Dietary and Lifestyle Strategies for Obesity
Abstract
1. Introduction
2. Methodology
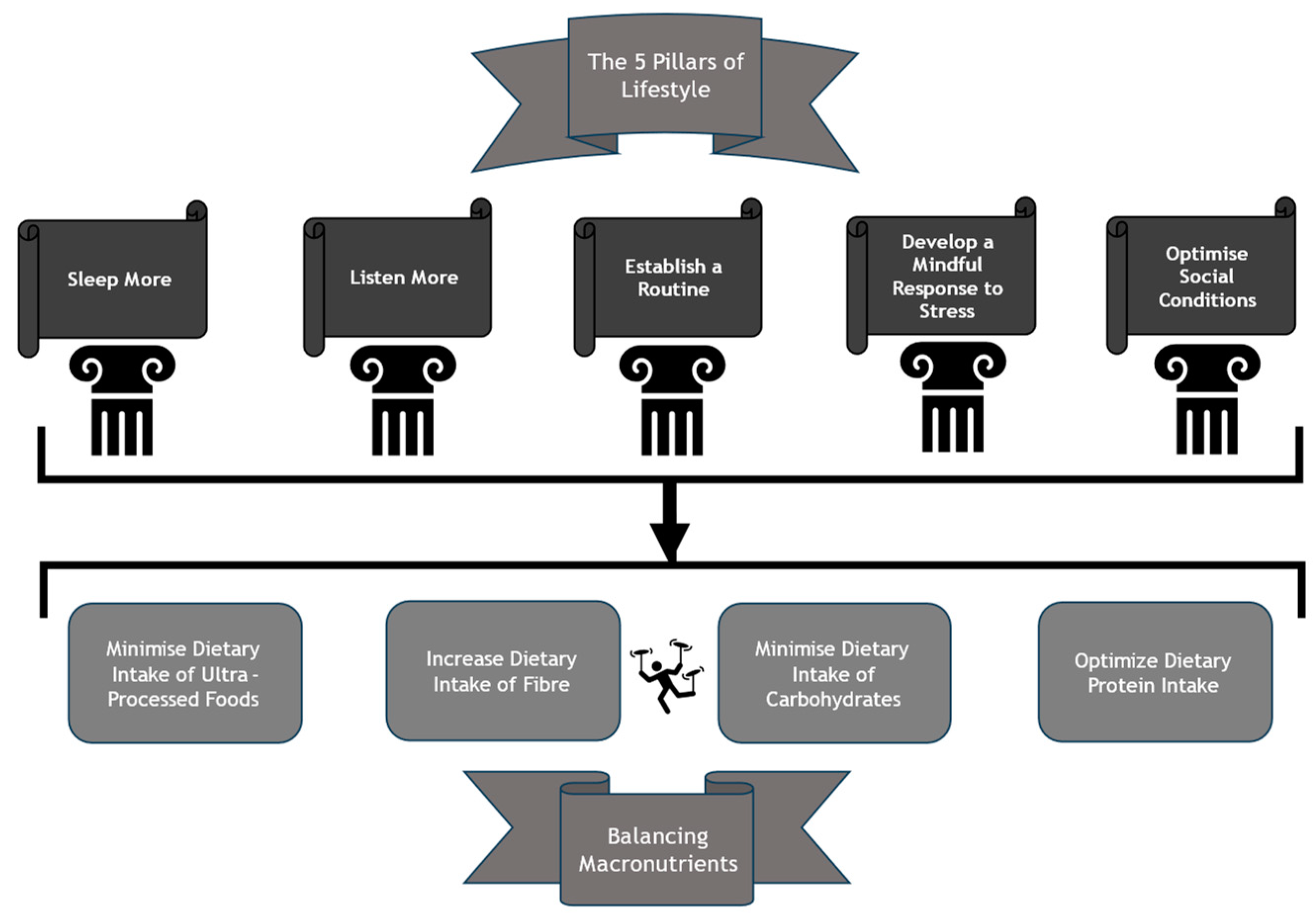
3. Building the Foundations for Dietary Modification
3.1. Sleeping
3.2. Listening
3.3. Routine
3.4. De-Stressing
3.5. Optimising Social Conditions
4. Balancing Macronutrients
4.1. Dietary Fibre
4.2. Carbohydrates
4.3. Protein
4.4. Ultra-Processed Foods (UPFs)
5. Suggested Dietary Strategy for Obesity
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight; World Health Organization: Geneva, Switzerland, 2019.
