Sportomics Analyses of the Exercise-Induced Impact on Amino Acid Metabolism and Acute-Phase Protein Kinetics in Female Olympic Athletes
Highlights
- Utilizing data from only male athletes to plan interventions for female athletes may be ineffective or even detrimental to their health, emphasizing the need for sex-specific approaches.
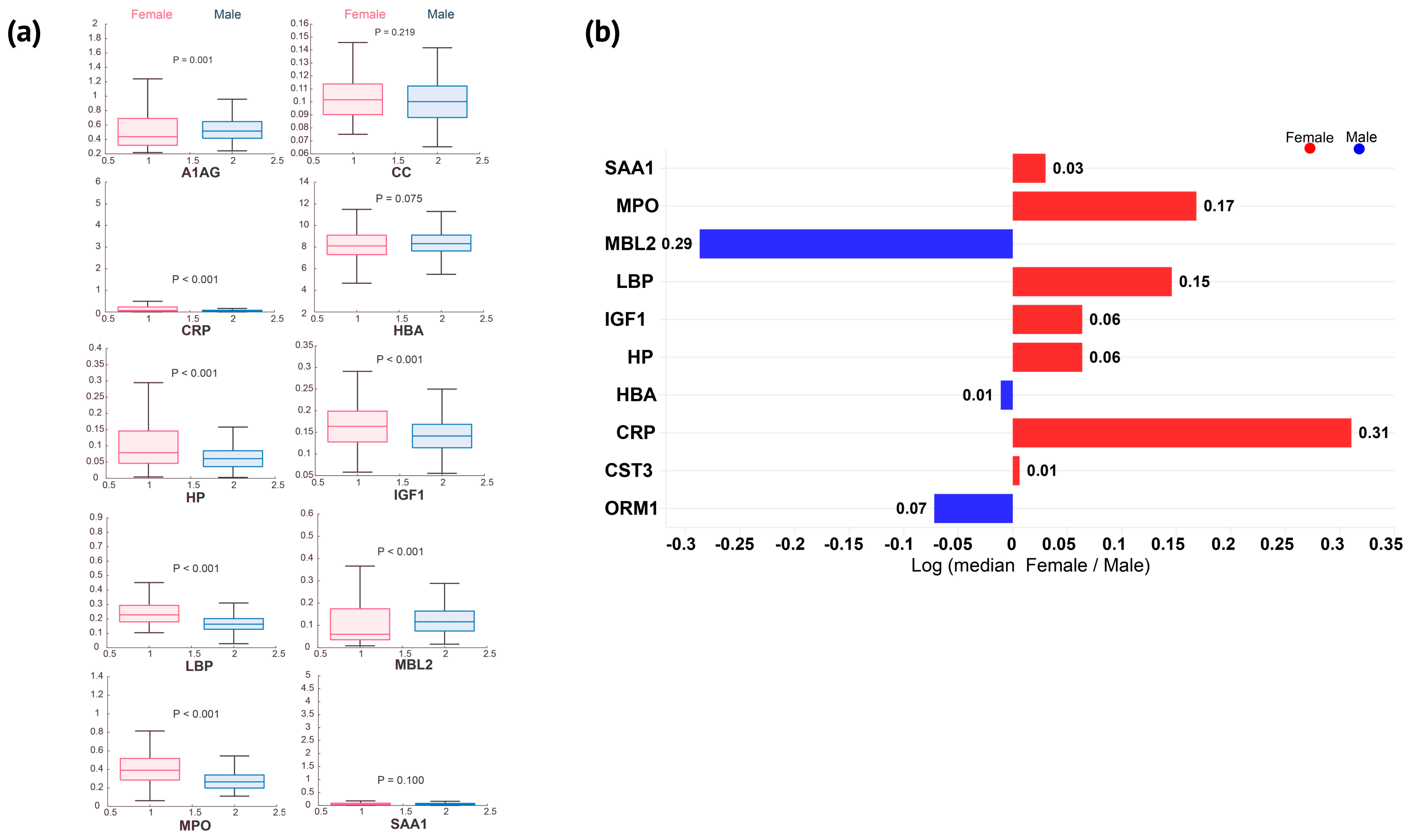
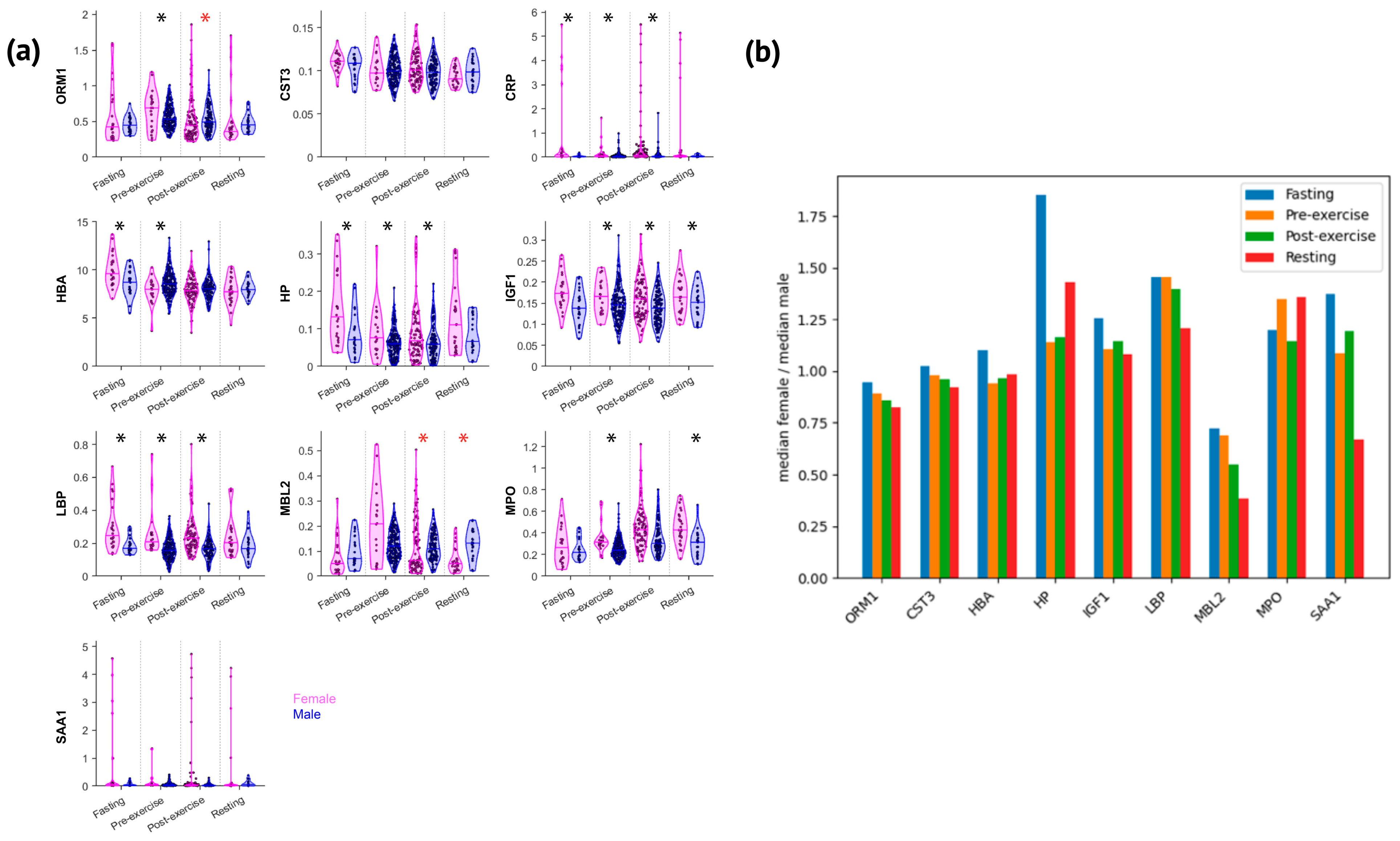
- Elite female athletes show elevated concentrations of most acute phase proteins, each with distinct biological significance (e.g., CRP, LBP, MPO, HP, and IGF1); exercise reduces sex differences and narrows the female-to-male ratio across various proteins.
