Protein Supplementation Increases Adaptations to Low-Volume, Intra-Session Concurrent Training in Untrained Healthy Adults: A Double-Blind, Placebo-Controlled, Randomized Trial
Abstract
1. Introduction
2. Materials and Methods
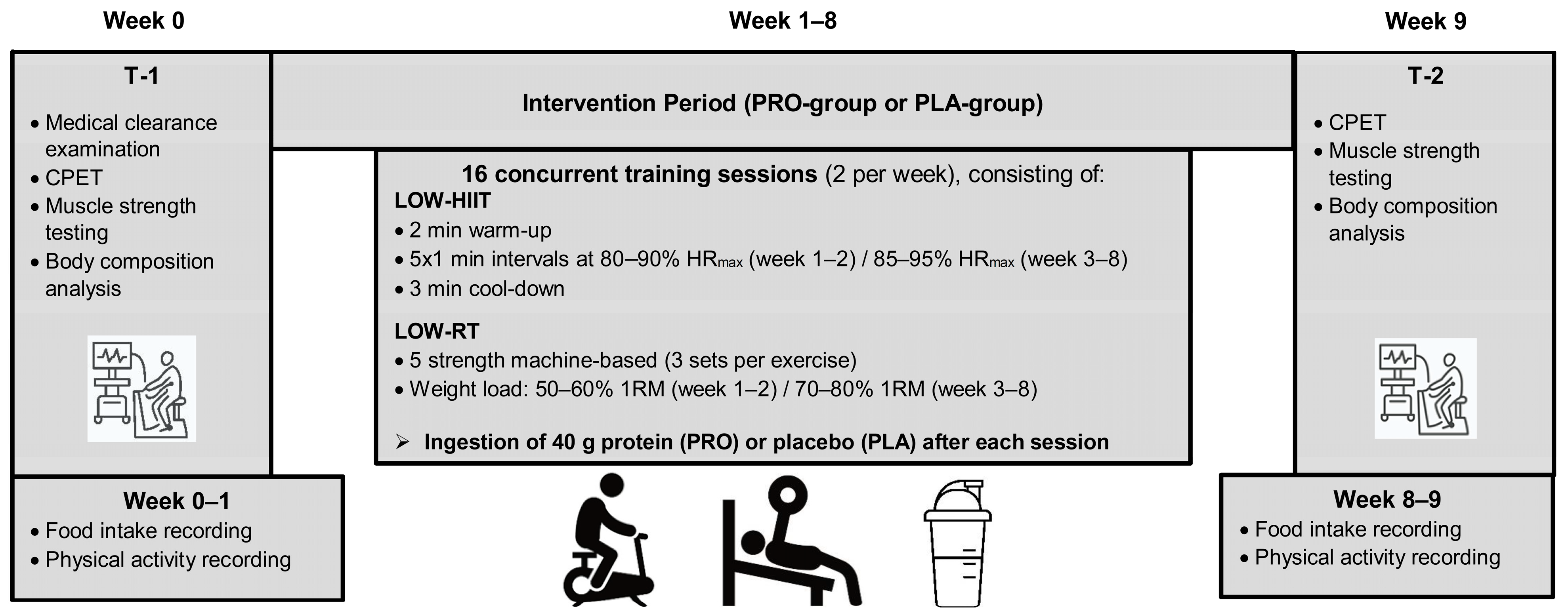
2.1. Design of the Study
2.2. Study Volunteers
2.3. Outcome Measurements
2.3.1. Body Composition Measurements
2.3.2. Cardiopulmonary Exercise Test (CPET)
2.3.3. Determination of One-Repetition Maximum Strength and Overall Fitness Z Score
2.4. Daily Nutrition and Physical Activity Monitoring
2.5. Concurrent Training Program
2.6. Supplementation
2.7. Statistical Analysis
3. Results
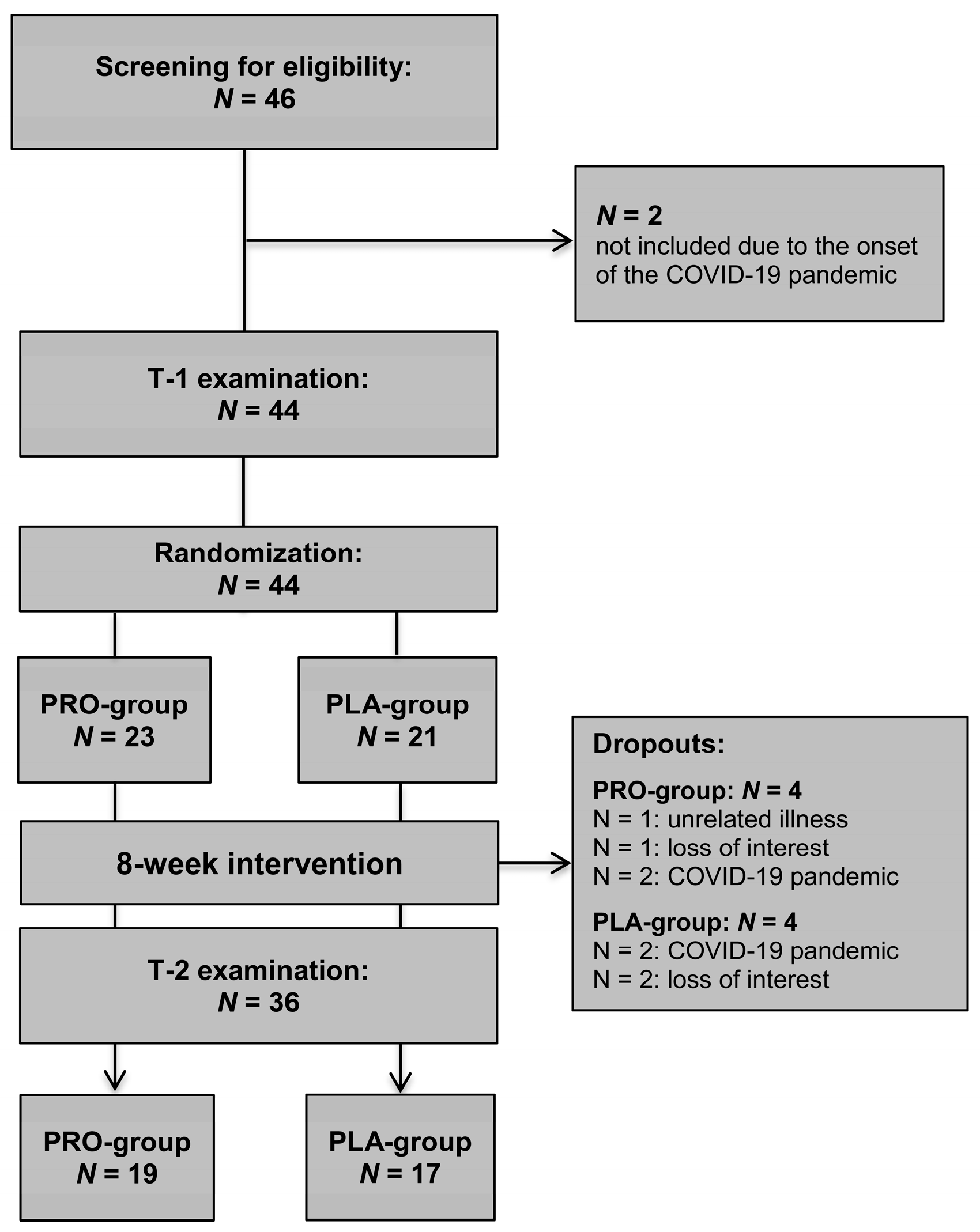
3.1. Study Flow
3.2. Training Data, Adverse Events, and Volunteers’ Evaluations
3.3. Nutritional Intake and Daily Physical Activity
3.4. Anthropometric Data
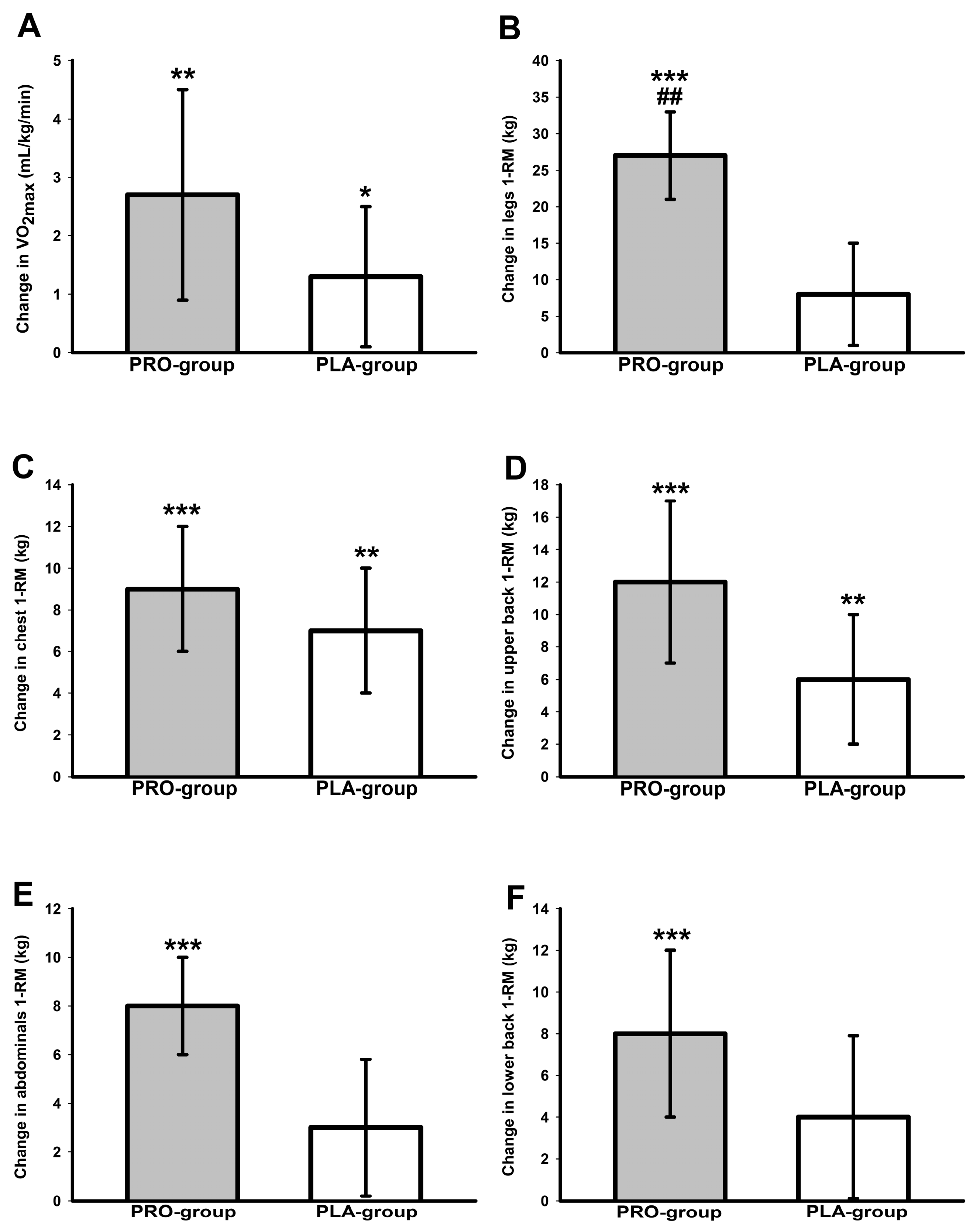
3.5. Cardiorespiratory Fitness Data
3.6. One-Repetition Maximum Strength Data
3.7. Overall Fitness Z Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Appelqvist-Schmidlechner, K.; Vaara, J.P.; Vasankari, T.; Häkkinen, A.; Mäntysaari, M.; Kyröläinen, H. Muscular and cardiorespiratory fitness are associated with health-related quality of life among young adult men. BMC Public Health 2020, 20, 842. [Google Scholar] [CrossRef] [PubMed]
- Franklin, B.A.; Wedig, I.J.; Sallis, R.E.; Lavie, C.J.; Elmer, S.J. Physical activity and cardiorespiratory fitness as modulators of health outcomes: A compelling research-based case presented to the medical community. Mayo Clin. Proc. 2023, 98, 316–331. [Google Scholar] [CrossRef]
- Kokkinos, P.; Faselis, C.; Samuel, I.B.H.; Pittaras, A.; Doumas, M.; Murphy, R.; Heimall, M.S.; Sui, X.; Zhang, J.; Myers, J. Cardiorespiratory fitness and mortality risk across the spectra of age, race, and sex. J. Am. Coll. Cardiol. 2022, 80, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Onerup, A.; Mehlig, K.; Geijerstam, A.A.; Eblom-Bak, E.; Kuhn, H.G.; Lissner, L.; Åberg, M.; Börjesson, M. Associations between cardiorespiratory fitness in youth and the incidence of site-specific cancer in men: A cohort study with register linkage. Br. J. Sports Med. 2023, 57, 1248–1256. [Google Scholar] [CrossRef]
- Bennie, J.A.; Shakespear-Druery, J.; De Cocker, K. Muscle-strengthening exercise epidemiology: A new frontier in chronic disease prevention. Sports Med. Open 2020, 6, 40. [Google Scholar] [CrossRef]
- Giovannucci, E.L.; Rezende, L.F.M.; Lee, D.H. Muscle-strengthening activities and risk of cardiovascular disease, type 2 diabetes, cancer and mortality: A review of prospective cohort studies. J. Intern. Med. 2021, 290, 789–805. [Google Scholar] [CrossRef]
- Myers, J.; McAuley, P.; Lavie, C.J.; Despres, J.P.; Arena, R.; Kokkinos, P. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk: Their independent and interwoven importance to health status. Prog. Cardiovasc. Dis. 2015, 57, 306–314. [Google Scholar] [CrossRef]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Després, J.P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. Importance of assessing cardiorespiratory fitness in clinical practice: A case for fitness as a clinical vital sign: A scientific statement from the American Heart Association. Circulation 2016, 134, e653–e699. [Google Scholar] [CrossRef] [PubMed]
- García-Hermoso, A.; Cavero-Redondo, I.; Ramírez-Vélez, R.; Ruiz, J.R.; Ortega, F.B.; Lee, D.C.; Martínez-Vizcaíno, V. Muscular strength as a predictor of all-cause mortality in an apparently healthy population: A systematic review and meta-analysis of data from approximately 2 million men and women. Arch. Phys. Med. Rehabil. 2018, 99, 2100–2113.e5. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P.; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 34, 1334–1359. [Google Scholar] [CrossRef]