- Sorensen, T.I.A.; Martinez, A.R.; Jorgensen, T.S.H. Epidemiology of Obesity. Handb. Exp. Pharmacol. 2022, 274, 3–27. [Google Scholar] [CrossRef]
- Pi-Sunyer, X. The medical risks of obesity. Postgrad. Med. 2009, 121, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Peeters, A.; Barendregt, J.; Willekens, F.; Mackenbach, J.; Al Mamun, A.; Bonneux, L. Obesity in adulthood and its consequences for life expectancy: A life-table analysis. Ann. Intern. Med. 2003, 138, 24–32. [Google Scholar] [CrossRef]
- Reilly, J.J.; Kelly, J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: Systematic review. Int. J. Obes. 2011, 35, 891–898. [Google Scholar] [CrossRef]
- Westbury, S.; Oyebode, O.; van Rens, T.; Barber, T.M. Obesity Stigma: Causes, Consequences, and Potential Solutions. Curr. Obes. Rep. 2023, 12, 10–23. [Google Scholar] [CrossRef]
- Wardle, J.; Cooke, L. The impact of obesity on psychological well-being. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 421–440. [Google Scholar] [CrossRef]
- Sarwer, D.B.; Polonsky, H.M. The Psychosocial Burden of Obesity. Endocrinol. Metab. Clin. N. Am. 2016, 45, 677–688. [Google Scholar] [CrossRef]
- Nigatu, Y.T.; van de Ven, H.A.; van der Klink, J.J.; Brouwer, S.; Reijneveld, S.A.; Bultmann, U. Overweight, obesity and work functioning: The role of working-time arrangements. Appl. Ergon. 2016, 52, 128–134. [Google Scholar] [CrossRef]
- Cecchini, M. Use of healthcare services and expenditure in the US in 2025: The effect of obesity and morbid obesity. PLoS ONE 2018, 13, e0206703. [Google Scholar] [CrossRef] [PubMed]
- Novelli, G.; Cassadonte, C.; Sbraccia, P.; Biancolella, M. Genetics: A Starting Point for the Prevention and the Treatment of Obesity. Nutrients 2023, 15, 2782. [Google Scholar] [CrossRef]
- Schultes, B.; Ernst, B.; Hallschmid, M.; Bueter, M.; Meyhofer, S.M. The ‘Behavioral Balance Model’: A new perspective on the aetiology and therapy of obesity. Diabetes Obes. Metab. 2023, 25, 3444–3452. [Google Scholar] [CrossRef]
- Ryan, D.H. Lifestyle-Based Obesity Care. Gastroenterol. Clin. N. Am. 2023, 52, 645–660. [Google Scholar] [CrossRef]
- Melson, E.; Miras, A.D.; Papamargaritis, D. Future therapies for obesity. Clin. Med. 2023, 23, 337–346. [Google Scholar] [CrossRef]
- Statham, L.; Pelling, M.; Hanson, P.; Kyrou, I.; Randeva, H.; Barber, T.M. Designer GLP1 poly-agonist peptides in the management of diabesity. Expert Rev. Endocrinol. Metab. 2023, 18, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.; Corcelles, R.; Jimenez, A.; Flores, L.; Lacy, A.M. Metabolic and Bariatric Surgery for Obesity. Gastroenterology 2017, 152, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, C.M.; Cohen, R.V.; Sumithran, P.; Clement, K.; Fruhbeck, G. Contemporary medical, device, and surgical therapies for obesity in adults. Lancet 2023, 401, 1116–1130. [Google Scholar] [CrossRef]
- Irfan, H. Obesity, Cardiovascular Disease, and the Promising Role of Semaglutide: Insights from the SELECT Trial. Curr. Probl. Cardiol. 2024, 49, 102060. [Google Scholar] [CrossRef]
- Teixeira, P.J.; Marques, M.M. Health Behavior Change for Obesity Management. Obes. Facts 2017, 10, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Denniss, E.; Lindberg, R.; Marchese, L.E.; McNaughton, S.A. #Fail: The quality and accuracy of nutrition-related information by influential Australian Instagram accounts. Int. J. Behav. Nutr. Phys. Act. 2024, 21, 16. [Google Scholar] [CrossRef]
- Post, R.E.; Mainous, A.G., 3rd. The accuracy of nutrition information on the Internet for type 2 diabetes. Arch. Intern. Med. 2010, 170, 1504–1506. [Google Scholar] [CrossRef]