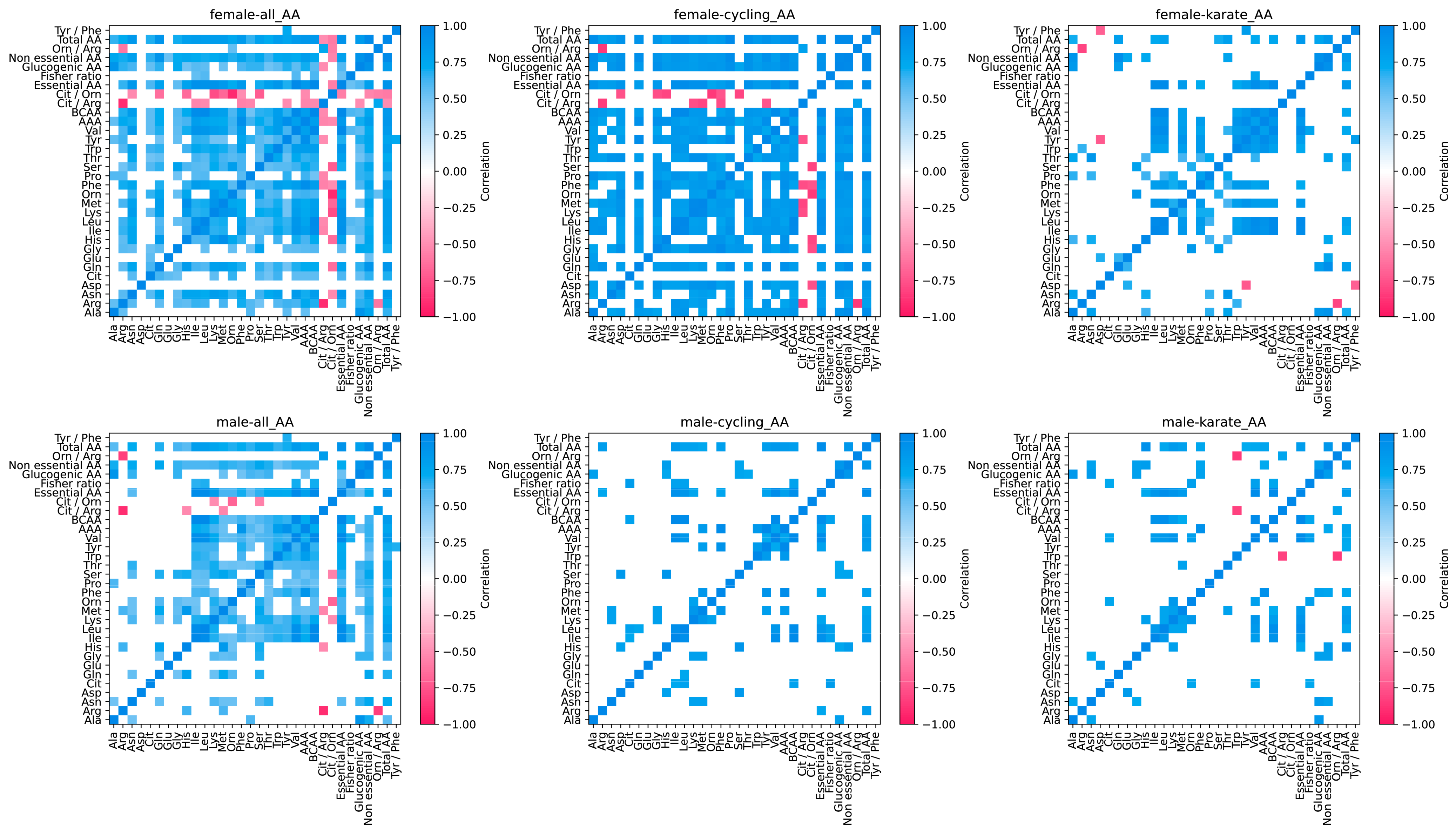
- Female athletes exhibit lower concentrations of nearly all amino acids except glutamate and alanine.
- Female athletes exhibit lower exercise-induced amino acid responses that are associated with the development of central fatigue; however, their pre-exercise profiles suggest a greater susceptibility to this type of fatigue.
- The extreme immunometabolic challenges experienced by elite athletes provide a valuable model for understanding metabolic stress in hypermetabolic and inflammatory conditions during health and disease, creating a bridge for translational studies.