- World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Cadore, E.L.; Izquierdo, M. How to simultaneously optimize muscle strength, power, functional capacity, and cardiovascular gains in the elderly: An update. Age 2013, 35, 2329–2344. [Google Scholar] [CrossRef]
- Chtara, M.; Chaouachi, A.; Levin, G.T.; Chaouachi, M.; Chamari, K.; Amri, M.; Laursen, P.B. Effect of concurrent endurance and circuit resistance training sequence on muscular strength and power development. J. Strength Cond. Res. 2008, 22, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Berryman, N.; Mujika, I.; Bosquet, L. Concurrent training for sports performance: The 2 sides of the medal. Int. J. Sports Physiol. Perform. 2019, 14, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, J.J.; Bishop, D.J.; Stepto, N.K. Interference between concurrent resistance and endurance exercise: Molecular bases and the role of individual training variables. Sports Med. 2014, 44, 743–762. [Google Scholar] [CrossRef] [PubMed]
- Knuiman, P.; Hopman, M.T.; Mensink, M. Glycogen availability and skeletal muscle adaptations with endurance and resistance exercise. Nutr. Metab. 2015, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Coffey, V.G.; Hawley, J.A. Concurrent exercise training: Do opposites distract? J. Physiol. 2017, 595, 2883–2896. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, T.R.; Feuerbacher, J.F.; Sünkeler, M.; Schumann, M. The effects of concurrent aerobic and strength training on muscle fiber hypertrophy: A systematic review and meta-analysis. Sports Med. 2022, 52, 2391–2403. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Marin, P.J.; Rhea, M.R.; Wilson, S.M.; Loenneke, J.P.; Anderson, J.C. Concurrent training: A meta-analysis examining interference of aerobic and resistance exercises. J. Strength Cond. Res. 2012, 26, 2293–2307. [Google Scholar] [CrossRef] [PubMed]
- Hickson, R.C. Interference of strength development by simultaneously training for strength and endurance. Eur. J. Appl. Physiol. Occup. Physiol. 1980, 45, 255–263. [Google Scholar] [CrossRef]
- Huiberts, R.O.; Wüst, R.C.; van der Zwaard, S. Concurrent strength and endurance training: A systematic review and meta-analysis on the impact of sex and training status. Sports Med. 2024, 54, 485–503. [Google Scholar] [CrossRef]
- Perez-Schindler, J.; Hamilton, D.L.; Moore, D.R.; Baar, K.; Philp, A. Nutritional strategies to support concurrent training. Eur. J. Sport Sci. 2015, 15, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Camera, D.M. Evaluating the effects of increased protein intake on muscle strength, hypertrophy and power adaptations with concurrent training: A narrative review. Sports Med. 2022, 52, 441–461. [Google Scholar] [CrossRef] [PubMed]
- Chapman, S.; Chung, H.C.; Rawcliffe, A.J.; Izard, R.; Smith, L.; Roberts, J.D. Does protein supplementation support adaptations to arduous concurrent exercise training? A systematic review and meta-analysis with military based applications. Nutrients 2021, 13, 1416. [Google Scholar] [CrossRef] [PubMed]
- Hartono, F.A.; Martin-Arrowsmith, P.W.; Peeters, W.M.; Churchward-Venne, T.A. The effects of dietary protein supplementation on acute changes in muscle protein synthesis and longer-term changes in muscle mass, strength, and aerobic capacity in response to concurrent resistance and endurance exercise in healthy adults: A systematic review. Sports Med. 2022, 52, 1295–1328. [Google Scholar] [CrossRef]
- Knuiman, P.; Hopman, M.T.; Verbruggen, C.; Mensink, M. Protein and the adaptive response with endurance training: Wishful thinking or a competitive edge? Front Physiol. 2018, 9, 598. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Sanders, M.S.; Ehler, L.A.; Uelmen, J.; Raether, J.B.; Stout, J.R. Effects of exercise training and amino-acid supplementation on body composition and physical performance in un-trained women. Nutrition 2000, 16, 1043–1046. [Google Scholar] [CrossRef]