- Denniss, E.; Lindberg, R.; McNaughton, S.A. Quality and accuracy of online nutrition-related information: A systematic review of content analysis studies. Public Health Nutr. 2023, 26, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Harcombe, Z. Dietary fat guidelines have no evidence base: Where next for public health nutritional advice? Br. J. Sports Med. 2017, 51, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Hinde, S. Understanding the role of carbohydrates in optimal nutrition. Nurs. Stand. 2019, 34, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Oppert, J.M.; Bellicha, A.; Ciangura, C. Physical activity in management of persons with obesity. Eur. J. Intern. Med. 2021, 93, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.C.; Aung, T. Sleep deprivation and its association with diseases—A review. Sleep Med. 2021, 77, 192–204. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Zheng, Q.; Gao, L.; Sun, Q. Sleep Deprivation and Central Appetite Regulation. Nutrients 2022, 14, 5196. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Tasali, E.; Penev, P.; Van Cauter, E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 2004, 141, 846–850. [Google Scholar] [CrossRef]
- Schoenborn, C.A.; Adams, P.E. Health behaviors of adults: United States, 2005–2007. Vital Health Stat. 2010, 10, 1–132. [Google Scholar]
- Nedeltcheva, A.V.; Kilkus, J.M.; Imperial, J.; Schoeller, D.A.; Penev, P.D. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann. Intern. Med. 2010, 153, 435–441. [Google Scholar] [CrossRef]
- Luyster, F.S.; Strollo, P.J., Jr.; Zee, P.C.; Walsh, J.K. Sleep: A health imperative. Sleep 2012, 35, 727–734. [Google Scholar] [CrossRef]
- Soong, C.; Burry, L.; Cho, H.J.; Gathecha, E.; Kisuule, F.; Tannenbaum, C.; Vijenthira, A.; Morgenthaler, T. An Implementation Guide to Promote Sleep and Reduce Sedative-Hypnotic Initiation for Noncritically Ill Inpatients. JAMA Intern. Med. 2019, 179, 965–972. [Google Scholar] [CrossRef]
- Duan, D.; Kim, L.J.; Jun, J.C.; Polotsky, V.Y. Connecting insufficient sleep and insomnia with metabolic dysfunction. Ann. N. Y. Acad. Sci. 2023, 1519, 94–117. [Google Scholar] [CrossRef]
- Zhao, M.; Tuo, H.; Wang, S.; Zhao, L. The Effects of Dietary Nutrition on Sleep and Sleep Disorders. Mediat. Inflamm. 2020, 2020, 3142874. [Google Scholar] [CrossRef]
- Hanson, P.; Shuttlewood, E.; Halder, L.; Shah, N.; Lam, F.T.; Menon, V.; Barber, T.M. Application of Mindfulness in a Tier 3 Obesity Service Improves Eating Behavior and Facilitates Successful Weight Loss. J. Clin. Endocrinol. Metab. 2019, 104, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Barrington, W.E.; Beresford, S.A.A. Eating Occasions, Obesity and Related Behaviors in Working Adults: Does it Matter When You Snack? Nutrients 2019, 11, 2320. [Google Scholar] [CrossRef] [PubMed]
- Barberis, N.; Gugliandolo, M.C.; Costa, S.; Cannavo, M. Healthy and binge eating behaviours: The motivational processes underlying peer pressure. Psychol. Health Med. 2022, 27, 1144–1153. [Google Scholar] [CrossRef]
- Chung, A.; Vieira, D.; Donley, T.; Tan, N.; Jean-Louis, G.; Kiely Gouley, K.; Seixas, A. Adolescent Peer Influence on Eating Behaviors via Social Media: Scoping Review. J. Med. Internet. Res. 2021, 23, e19697. [Google Scholar] [CrossRef] [PubMed]
- Vasim, I.; Majeed, C.N.; DeBoer, M.D. Intermittent Fasting and Metabolic Health. Nutrients 2022, 14, 631. [Google Scholar] [CrossRef]
- Van Dyke, N.; Drinkwater, E.J. Relationships between intuitive eating and health indicators: Literature review. Public Health Nutr. 2014, 17, 1757–1766. [Google Scholar] [CrossRef]
- Meyer, N.; Harvey, A.G.; Lockley, S.W.; Dijk, D.J. Circadian rhythms and disorders of the timing of sleep. Lancet 2022, 400, 1061–1078. [Google Scholar] [CrossRef]
- Stenvers, D.J.; Scheer, F.; Schrauwen, P.; la Fleur, S.E.; Kalsbeek, A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 2019, 15, 75–89. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.; Stern, N.; Bilu, C.; El-Osta, A.; Einat, H.; Kronfeld-Schor, N. The Circadian Syndrome: Is the Metabolic Syndrome and much more! J. Intern. Med. 2019, 286, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Gu, C.; Polotsky, V.Y.; Jun, J.C.; Pham, L.V. Effects of Dinner Timing on Sleep Stage Distribution and EEG Power Spectrum in Healthy Volunteers. Nat. Sci. Sleep 2021, 13, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Yau, Y.H.; Potenza, M.N. Stress and eating behaviors. Minerva Endocrinol. 2013, 38, 255–267. [Google Scholar]