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Sample Collection and Amino Acid and APP Quantifications
2.3. Bioinformatic Analysis
3. Results
3.1. Acute-Phase Proteins
3.2. Amino Acids
4. Discussion
4.1. Acute-Phase Protein Response in Elite Female Athletes
4.2. Amino Acid Metabolism in Elite Female Athletes
4.3. The Immune-Metabolic Response
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xing, X.; Sun, Q.; Wang, R.; Wang, Y. Impacts of glutamate, an exercise-responsive metabolite on insulin signaling. Life Sci. 2024, 341, 122471. [Google Scholar] [CrossRef] [PubMed]
- Pataky, M.W.; Kumar, A.P.; Gaul, D.A.; Dasari, S.; Robinson, M.M.; Klaus, K.A.; Kumar, A.A.; Fernandez, F.M.; Nair, K.S. Divergent Skeletal Muscle Metabolomic Signatures of Different Exercise Training Modes Independently Predict Cardiometabolic Risk Factors. Diabetes 2024, 73, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Terink, R.; Ten Haaf, D.; Bongers, C.W.G.; Balvers, M.G.J.; Witkamp, R.F.; Mensink, M.; Eijsvogels, T.M.H.; Klein Gunnewiek, J.M.T.; Hopman, M.T.E. Changes in iron metabolism during prolonged repeated walking exercise in middle-aged men and women. Eur. J. Appl. Physiol. 2018, 118, 2349–2357. [Google Scholar] [CrossRef] [PubMed]
- Maestre-Hernández, A.B.; Pérez-Piñero, S.; López-Román, F.J.; Andreu-Caravaca, L.; Luque-Rubia, A.J.; Ramos-Campo, D.J.; Díaz-Silvestre, M.J.; Ávila-Gandía, V. Effect of a sustained-release formulation of β-alanine on laboratory parameters and paresthesia in recreational trained men: A randomized double-blind placebo-controlled study. Front. Nutr. 2023, 10, 1213105. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.L.; Zheng, A.C.; Suzuki, K.; Lu, C.C.; Wang, C.Y.; Fang, S.H. Supplementation of L-glutamine enhanced mucosal immunity and improved hormonal status of combat-sport athletes. J. Int. Soc. Sports Nutr. 2024, 21, 2300259. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Fiandor, E.M.; García, J.F.; Fernández-Lázaro, D.; Fiandor, E.M.; García, J.F.; Busto, N.; Santamaría-Peláez, M.; Gutiérrez-Abejón, E.; Roche, E.; et al. β-Alanine Supplementation in Combat Sports: Evaluation of Sports Performance, Perception, and Anthropometric Parameters and Biochemical Markers-A Systematic Review of Clinical Trials. Nutrients 2023, 15, 3755. [Google Scholar] [CrossRef]
- Corwin, D.J.; Myers, S.R.; Arbogast, K.B.; Lim, M.M.; Elliott, J.E.; Metzger, K.B.; LeRoux, P.; Elkind, J.; Metheny, H.; Berg, J.; et al. Head Injury Treatment with HEalthy and Advanced Dietary Supplements (HIT HEADS): A pilot randomized controlled trial of the tolerability, safety, and efficacy of branched chain amino acids (BCAAs) in the treatment of concussion in adolescents and young adults. J. Neurotrauma 2024, 41, 1299–1309. [Google Scholar] [CrossRef]
- França, T.C.L.; Muniz-Santos, R.; Caetano, L.C.; Souza, G.H.M.F.; Goulart, H.F.; Assis, M.; Bottino, A.; Bassini, A.; Santana, A.E.G.; Prado, E.S.; et al. A sportomics soccer investigation unveils an exercise-induced shift in tyrosine metabolism leading to hawkinsinuria. Front. Nutr. 2023, 10, 1169188. [Google Scholar] [CrossRef]
- Socha, E.; Koba, M.; Kośliński, P. Amino acid profiling as a method of discovering biomarkers for diagnosis of neurodegenerative diseases. Amino Acids 2019, 51, 367–371. [Google Scholar] [CrossRef]
- Sato, H.; Takado, Y.; Toyoda, S.; Tsukamoto-Yasui, M.; Minatohara, K.; Takuwa, H.; Urushihata, T.; Takahashi, M.; Shimojo, M.; Ono, M.; et al. Neurodegenerative processes accelerated by protein malnutrition and decelerated by essential amino acids in a tauopathy mouse model. Sci. Adv. 2021, 7, eabd5046. [Google Scholar] [CrossRef]
- Chen, S.; Minegishi, Y.; Hasumura, T.; Shimotoyodome, A.; Ota, N. Involvement of ammonia metabolism in the improvement of endurance performance by tea catechins in mice. Sci. Rep. 2020, 10, 6065. [Google Scholar] [CrossRef] [PubMed]