- Arciero, P.J.; Ives, S.J.; Norton, C.; Escudero, D.; Minicucci, O.; O’Brien, G.; Paul, M.; Ormsbee, M.J.; Miller, V.; Sheridan, C.; et al. Protein-pacing and multi-component exercise training improves physical performance outcomes in exercise-trained women: The PRISE 3 Study. Nutrients 2016, 8, 332. [Google Scholar] [CrossRef]
- Beelen, M.; Tieland, M.; Gijsen, A.P.; Vandereyt, H.; Kies, A.K.; Kuipers, H.; Saris, W.H.; Koopman, R.; van Loon, L.J. Coingestion of carbohydrate and protein hydrolysate stimulates muscle protein synthesis during exercise in young men, with no further increase during subsequent overnight recovery. J. Nutr. 2008, 138, 2198–2204. [Google Scholar] [CrossRef]
- Camera, D.M.; West, D.W.; Phillips, S.M.; Rerecich, T.; Stellingwerff, T.; Hawley, J.A.; Coffey, V.G. Protein ingestion increases myofibrillar protein synthesis after concurrent exercise. Med. Sci. Sports Exerc. 2015, 47, 82–91. [Google Scholar] [CrossRef]
- Camera, D.M.; West, D.W.; Burd, N.A.; Phillips, S.M.; Garnham, A.P.; Hawley, J.A.; Coffey, V.G. Low muscle glycogen concentration does not suppress the anabolic response to resistance exercise. J. Appl. Physiol. 2012, 113, 206–214. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Pinckaers, P.J.M.; Smeets, J.S.J.; Peeters, W.M.; Zorenc, A.H.; Schierbeek, H.; Rollo, I.; Verdijk, L.B.; van Loon, L.J.C. Myofibrillar and mitochondrial protein synthesis rates do not differ in young men following the ingestion of carbohydrate with milk protein, whey, or micellar casein after concurrent resistance- and endurance-type exercise. J. Nutr. 2019, 149, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Crowe, M.J.; Weatherson, J.N.; Bowden, B.F. Effects of dietary leucine supplementation on exercise performance. Eur. J. Appl. Physiol. 2006, 97, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Dicks, N.D.; Kotarsky, C.J.; Trautman, K.A.; Barry, A.M.; Keith, J.F.; Mitchell, S.; Byun, W.; Stastny, S.N.; Hackney, K.J. Contribution of protein intake and concurrent exercise to skeletal muscle quality with aging. J. Frailty Aging 2020, 9, 51–56. [Google Scholar] [CrossRef]
- Eddens, L.; Browne, S.; Stevenson, E.J.; Sanderson, B.; van Someren, K.; Howatson, G. The efficacy of protein supplementation during recovery from muscle-damaging concurrent exercise. Appl. Physiol. Nutr. Metab. 2017, 42, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.C.; Bell, G.J. Whey protein isolate or concentrate combined with concurrent training does not augment performance, cardiorespiratory fitness, or strength adaptations. J. Sports Med. Phys. Fit. 2020, 60, 832–840. [Google Scholar] [CrossRef]
- Ives, S.J.; Norton, C.; Miller, V.; Minicucci, O.; Robinson, J.; O’Brien, G.; Escudero, D.; Paul, M.; Sheridan, C.; Curran, K.; et al. Multi-modal exercise training and protein-pacing enhances physical performance adaptations independent of growth hor-mone and BDNF but may be dependent on IGF-1 in exercise-trained men. Growth Horm. IGF Res. 2017, 32, 60–70. [Google Scholar] [CrossRef]
- Jendricke, P.; Kohl, J.; Centner, C.; Gollhofer, A.; König, D. Influence of specific collagen peptides and concurrent training on cardiometabolic parameters and performance indices in women: A randomized controlled trial. Front. Nutr. 2020, 7, 580918. [Google Scholar] [CrossRef]
- Knuiman, P.; Hopman, M.T.E.; Hangelbroek, R.; Mensink, M. Plasma cytokine responses to re-sistance exercise with different nutrient availability on a concurrent exercise day in trained healthy males. Physiol. Rep. 2018, 6, e13708. [Google Scholar] [CrossRef]