- Hanson, P.; Lange, M.; Oduro-Donkor, D.; Shuttlewood, E.; Weickert, M.O.; Randeva, H.S.; Menon, V.; Alexander, R.T.; Basset, P.; Shankar, R.; et al. The role of mindfulness training in sustaining weight reduction: Retrospective cohort analysis. BJPsych Open 2022, 8, e198. [Google Scholar] [CrossRef] [PubMed]
- Muhiyaddin, R.; Abd-Alrazaq, A.; Shah, Z.; Alam, T.; Househ, M. Evaluation of Meditation Apps Available on Google Play and Apple Store: An App Review. Stud. Health Technol. Inform. 2022, 289, 376–379. [Google Scholar] [CrossRef]
- Huberty, J.; Green, J.; Glissmann, C.; Larkey, L.; Puzia, M.; Lee, C. Efficacy of the Mindfulness Meditation Mobile App “Calm” to Reduce Stress Among College Students: Randomized Controlled Trial. JMIR Mhealth Uhealth 2019, 7, e14273. [Google Scholar] [CrossRef] [PubMed]
- Najafi Ghezeljeh, T.; Kohandany, M.; Oskouei, F.H.; Malek, M. The effect of progressive muscle relaxation on glycated hemoglobin and health-related quality of life in patients with type 2 diabetes mellitus. Appl. Nurs. Res. 2017, 33, 142–148. [Google Scholar] [CrossRef]
- Pascoe, M.C.; Thompson, D.R.; Ski, C.F. Yoga, mindfulness-based stress reduction and stress-related physiological measures: A meta-analysis. Psychoneuroendocrinology 2017, 86, 152–168. [Google Scholar] [CrossRef]
- Inagaki, K.; Ohta, Y. Capacity of Autonomous Sensory Meridian Response on the Reduction of Mental Stress. Int. J. Environ. Res. Public Health 2022, 19, 4577. [Google Scholar] [CrossRef]
- Johnson, J.S.; Nobmann, E.D.; Asay, E.; Lanier, A.P. Dietary intake of Alaska Native people in two regions and implications for health: The Alaska Native Dietary and Subsistence Food Assessment Project. Int. J. Circumpolar Health 2009, 68, 109–122. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Effects of the Mediterranean Diet on Health and Gut Microbiota. Nutrients 2023, 15, 2150. [Google Scholar] [CrossRef]
- Barber, T.M.; Valsamakis, G.; Mastorakos, G.; Hanson, P.; Kyrou, I.; Randeva, H.S.; Weickert, M.O. Dietary Influences on the Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2021, 22, 3502. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Hijova, E.; Bertkova, I.; Stofilova, J. Dietary fibre as prebiotics in nutrition. Cent. Eur. J. Public Health 2019, 27, 251–255. [Google Scholar] [CrossRef]
- Stephen, A.M.; Champ, M.M.; Cloran, S.J.; Fleith, M.; van Lieshout, L.; Mejborn, H.; Burley, V.J. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017, 30, 149–190. [Google Scholar] [CrossRef] [PubMed]
- Burkitt, D.P.; Trowell, H.C. Dietary fibre and western diseases. Ir. Med. J. 1977, 70, 272–277. [Google Scholar]
- Weickert, M.O.; Mohlig, M.; Koebnick, C.; Holst, J.J.; Namsolleck, P.; Ristow, M.; Osterhoff, M.; Rochlitz, H.; Rudovich, N.; Spranger, J.; et al. Impact of cereal fibre on glucose-regulating factors. Diabetologia 2005, 48, 2343–2353. [Google Scholar] [CrossRef]
- Weickert, M.O.; Mohlig, M.; Schofl, C.; Arafat, A.M.; Otto, B.; Viehoff, H.; Koebnick, C.; Kohl, A.; Spranger, J.; Pfeiffer, A.F. Cereal fiber improves whole-body insulin sensitivity in overweight and obese women. Diabetes Care 2006, 29, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Honsek, C.; Kabisch, S.; Kemper, M.; Gerbracht, C.; Arafat, A.M.; Birkenfeld, A.L.; Dambeck, U.; Osterhoff, M.A.; Weickert, M.O.; Pfeiffer, A.F.H. Fibre supplementation for the prevention of type 2 diabetes and improvement of glucose metabolism: The randomised controlled Optimal Fibre Trial (OptiFiT). Diabetologia 2018, 61, 1295–1305. [Google Scholar] [CrossRef]
- Russell, W.R.; Baka, A.; Bjorck, I.; Delzenne, N.; Gao, D.; Griffiths, H.R.; Hadjilucas, E.; Juvonen, K.; Lahtinen, S.; Lansink, M.; et al. Impact of Diet Composition on Blood Glucose Regulation. Crit. Rev. Food Sci. Nutr. 2016, 56, 541–590. [Google Scholar] [CrossRef] [PubMed]
