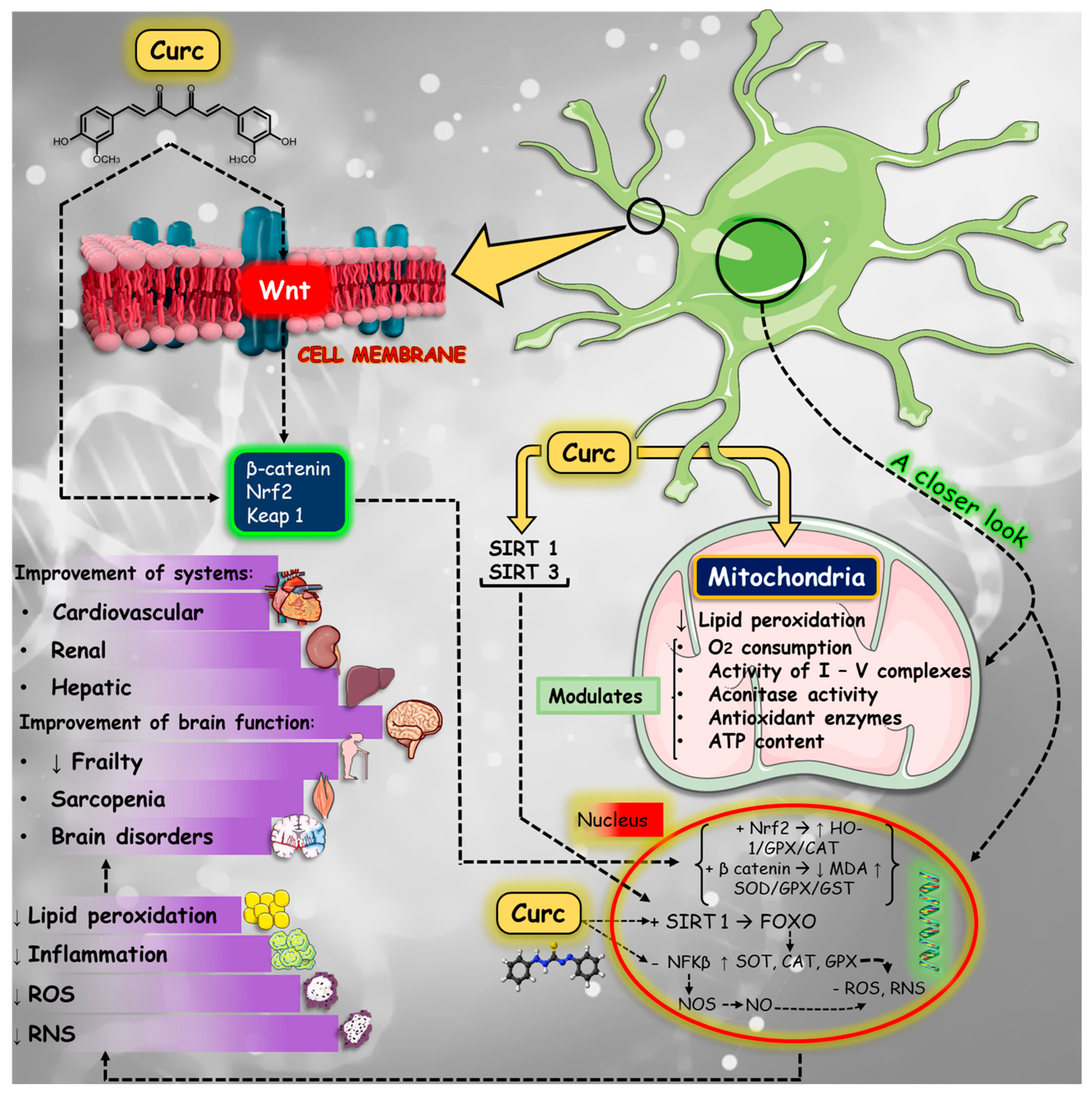
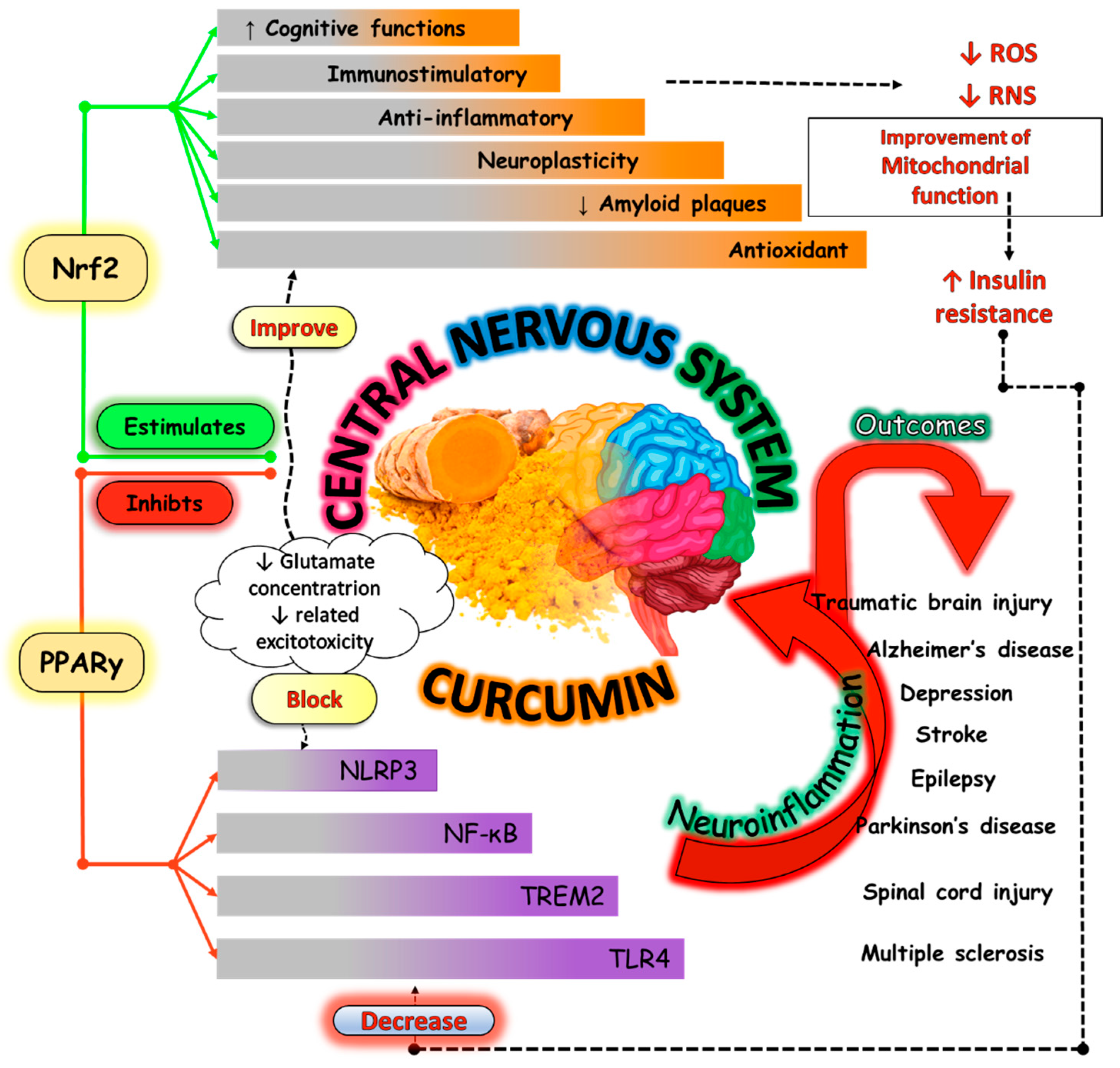

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, Y.C.; Mendes, N.M.; Pereira de Lima, E.; Chehadi, A.C.; Lamas, C.B.; Haber, J.F.S.; dos Santos Bueno, M.; Araújo, A.C.; Catharin, V.C.S.; Detregiachi, C.R.P.; et al. Curcumin: A Golden Approach to Healthy Aging: A Systematic Review of the Evidence. Nutrients 2024, 16, 2721. https://doi.org/10.3390/nu16162721
Nunes YC, Mendes NM, Pereira de Lima E, Chehadi AC, Lamas CB, Haber JFS, dos Santos Bueno M, Araújo AC, Catharin VCS, Detregiachi CRP, et al. Curcumin: A Golden Approach to Healthy Aging: A Systematic Review of the Evidence. Nutrients. 2024; 16(16):2721. https://doi.org/10.3390/nu16162721
Chicago/Turabian StyleNunes, Yandra Cervelim, Nathalia M. Mendes, Enzo Pereira de Lima, Amanda Chabrour Chehadi, Caroline Barbalho Lamas, Jesselina F. S. Haber, Manoela dos Santos Bueno, Adriano Cressoni Araújo, Vitor C. Strozze Catharin, Claudia Rucco P. Detregiachi, and et al. 2024. "Curcumin: A Golden Approach to Healthy Aging: A Systematic Review of the Evidence" Nutrients 16, no. 16: 2721. https://doi.org/10.3390/nu16162721
APA StyleNunes, Y. C., Mendes, N. M., Pereira de Lima, E., Chehadi, A. C., Lamas, C. B., Haber, J. F. S., dos Santos Bueno, M., Araújo, A. C., Catharin, V. C. S., Detregiachi, C. R. P., Laurindo, L. F., Tanaka, M., Barbalho, S. M., & Marin, M. J. S. (2024). Curcumin: A Golden Approach to Healthy Aging: A Systematic Review of the Evidence. Nutrients, 16(16), 2721. https://doi.org/10.3390/nu16162721