- Lee, M.J.; Ballantyne, J.K.; Chagolla, J.; Hopkins, W.G.; Fyfe, J.J.; Phillips, S.M.; Bishop, D.J.; Bartlett, J.D. Order of same-day concurrent training influences some indices of power development, but not strength, lean mass, or aerobic fitness in healthy, moderately-active men after 9 weeks of training. PLoS ONE 2020, 15, e0233134. [Google Scholar] [CrossRef]
- Longland, T.M.; Oikawa, S.Y.; Mitchell, C.J.; Devries, M.C.; Phillips, S.M. Higher compared with lower dietary protein during an energy deficit combined with intense exercise pro-motes greater lean mass gain and fat mass loss: A randomized trial. Am. J. Clin. Nutr. 2016, 103, 738–746. [Google Scholar] [CrossRef]
- McAdam, J.S.; McGinnis, K.D.; Beck, D.T.; Haun, C.T.; Romero, M.A.; Mumford, P.W.; Roberson, P.A.; Young, K.C.; Lohse, K.R.; Lockwood, C.M.; et al. Effect of whey protein supplementation on physical performance and body composition in army initial entry training soldiers. Nutrients 2018, 10, 1248. [Google Scholar] [CrossRef] [PubMed]
- Parr, E.B.; Camera, D.M.; Areta, J.L.; Burke, L.M.; Phillips, S.M.; Hawley, J.A.; Coffey, V.G. Alcohol in-gestion impairs maximal post-exercise rates of myofibrillar protein synthesis following a single bout of concurrent training. PLoS ONE 2014, 9, e88384. [Google Scholar] [CrossRef]
- Pasiakos, S.M.; Cao, J.J.; Margolis, L.M.; Sauter, E.R.; Whigham, L.D.; McClung, J.P.; Rood, J.C.; Carbone, J.W.; Combs, G.F., Jr.; Young, A.J. Effects of high-protein diets on fat-free mass and muscle protein synthesis following weight loss: A randomized controlled trial. FASEB J. 2013, 27, 3837–3847. [Google Scholar] [CrossRef]
- Shamim, B.; Devlin, B.L.; Timmins, R.G.; Tofari, P.; Lee Dow, C.; Coffey, V.G.; Hawley, J.A.; Camera, D.M. Adaptations to concurrent training in combination with high protein availability: A comparative trial in healthy, recreationally active men. Sports Med. 2018, 48, 2869–2883. [Google Scholar] [CrossRef]
- Taylor, L.W.; Wilborn, C.; Roberts, M.D.; White, A.; Dugan, K. Eight weeks of pre- and postexercise whey protein supplementation increases lean body mass and improves per-formance in Division III collegiate female basketball players. Appl. Physiol. Nutr. Metab. 2016, 41, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.B.; Smith, J.; Herrera, M.; Lebegue, B.; Pinchak, A.; Fischer, J. The influence of 8 weeks of whey-protein and leucine supplementation on physical and cognitive performance. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 409–417. [Google Scholar] [CrossRef]
- Cronin, O.; Barton, W.; Skuse, P.; Penney, N.C.; Garcia-Perez, I.; Murphy, E.F.; Woods, T.; Nugent, H.; Fanning, A.; Melgar, S.; et al. A prospective metagenomic and metabolomic analysis of the impact of exercise and/or whey protein supplementation on the gut microbiome of sedentary adults. mSystems 2018, 3, e00044-18. [Google Scholar] [CrossRef] [PubMed]
- Donges, C.E.; Burd, N.A.; Duffield, R.; Smith, G.C.; West, D.W.; Short, M.J.; Mackenzie, R.; Plank, L.D.; Shepherd, P.R.; Phillips, S.M.; et al. Concurrent resistance and aerobic exercise stimulates both myofibrillar and mitochondrial protein synthesis in sedentary middle-aged men. J. Appl. Physiol. 2012, 112, 1992–2001. [Google Scholar] [CrossRef] [PubMed]
- Gryson, C.; Ratel, S.; Rance, M.; Penando, S.; Bonhomme, C.; Le Ruyet, P.; Duclos, M.; Boirie, Y.; Walrand, S. Four-month course of soluble milk proteins interacts with exercise to improve muscle strength and delay fatigue in elderly participants. J. Am. Med. Dir. Assoc. 2014, 15, 958.e1–958.e9. [Google Scholar] [CrossRef]
- Lockwood, C.M.; Moon, J.R.; Tobkin, S.E.; Walter, A.A.; Smith, A.E.; Dalbo, V.J.; Cramer, J.T.; Stout, J.R. Minimal nutrition intervention with high-protein/low-carbohydrate and low-fat, nutrient-dense food supplement improves body composition and exercise benefits in overweight adults: A randomized controlled trial. Nutr. Metab. 2008, 5, 11. [Google Scholar] [CrossRef]