- Kabisch, S.; Meyer, N.M.T.; Honsek, C.; Gerbracht, C.; Dambeck, U.; Kemper, M.; Osterhoff, M.A.; Birkenfeld, A.L.; Arafat, A.M.; Hjorth, M.F.; et al. Fasting Glucose State Determines Metabolic Response to Supplementation with Insoluble Cereal Fibre: A Secondary Analysis of the Optimal Fibre Trial (OptiFiT). Nutrients 2019, 11, 2385. [Google Scholar] [CrossRef] [PubMed]
- Kabisch, S.; Meyer, N.M.T.; Honsek, C.; Gerbracht, C.; Dambeck, U.; Kemper, M.; Osterhoff, M.A.; Birkenfeld, A.L.; Arafat, A.M.; Weickert, M.O.; et al. Obesity Does Not Modulate the Glycometabolic Benefit of Insoluble Cereal Fibre in Subjects with Prediabetes-A Stratified Post Hoc Analysis of the Optimal Fibre Trial (OptiFiT). Nutrients 2019, 11, 2726. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, N.; Kasuga, C.; Tanaka, A.; Kamachi, K.; Ai, M.; Urayama, K.Y.; Tanaka, A. Association between dietary fibre:carbohydrate intake ratio and insulin resistance in Japanese adults without type 2 diabetes. Br. J. Nutr. 2018, 119, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Weickert, M.O.; Roden, M.; Isken, F.; Hoffmann, D.; Nowotny, P.; Osterhoff, M.; Blaut, M.; Alpert, C.; Gogebakan, O.; Bumke-Vogt, C.; et al. Effects of supplemented isoenergetic diets differing in cereal fiber and protein content on insulin sensitivity in overweight humans. Am. J. Clin. Nutr. 2011, 94, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef] [PubMed]
- Isken, F.; Klaus, S.; Petzke, K.J.; Loddenkemper, C.; Pfeiffer, A.F.; Weickert, M.O. Impairment of fat oxidation under high- vs. low-glycemic index diet occurs before the development of an obese phenotype. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E287–E295. [Google Scholar] [CrossRef]
- Gruendel, S.; Otto, B.; Garcia, A.L.; Wagner, K.; Mueller, C.; Weickert, M.O.; Heldwein, W.; Koebnick, C. Carob pulp preparation rich in insoluble dietary fibre and polyphenols increases plasma glucose and serum insulin responses in combination with a glucose load in humans. Br. J. Nutr. 2007, 98, 101–105. [Google Scholar] [CrossRef]
- Weickert, M.O. High fiber intake, dietary protein, and prevention of type 2 diabetes. Expert Rev. Endocrinol. Metab. 2018, 13, 223–224. [Google Scholar] [CrossRef]
- Isken, F.; Klaus, S.; Osterhoff, M.; Pfeiffer, A.F.; Weickert, M.O. Effects of long-term soluble vs. insoluble dietary fiber intake on high-fat diet-induced obesity in C57BL/6J mice. J. Nutr. Biochem. 2010, 21, 278–284. [Google Scholar] [CrossRef]
- Kim, S.J.; de Souza, R.J.; Choo, V.L.; Ha, V.; Cozma, A.I.; Chiavaroli, L.; Mirrahimi, A.; Blanco Mejia, S.; Di Buono, M.; Bernstein, A.M.; et al. Effects of dietary pulse consumption on body weight: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2016, 103, 1213–1223. [Google Scholar] [CrossRef]
- Jovanovski, E.; Mazhar, N.; Komishon, A.; Khayyat, R.; Li, D.; Mejia, S.B.; Khan, T.; Jenkins, A.L.; Smircic-Duvnjak, L.; Sievenpiper, J.L.; et al. Can dietary viscous fiber affect body weight independently of an energy-restrictive diet? A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2020, 111, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Miketinas, D.C.; Bray, G.A.; Beyl, R.A.; Ryan, D.H.; Sacks, F.M.; Champagne, C.M. Fiber Intake Predicts Weight Loss and Dietary Adherence in Adults Consuming Calorie-Restricted Diets: The POUNDS Lost (Preventing Overweight Using Novel Dietary Strategies) Study. J. Nutr. 2019, 149, 1742–1748. [Google Scholar] [CrossRef]
- Track, N.S.; Cawkwell, M.E.; Chin, B.C.; Chiu, S.S.; Haberer, S.A.; Honey, C.R. Guar gum consumption in adolescent and adult rats: Short- and long-term metabolic effects. Can. J. Physiol. Pharmacol. 1985, 63, 1113–1121. [Google Scholar] [CrossRef]