- Ormsbee, M.J.; Willingham, B.D.; Marchant, T.; Binkley, T.L.; Specker, B.L.; Vukovich, M.D. Protein supplementation during a 6-month concurrent training program: Effect on body composition and muscular strength in sedentary individuals. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Weinheimer, E.M.; Conley, T.B.; Kobza, V.M.; Sands, L.P.; Lim, E.; Janle, E.M.; Campbell, W.W. Whey protein supplementation does not affect exercise training-induced changes in body com-position and indices of metabolic syndrome in middle-aged overweight and obese adults. J. Nutr. 2012, 142, 1532–1539. [Google Scholar] [CrossRef]
- Gibala, M.J.; Gillen, J.B.; Percival, M.E. Physiological and health-related adaptations to low-volume interval training: Influences of nutrition and sex. Sports Med. 2014, 44, S127–S137. [Google Scholar] [CrossRef]
- Gillen, J.B.; Gibala, M.J. Is high-intensity interval training a time-efficient exercise strategy to improve health and fitness? Appl. Physiol. Nutr. Metab. 2014, 39, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Baz-Valle, E.; Balsalobre-Fernández, C.; Alix-Fages, C.; Santos-Concejero, J. A systematic review of the effects of different resistance training volumes on muscle hypertrophy. J. Hum. Kinet. 2022, 81, 199–210. [Google Scholar] [CrossRef]
- Gibala, M.J.; Little, J.P. Physiological basis of brief vigorous exercise to improve health. J. Physiol. 2020, 598, 61–69. [Google Scholar] [CrossRef]
- Fyfe, J.J.; Hamilton, D.L.; Daly, R.M. Minimal-dose resistance training for improving muscle mass, strength, and function: A narrative review of current evidence and practical considerations. Sports Med. 2022, 52, 463–479. [Google Scholar] [CrossRef] [PubMed]
- Saghaei, M.; Saghaei, S. Implementation of an open-source customizable minimization program for allocation of patients to parallel groups in clinical trials. J. Biomed. Sci. Eng. 2011, 4, 734–739. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 8th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010; pp. 26–27. [Google Scholar]
- Bosy-Westphal, A.; Jensen, B.; Braun, W.; Pourhassan, M.; Gallagher, D.; Müller, M.J. Quantification of whole-body and segmental skeletal muscle mass using phase-sensitive 8-electrode medical bioelectrical impedance devices. Eur. J. Clin. Nutr. 2017, 71, 1061–1067. [Google Scholar] [CrossRef]
- Borg, G. Ratings of perceived exertion and heart rates during shortterm cycle exercise and their use in a new cycling strength test. Int. J. Sports Med. 1982, 3, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Howley, E.T.; Bassett, D.R.; Welch, H.G. Criteria for maximal oxygen uptake: Review and commentary. Med. Sci. Sports Exer. 1995, 27, 1292–1301. [Google Scholar] [CrossRef]
- Reynolds, J.M.; Gordon, T.J.; Robergs, R.A. Prediction of one repetition maximum strength from multiple repetition maximum testing and anthropometry. J. Strength Cond. Res. 2006, 20, 584–592. [Google Scholar] [PubMed]
- Dohoney, P.; Chromiak, J.A.; Lemire, D.; Abadie, B.R.; Kovacs, C. Prediction of one repetition maximum (1-RM) strength from a 4–6 RManda7–10RMsubmaximalstrength test in healthy young adult males. J. Exerc. Physiol. 2002, 5, 54–59. [Google Scholar]
- Brzycki, M. Strength testing: Predicting a one-rep max from repetitions to fatigue. J. Phys. Educ. Recreat. Dance 1993, 64, 88–90. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R., Jr.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of physical activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000, 32, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.dge.de/wissenschaft/referenzwerte/tool/ (accessed on 6 May 2024).
- Braun, H.; Carlsohn, A.; Großhauser, M.; König, D.; Lampen, A.; Mosler, S.; Nieß, A.; Oberritter, H.; Schäbethal, K.; Schek, A.; et al. Position of the working group sports nutrition of the German Nutrition Society (DGE): Energy needs in sports. Dtsch. Z. Sportmed. 2020, 71, 171–177. [Google Scholar] [CrossRef]
- Harris, J.A.; Benedict, F.G. A biometric study of basal metabolism in man. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef]
- Reljic, D.; Wittmann, F.; Fischer, J.E. Effects of low-volume high-intensity interval training in a community setting: A pilot study. Eur. J. Appl. Physiol. 2018, 118, 1153–1167. [Google Scholar] [CrossRef]
- American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med. Sci. Sports Exerc. 2009, 41, 687–708. [Google Scholar]
- Macnaughton, L.S.; Wardle, S.L.; Witard, O.C.; McGlory, C.; Hamilton, D.L.; Jeromson, S.; Lawrence, C.E.; Wallis, G.A.; Tipton, K.D. The response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g than 20 g of ingested whey protein. Physiol. Rep. 2016, 4, e12893. [Google Scholar] [CrossRef]
- Tybout, A.; Sternthal, B. Can I test for simple effects in the presence of an insignificant interaction? J. Consum. Psychol. 2001, 10, 9–10. [Google Scholar]
- Wei, J.; Carroll, R.J.; Harden, K.K.; Wu, G. Comparisons of treatment means when factors do not interact in two-factorial studies. Amino Acids. 2012, 42, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Taylor and Francis: Routledge, UK, 1988. [Google Scholar]
- Gillen, J.B.; Martin, B.J.; MacInnis, M.J.; Skelly, L.E.; Tarnopolsky, M.A.; Gibala, M.J. Twelve weeks of sprint interval training improves indices of cardiometabolic health similar to traditional endurance training despite a five-fold lower exercise volume and time commitment. PLoS ONE 2016, 11, e0154075. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, R.S.; Babraj, J.A.; Fawkner, S.G.; Vollaard, N.B. Towards the minimal amount of exercise for improving metabolic health: Beneficial effects of reduced-exertion high-intensity interval training. Eur. J. Appl. Physiol. 2012, 112, 2767–2775. [Google Scholar] [CrossRef]
- Reljic, D.; Eichhorn, A.; Herrmann, H.J.; Neurath, M.F.; Zopf, Y. Very low-volume, high-intensity interval training mitigates negative health impacts of COVID-19 pandemic-induced physical inactivity. Int. J. Environ. Res. Public Health 2022, 19, 12308. [Google Scholar] [CrossRef]
- Reljic, D.; Zieseniss, N.; Herrmann, H.J.; Neurath, M.F.; Zopf, Y. Protein supplementation does not maximize adaptations to low-volume high-intensity interval training in sedentary, healthy adults: A placebo-controlled double-blind randomized study. Nutrients 2022, 14, 3883. [Google Scholar] [CrossRef]
- Schubert, M.M.; Clarke, H.E.; Seay, R.F.; Spain, K.K. Impact of 4 weeks of interval training on resting metabolic rate, fitness, and health-related outcomes. Appl. Physiol. Nutr. Metab. 2017, 42, 1073–1081. [Google Scholar] [CrossRef]
- Scoubeau, C.; Carpentier, J.; Baudry, S.; Faoro, V.; Klass, M. Body composition, cardiorespiratory fitness, and neuromuscular adaptations induced by a home-based whole-body high intensity interval training. J. Exerc. Sci. Fit. 2023, 21, 226–236. [Google Scholar] [CrossRef]
- Venegas-Carro, M.; Herring, J.T.; Riehle, S.; Kramer, A. Jumping vs. running: Effects of exercise modality on aerobic capacity and neuromuscular performance after a six-week high-intensity interval training. PLoS ONE 2023, 18, e0281737. [Google Scholar] [CrossRef]
- Grgic, J.; Schoenfeld, B.J.; Orazem, J.; Sabol, F. Effects of resistance training performed to repetition failure or non-failure on muscular strength and hypertrophy: A systematic review and meta-analysis. J. Sport Health Sci. 2022, 11, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Grgic, J.; Ogborn, D.; Krieger, J.W. Strength and hypertrophy adaptations between low- vs. high-load resistance training: A systematic review and meta-analysis. J. Strength Cond. Res. 2017, 31, 3508–3523. [Google Scholar] [CrossRef]
- Jenkins, N.D.; Housh, T.J.; Bergstrom, H.C.; Cochrane, K.C.; Hill, E.C.; Smith, C.M.; Johnson, G.O.; Schmidt, R.J.; Cramer, J.T. Muscle activation during three sets to failure at 80 vs. 30% 1RM resistance exercise. Eur. J. Appl. Physiol. 2015, 115, 2335–2347. [Google Scholar] [CrossRef] [PubMed]
- Volaklis, K.A.; Halle, M.; Meisinger, C. Muscular strength as a strong predictor of mortality: A narrative review. Eur. J. Intern. Med. 2015, 26, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Strasser, B.; Pesta, D. Resistance training for diabetes prevention and therapy: Experimental findings and molecular mechanisms. BioMed Res. Int. 2013, 2013, 805217. [Google Scholar] [CrossRef]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A., Jr.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.M.; Goodpaster, B.H.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Camara, M.; Lima, K.C.; Freire, Y.A.; Souto, G.C.; Macêdo, G.A.; Silva, R.M.; Cabral, L.L.; Browne, R.A.; Lemos, T.M.; Waters, D.L.; et al. Independent and joint associations of cardiorespiratory fitness and lower-limb muscle strength with cardiometabolic risk in older adults. PLoS ONE 2023, 18, e0292957. [Google Scholar] [CrossRef]