- Oduro-Donkor, D.; Turner, M.C.; Farnaud, S.; Renshaw, D.; Kyrou, I.; Hanson, P.; Hattersley, J.; Weickert, M.O.; Menon, V.; Randeva, H.S.; et al. Modification of fecal microbiota as a mediator of effective weight loss and metabolic benefits following bariatric surgery. Expert Rev. Endocrinol. Metab. 2020, 15, 363–373. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e1321. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Backhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Haghikia, A.; Jörg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A.; Hammer, A.; Lee, D.-H.; May, C.; Wilck, N. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 2015, 43, 817–829. [Google Scholar] [CrossRef]
- Kabisch, S.; Wenschuh, S.; Buccellato, P.; Spranger, J.; Pfeiffer, A.F.H. Affordability of Different Isocaloric Healthy Diets in Germany-An Assessment of Food Prices for Seven Distinct Food Patterns. Nutrients 2021, 13, 3037. [Google Scholar] [CrossRef] [PubMed]
- Fulgoni, V., 3rd; Drewnowski, A. An Economic Gap Between the Recommended Healthy Food Patterns and Existing Diets of Minority Groups in the US National Health and Nutrition Examination Survey 2013–2014. Front. Nutr. 2019, 6, 37. [Google Scholar] [CrossRef]
- Schroder, H.; Gomez, S.F.; Ribas-Barba, L.; Perez-Rodrigo, C.; Bawaked, R.A.; Fito, M.; Serra-Majem, L. Monetary Diet Cost, Diet Quality, and Parental Socioeconomic Status in Spanish Youth. PLoS ONE 2016, 11, e0161422. [Google Scholar] [CrossRef]
- Barber, T.M.; Hanson, P.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Low-Carbohydrate Diet: Short-Term Metabolic Efficacy Versus Longer-Term Limitations. Nutrients 2021, 13, 1187. [Google Scholar] [CrossRef]
- Meldrum, D.R.; Morris, M.A.; Gambone, J.C. Obesity pandemic: Causes, consequences, and solutions-but do we have the will? Fertil. Steril. 2017, 107, 833–839. [Google Scholar] [CrossRef]
- Brouns, F. Overweight and diabetes prevention: Is a low-carbohydrate-high-fat diet recommendable? Eur. J. Nutr. 2018, 57, 1301–1312. [Google Scholar] [CrossRef]
- Pliquett, R.U.; Fuhrer, D.; Falk, S.; Zysset, S.; von Cramon, D.Y.; Stumvoll, M. The effects of insulin on the central nervous system--focus on appetite regulation. Horm. Metab. Res. 2006, 38, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, J.Z.; Day, A.; Brinkworth, G.D.; Sato, J.; Yamada, S.; Jonsson, T.; Beardsley, J.; Johnson, J.A.; Thabane, L.; Johnston, B.C. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: Systematic review and meta-analysis of published and unpublished randomized trial data. BMJ 2021, 372, m4743. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.M. Which diet is better--low-fat or low-carb? JAAPA 2006, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Fukuda, T.; Oyabu, C.; Tanaka, M.; Asano, M.; Yamazaki, M.; Fukui, M. Impact of low-carbohydrate diet on body composition: Meta-analysis of randomized controlled studies. Obes. Rev. 2016, 17, 499–509. [Google Scholar] [CrossRef]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef]
- Burke, L.M.; Ross, M.L.; Garvican-Lewis, L.A.; Welvaert, M.; Heikura, I.A.; Forbes, S.G.; Mirtschin, J.G.; Cato, L.E.; Strobel, N.; Sharma, A.P.; et al. Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. J. Physiol. 2017, 595, 2785–2807. [Google Scholar] [CrossRef] [PubMed]
- Raffensperger, J.F. The least-cost low-carbohydrate diet is expensive. Nutr. Res. 2008, 28, 6–12. [Google Scholar] [CrossRef]
- Feinman, R.D.; Pogozelski, W.K.; Astrup, A.; Bernstein, R.K.; Fine, E.J.; Westman, E.C.; Accurso, A.; Frassetto, L.; Gower, B.A.; McFarlane, S.I.; et al. Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base. Nutrition 2015, 31, 1–13. [Google Scholar] [CrossRef]