- Feng, S.; Wang, J.; Yin, C.; Li, H.; Wang, T.; Liu, J.; Liang, Y.; Liu, J.; Han, D. The association between lower extremity function and cardiovascular diseases risk in older Chinese adults: Longitudinal evidence from a nationwide cohort. Arch. Gerontol. Geriatr. 2024, 124, 105463. [Google Scholar] [CrossRef]
- Sharma, H. Statistical significance or clinical significance? A researcher’s dilemma for appropriate interpretation of research results. Saudi J. Anaesth. 2021, 15, 431–434. [Google Scholar] [CrossRef]
- Kavanagh, T.; Mertens, D.J.; Hamm, L.F.; Beyene, J.; Kennedy, J.; Corey, P.; Shephard, R.J. Prediction of long-term prognosis in 12 169 men referred for cardiac rehabilitation. Circulation 2002, 106, 666–671. [Google Scholar] [CrossRef]
- Araújo Oliveira, A.L.; Rebelo, P.; Paixão, C.; Jácome, C.; Cruz, J.; Valente, C.; Andrade, L.; Brooks, D.; Marques, A. Minmal clinically important difference using one-repetition maximum in COPD. Eur. Respir. J. 2019, 54, PA1205. [Google Scholar] [CrossRef]
- Androulakis-Korakakis, P.; Fisher, J.P.; Steele, J. The minimum effective training dose required to increase 1RM strength in resistance-trained men: A systematic review and meta-analysis. Sports Med. 2020, 50, 751–765. [Google Scholar] [CrossRef]
- Torre-Villalvazo, I.; Alemán-Escondrillas, G.; Valle-Ríos, R.; Noriega, L.G. Protein intake and amino acid supplementation regulate exercise recovery and performance through the modulation of mTOR, AMPK, FGF21, and immunity. Nutr. Res. 2019, 72, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pasiakos, S.M.; Lieberman, H.R.; McLellan, T.M. Effects of protein supplements on muscle damage, soreness and recovery of muscle function and physical performance: A systematic review. Sports Med. 2014, 44, 655–670. [Google Scholar] [CrossRef] [PubMed]
- West, D.W.; Burd, N.A.; Tang, J.E.; Moore, D.R.; Staples, A.W.; Holwerda, A.M.; Baker, S.K.; Phillips, S.M. Elevations in ostensibly anabolic hormones with resistance exercise enhance neither training-induced muscle hypertrophy nor strength of the elbow flexors. J. Appl. Physiol. 2010, 108, 60–67. [Google Scholar] [CrossRef]
- Folland, J.P.; Williams, A.G. The adaptations to strength training: Morphological and neurological contributions to increased strength. Sports Med. 2007, 37, 145–168. [Google Scholar] [CrossRef]
- Watanabe, K.; Holobar, A.; Mita, Y.; Kouzaki, M.; Ogawa, M.; Akima, H.; Moritani, T. Effect of resistance training and fish protein intake on motor unit firing pattern and motor function of elderly. Front. Physiol. 2018, 9, 1733. [Google Scholar] [CrossRef]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Spina, R.J.; Martin, W.H., 3rd; Kohrt, W.M.; Schechtman, K.B.; Holloszy, J.O.; Ehsani, A.A. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation 1992, 86, 494–503. [Google Scholar] [CrossRef]
- Moore, D.R.; Camera, D.M.; Areta, J.L.; Hawley, J.A. Beyond muscle hypertrophy: Why dietary protein is important for endurance athletes. Appl. Physiol. Nutr. Metab. 2014, 39, 987–997. [Google Scholar] [CrossRef]
- Cintineo, H.P.; Arent, M.A.; Antionio, J.; Arent, S.M. Effects of protein supplementation on performance and recovery in resistance and endurance training. Front. Nutr. 2018, 5, 83. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, D.S.; Thomson, J.S.; Timmons, B.W.; Raymond, F.; Fuerholz, A.; Mansourian, R.; Zwahlen, M.C.; Métairon, S.; Glover, E.; Stellingwerff, T.; et al. Transcriptome and translational signaling following endurance exercise in trained skeletal muscle: Impact of dietary protein. Physiol Genom. 2011, 43, 1004–1020. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, M.N.; Schoeller, D.A. Traditional self-reported dietary instruments are prone to inaccuracies and new approaches are needed. Front Nutr. 2020, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jäger, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN exercise & sports nutrition review update: Research & recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Breen, L.; Philp, A.; Witard, O.C.; Jackman, S.R.; Selby, A.; Smith, K.; Baar, K.; Tipton, K.D. The influence of carbohydrate-protein co-ingestion following endurance exercise on myofibrillar and mitochondrial protein synthesis. J. Physiol. 2011, 589, 4011–4025. [Google Scholar] [CrossRef] [PubMed]
- Knuiman, P.; van Loon, L.J.C.; Wouters, J.; Hopman, M.; Mensink, M. Protein supplementation elicits greater gains in maximal oxygen uptake capacity and stimulates lean mass accretion during prolonged endurance training: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2019, 110, 508–518. [Google Scholar] [CrossRef]
- Robinson, M.M.; Turner, S.M.; Hellerstein, M.K.; Hamilton, K.L.; Miller, B.F. Long-term synthesis rates of skeletal muscle DNA and protein are higher during aerobic training in older humans than in sedentary young subjects but are not altered by protein supplementation. FASEB J. 2011, 25, 3240–3249. [Google Scholar] [CrossRef]
- Wagner, D.R. Bioelectrical impedance changes of the trunk are opposite the limbs following acute hydration change. Electr. Bioimpedance 2022, 13, 25–30. [Google Scholar] [CrossRef]

| Variable | Protein Shake 1,3 | Placebo Shake 2,3 |
|---|---|---|
| Caloric value (kcal) | 213 | 222 |
| Protein (g) | 40 | 5 |
| Carbohydrates (g) | 7.5 | 46 |
| Fat (g) | 2.6 | 2 |
| Outcome | PRO Group (N = 19) | PLA Group (N = 17) | Main Effect of Time (p-Value) | Group × Time Interaction (p-Value) | ||
|---|---|---|---|---|---|---|
| Week 0 | Week 8 | Week 0 | Week 8 | |||
| Nutrition 1 | ||||||
| Energy (kcal/day) | 1987 ± 482 | 1965 ± 406 | 2052 ± 473 | 2000 ± 528 | 0.548 | 0.798 |
| Protein (g/day) | 81 ± 17 | 76 ± 11 | 79 ± 22 | 81 ± 28 | 0.656 | 0.237 |
| Protein (g/kg/day) | 1.2 ± 0.2 | 1.2 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.3 | 0.786 | 0.511 |
| Fat (g/day) | 81 ± 28 | 78 ± 22 | 81 ± 23 | 80 ± 33 | 0.632 | 0.723 |
| Fat (g/kg/day) | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.1 ± 0.4 | 1.1 ± 0.4 | 0.644 | 0.930 |
| Carbohydrates (g/day) | 207 ± 52 | 210 ± 78 | 216 ± 70 | 219 ± 63 | 0.745 | 0.995 |
| Carbohydrates (g/kg/day) | 3.2 ± 0.9 | 3.2 ± 1.0 | 3.0 ± 1.2 | 3.0 ± 1.0 | 0.929 | 0.990 |
| Fiber (g/day) | 21 ± 8 | 20 ± 8 | 22 ± 9 | 21 ± 9 | 0.527 | 0.830 |
| Physical activity 2 | ||||||
| Light PA (h/week) | 2.3 ± 1.4 | 2.4 ± 0.7 | 2.9 ± 0.7 | 2.9 ± 0.7 | 0.310 | 0.640 |
| Moderate PA (h/week) | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.2 ± 0.5 | 1.4 ± 0.9 | 0.063 | 0.781 |
| PAL | 1.40 ± 0.02 | 1.41 ± 0.02 | 1.47 ± 0.01 | 1.47 ± 0.01 | 0.234 | 3 |
| Outcome | PRO Group (N = 19) | PLA Group (N = 17) | Main Effect of Time (p-Value) | Group × Time Interaction (p-Value) | ||
|---|---|---|---|---|---|---|
| T-1 | T-2 | T-1 | T-2 | |||
| Body weight (kg) | 65.9 ± 11.16 | 65.7 ± 11.6 | 75.9 ± 13.7 | 76.0 ± 12.9 | 0.936 | 0.530 |
| Body mass index (kg/m2) | 21.8 ± 2.2 | 21.8 ± 2.3 | 25.0 ± 4.3 | 25.0 ± 4.3 | 0.426 | 0.982 |
| Fat mass (kg) | 15.7 ± 4.3 | 15.4 ± 4.6 | 21.9 ± 10.2 | 22.0 ± 10.3 | 0.726 | 0.340 |
| Fat mass (%) | 24.0 ± 6.6 | 23.6 ± 6.6 | 28.3 ± 10.2 | 28.4 ± 10.4 | 0.535 | 0.238 |
| Skeletal muscle mass (kg) | 23.9 ± 6.1 | 24.0 ± 6.2 | 25.7 ± 5.5 | 25.9 ± 5.4 | 0.304 | 0.344 |
| Total body water (L) | 36.9 ± 7.6 | 36.0 ± 10.6 | 39.7 ± 7.2 | 39.8 ± 7.1 | 0.381 | 0.321 |
| Waist circumference (cm) | 74 ± 8 | 72 ± 8 a | 81 ± 7 | 80 ± 7 | 0.033 | 0.291 |
| Outcome | PRO Group (N = 19) | PLA Group (N = 17) | Main Effect of Time (p-Value) | Group × Time Interaction (p-Value) | ||
|---|---|---|---|---|---|---|
| T-1 | T-2 | T-1 | T-2 | |||
| VO2max (mL/kg/min) | 40.1 ± 6.3 | 42.8 ± 7.2 b | 36.8 ± 8.2 | 38.2 ± 8.1 a | <0.001 | 0.210 |
| VO2max (L/min) | 2.6 ± 0.8 | 2.9 ± 0.8 b | 2.8 ± 0.7 | 2.9 ± 0.7 a | <0.001 | 0.166 |
| Wmax (W/kg) | 3.2 ± 0.5 | 3.6 ± 0.5 c | 2.8 ± 0.6 | 3.0 ± 0.6 c | <0.001 | 0.062 |
| Wmax (W) | 213 ± 60 | 235 ± 64 c | 211 ± 43 | 228 ± 52 c | <0.001 | 0.306 |
| WVT (W) | 82 ± 36 | 90 ± 49 | 76 ± 12 | 78 ± 19 | 0.275 | 0.532 |
| Outcome | PRO Group (N = 19) | PLA Group (N = 17) | Main Effect of Time (p-Value) | Group × Time Interaction (p-Value) | ||
|---|---|---|---|---|---|---|
| pre | post | pre | post | |||
| Abdominals (kg) | 28 ± 12 | 35 ± 14 c | 30 ± 11 | 34 ± 10 | <0.001 | 0.050 |
| Lower back(kg) | 39 ± 13 | 47 ± 15 c | 49 ± 15 | 53 ± 14 | 0.001 | 0.276 |
| Chest (kg) | 34 ± 16 | 43 ± 16 c | 39 ± 19 | 47 ± 20 b | <0.001 | 1 |
| Upper back (kg) | 47 ± 18 | 58 ± 22 c | 50 ± 20 | 57 ± 2 b | <0.001 | 1 |
| Legs (kg) | 125 ± 42 | 152 ± 43 c | 132 ± 32 | 139 ± 35 | <0.001 | 0.007 |
| Fit score | 47 ± 11 | 54 ± 13 c | 48 ± 9 | 52 ± 11 b | <0.001 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reljic, D.; Zieseniss, N.; Herrmann, H.J.; Neurath, M.F.; Zopf, Y. Protein Supplementation Increases Adaptations to Low-Volume, Intra-Session Concurrent Training in Untrained Healthy Adults: A Double-Blind, Placebo-Controlled, Randomized Trial. Nutrients 2024, 16, 2713. https://doi.org/10.3390/nu16162713
Reljic D, Zieseniss N, Herrmann HJ, Neurath MF, Zopf Y. Protein Supplementation Increases Adaptations to Low-Volume, Intra-Session Concurrent Training in Untrained Healthy Adults: A Double-Blind, Placebo-Controlled, Randomized Trial. Nutrients. 2024; 16(16):2713. https://doi.org/10.3390/nu16162713
Chicago/Turabian StyleReljic, Dejan, Nilas Zieseniss, Hans Joachim Herrmann, Markus Friedrich Neurath, and Yurdagül Zopf. 2024. "Protein Supplementation Increases Adaptations to Low-Volume, Intra-Session Concurrent Training in Untrained Healthy Adults: A Double-Blind, Placebo-Controlled, Randomized Trial" Nutrients 16, no. 16: 2713. https://doi.org/10.3390/nu16162713
APA StyleReljic, D., Zieseniss, N., Herrmann, H. J., Neurath, M. F., & Zopf, Y. (2024). Protein Supplementation Increases Adaptations to Low-Volume, Intra-Session Concurrent Training in Untrained Healthy Adults: A Double-Blind, Placebo-Controlled, Randomized Trial. Nutrients, 16(16), 2713. https://doi.org/10.3390/nu16162713