- Accurso, A.; Bernstein, R.K.; Dahlqvist, A.; Draznin, B.; Feinman, R.D.; Fine, E.J.; Gleed, A.; Jacobs, D.B.; Larson, G.; Lustig, R.H.; et al. Dietary carbohydrate restriction in type 2 diabetes mellitus and metabolic syndrome: Time for a critical appraisal. Nutr. Metab. 2008, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Raben, A.; Vestentoft, P.S.; Brand-Miller, J.; Jalo, E.; Drummen, M.; Simpson, L.; Martinez, J.A.; Handjieva-Darlenska, T.; Stratton, G.; Huttunen-Lenz, M.; et al. The PREVIEW intervention study: Results from a 3-year randomized 2 × 2 factorial multinational trial investigating the role of protein, glycaemic index and physical activity for prevention of type 2 diabetes. Diabetes Obes. Metab. 2021, 23, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Penalvo, J.L.; Cudhea, F.; Imamura, F.; Rehm, C.D.; Mozaffarian, D. Association Between Dietary Factors and Mortality From Heart Disease, Stroke, and Type 2 Diabetes in the United States. JAMA 2017, 317, 912–924. [Google Scholar] [CrossRef]
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [PubMed]
- Madiraju, A.K.; Alves, T.; Zhao, X.; Cline, G.W.; Zhang, D.; Bhanot, S.; Samuel, V.T.; Kibbey, R.G.; Shulman, G.I. Argininosuccinate synthetase regulates hepatic AMPK linking protein catabolism and ureagenesis to hepatic lipid metabolism. Proc. Natl. Acad. Sci. USA 2016, 113, E3423–E3430. [Google Scholar] [CrossRef]
- Muller, M.J.; Illner, K.; Bosy-Westphal, A.; Brinkmann, G.; Heller, M. Regional lean body mass and resting energy expenditure in non-obese adults. Eur. J. Nutr. 2001, 40, 93–97. [Google Scholar] [CrossRef]
- Cuenca-Sanchez, M.; Navas-Carrillo, D.; Orenes-Pinero, E. Controversies surrounding high-protein diet intake: Satiating effect and kidney and bone health. Adv. Nutr. 2015, 6, 260–266. [Google Scholar] [CrossRef]
- Vogtschmidt, Y.D.; Raben, A.; Faber, I.; de Wilde, C.; Lovegrove, J.A.; Givens, D.I.; Pfeiffer, A.F.H.; Soedamah-Muthu, S.S. Is protein the forgotten ingredient: Effects of higher compared to lower protein diets on cardiometabolic risk factors. A systematic review and meta-analysis of randomised controlled trials. Atherosclerosis 2021, 328, 124–135. [Google Scholar] [CrossRef]
- Ko, G.J.; Rhee, C.M.; Kalantar-Zadeh, K.; Joshi, S. The Effects of High-Protein Diets on Kidney Health and Longevity. J. Am. Soc. Nephrol. 2020, 31, 1667–1679. [Google Scholar] [CrossRef]
- Tanaka, S.; Wakui, H.; Azushima, K.; Tsukamoto, S.; Yamaji, T.; Urate, S.; Suzuki, T.; Abe, E.; Taguchi, S.; Yamada, T.; et al. Effects of a High-Protein Diet on Kidney Injury under Conditions of Non-CKD or CKD in Mice. Int. J. Mol. Sci. 2023, 24, 7778. [Google Scholar] [CrossRef]
- Hahn, D.; Hodson, E.M.; Fouque, D. Low protein diets for non-diabetic adults with chronic kidney disease. Cochrane Database Syst. Rev. 2020, 10, CD001892. [Google Scholar] [CrossRef]
- Jiang, S.; Fang, J.; Li, W. Protein restriction for diabetic kidney disease. Cochrane Database Syst. Rev. 2023, 1, CD014906. [Google Scholar] [CrossRef]
- Yue, H.; Zhou, P.; Xu, Z.; Liu, L.; Zong, A.; Qiu, B.; Liu, W.; Jia, M.; Du, F.; Xu, T. Effect of low-protein diet on kidney function and nutrition in nephropathy: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2020, 39, 2675–2685. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, A.F.H.; Pedersen, E.; Schwab, U.; Riserus, U.; Aas, A.M.; Uusitupa, M.; Thanopoulou, A.; Kendall, C.; Sievenpiper, J.L.; Kahleova, H.; et al. The Effects of Different Quantities and Qualities of Protein Intake in People with Diabetes Mellitus. Nutrients 2020, 12, 365. [Google Scholar] [CrossRef] [PubMed]
- Verzola, D.; Picciotto, D.; Saio, M.; Aimasso, F.; Bruzzone, F.; Sukkar, S.G.; Massarino, F.; Esposito, P.; Viazzi, F.; Garibotto, G. Low Protein Diets and Plant-Based Low Protein Diets: Do They Meet Protein Requirements of Patients with Chronic Kidney Disease? Nutrients 2020, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- De Las Heras-Delgado, S.; Shyam, S.; Cunillera, E.; Dragusan, N.; Salas-Salvado, J.; Babio, N. Are plant-based alternatives healthier? A two-dimensional evaluation from nutritional and processing standpoints. Food Res. Int. 2023, 169, 112857. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M. Is obesity a disease? Expert Rev. Endocrinol. Metab. 2018, 13, 59–61. [Google Scholar] [CrossRef]
- Cordova, R.; Viallon, V.; Fontvieille, E.; Peruchet-Noray, L.; Jansana, A.; Wagner, K.H.; Kyro, C.; Tjonneland, A.; Katzke, V.; Bajracharya, R.; et al. Consumption of ultra-processed foods and risk of multimorbidity of cancer and cardiometabolic diseases: A multinational cohort study. Lancet Reg. Health Eur. 2023, 35, 100771. [Google Scholar] [CrossRef]
- Gibney, M.J. Ultra-Processed Foods: Definitions and Policy Issues. Curr. Dev. Nutr. 2019, 3, nzy077. [Google Scholar] [CrossRef]
- Lane, M.M.; Davis, J.A.; Beattie, S.; Gomez-Donoso, C.; Loughman, A.; O’Neil, A.; Jacka, F.; Berk, M.; Page, R.; Marx, W.; et al. Ultraprocessed food and chronic noncommunicable diseases: A systematic review and meta-analysis of 43 observational studies. Obes. Rev. 2021, 22, e13146. [Google Scholar] [CrossRef]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019, 30, 67–77.e63. [Google Scholar] [CrossRef] [PubMed]
| Strategy | Modern-Day Problem | Impact on Propensity for Weight Gain and Obesity | Potential Solution |
|---|---|---|---|
| Sleep | Deprivation | Enhanced appetite | Need for adults to get 7–8 h sleep per night |
| Listening | Distractions; Relative deafness to internal and external signals | Appetitive dysregulation; Mindless eating | Actively listen to internal (appetitive) signals; Eat to hunger |
| Routine | Busy, chaotic and unpredictable lives | Dysregulated sleep–wake cycle; Predisposition to disturbed and mindless eating behaviours | Establish and maintain regular sleep–wake cycle; Individualize lifestyle recommendations |
| De-stressing | Stress as an inherent component of modern-day life | Mindless eating and over-consumption of hyper-palatable foods; Altered neurobiology with increasingly compulsive behaviours | Implement techniques to alleviate response to stress (such as mindfulness or yoga) to improve healthy eating behaviours |
| Optimize Social Conditions | Profit-driven and irresponsible food companies; Poor governmental control and regulation of our nutrition; Stigmatization and expense of healthy foods | Unaffordability of healthy foods; Easier choice of cheaper, unhealthy foods with hedonic and addictive effects | Implement much stricter controls and regulation of food companies; Government input to improve affordability of healthy foods |
| Dietary Fibre | Impoverishment | Plethoric negative impact on health and gut microbiota; Worsened CV and inflammatory outcomes | Improve dietary fibre intake generally; Increased availability and consumption of plant-based foods; Added fibre to UPFs |
| Carbohydrate | Over-consumption | Weight gain; Predisposition to T2D and other CV RFs | LCD in shorter term; Generalized restriction of carbohydrate intake in longer term |
| Protein | Relative restriction of plant-based protein intake | Reduced muscle bulk; Reduced metabolic efficiency and appetite control | Increase availability and consumption of plant-based protein |
| UPFs | Ubiquitous availability and over-consumption | Weight gain; Development of Metabolic Syndrome; Increased mortality | Restrict consumption of UPFs; Food companies to fortify UPFs with key micronutrients and fibre |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. Dietary and Lifestyle Strategies for Obesity. Nutrients 2024, 16, 2714. https://doi.org/10.3390/nu16162714
Barber TM, Kabisch S, Pfeiffer AFH, Weickert MO. Dietary and Lifestyle Strategies for Obesity. Nutrients. 2024; 16(16):2714. https://doi.org/10.3390/nu16162714
Chicago/Turabian StyleBarber, Thomas M., Stefan Kabisch, Andreas F. H. Pfeiffer, and Martin O. Weickert. 2024. "Dietary and Lifestyle Strategies for Obesity" Nutrients 16, no. 16: 2714. https://doi.org/10.3390/nu16162714
APA StyleBarber, T. M., Kabisch, S., Pfeiffer, A. F. H., & Weickert, M. O. (2024). Dietary and Lifestyle Strategies for Obesity. Nutrients, 16(16), 2714. https://doi.org/10.3390/nu16162714