- Blomstrand, E. A role for branched-chain amino acids in reducing central fatigue. J. Nutr. 2006, 136, 544S–547S. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Muscle Amino Acid and Adenine Nucleotide Metabolism during Exercise and in Liver Cirrhosis: Speculations on How to Reduce the Harmful Effects of Ammonia. Metabolites 2022, 12, 971. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Smeeton, N.J.; Watt, P.W. Ammonia metabolism, the brain and fatigue; revisiting the link. Prog. Neurobiol. 2010, 91, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, K.; Shimizu, Y. Branched-chain amino acids in liver diseases. Transl. Gastroenterol. Hepatol. 2018, 3, 47. [Google Scholar] [CrossRef]
- Bassini-Cameron, A.; Monteiro, A.; Gomes, A.; Werneck-de-Castro, J.P.; Cameron, L. Glutamine protects against increases in blood ammonia in football players in an exercise intensity-dependent way. Br. J. Sports Med. 2008, 42, 260–266. [Google Scholar] [CrossRef]
- Fischer, J.E.; Baldessarini, R.J. False neurotransmitters and hepatic failure. Lancet 1971, 2, 75–80. [Google Scholar] [CrossRef]
- Newsholme, E.A.; Acworth, I.N.; Blomstrand, E. Amino acids, brain neurotransmitters and a functional link between muscle and brain that is important in sustained exercise. In Advances in Myochemistry; Benzi, G., Ed.; John Libbey Eurotext Ltd.: London, UK, 1987; pp. 127–133. [Google Scholar]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel metabolic and physiological functions of branched chain amino acids: A review. J. Anim. Sci. Biotechnol. 2017, 8, 10. [Google Scholar] [CrossRef]
- Schranner, D.; Kastenmüller, G.; Schönfelder, M.; Römisch-Margl, W.; Wackerhage, H. Metabolite Concentration Changes in Humans After a Bout of Exercise: A Systematic Review of Exercise Metabolomics Studies. Sports Med. Open 2020, 6, 11. [Google Scholar] [CrossRef]
- Mantovani, A.; Garlanda, C. Humoral Innate Immunity and Acute-Phase Proteins. N. Engl. J. Med. 2023, 388, 439–452. [Google Scholar] [CrossRef]
- Weight, L.M.; Alexander, D.; Jacobs, P. Strenuous exercise: Analogous to the acute-phase response? Clin. Sci. 1991, 81, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Bassini, A.; Sartoretto, S.; Jurisica, L.; Magno-França, A.; Anderson, L.; Pearson, T.; Razavi, M.; Chandran, V.; Martin, L., 3rd; Jurisica, I.; et al. Sportomics method to assess acute phase proteins in Olympic level athletes using dried blood spots and multiplex assay. Sci. Rep. 2022, 12, 19824. [Google Scholar] [CrossRef] [PubMed]
- Pakula, P.D.; Halama, A.; Al-Dous, E.K.; Al-Dous, E.K.; Johnson, S.J.; Filho, S.A.; Suhre, K.; Vinardell, T. Characterization of exercise-induced hemolysis in endurance horses. Front. Vet. Sci. 2023, 10, 1115776. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Anderson, N.L.; Yip, R.; Pope, M.E.; Pearson, T.W. Multiplexed longitudinal measurement of protein biomarkers in DBS using an automated SISCAPA workflow. Bioanalysis 2016, 8, 1597–1609. [Google Scholar] [CrossRef]
- Fallon, K.E.; Fallon, S.K.; Boston, T. The acute phase response and exercise: Court and field sports. Br. J. Sports Med. 2001, 35, 170–173. [Google Scholar] [CrossRef]
- Carvalho Filho, W.P.; Girardi, F.M.; Souto, P.C.; Ortega Orozco, A.M.; de Oliveira, T.; Dornelas, L.R.S.M.; Argumedo Jimenez, A.K.; Fonseca, L.A. Profile of Acute-Phase Proteins of Horses Submitted to Low-Level Show Jumping Classes. J. Equine. Vet. Sci. 2020, 91, 103105. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, J.; Jiang, Y.; Ju, Y.; He, J.; Yu, K.; Kan, G.; Zhang, H. Determination of amino acid metabolic diseases from dried blood spots with a rapid extraction method coupled with nanoelectrospray ionization mass spectrometry. Talanta 2024, 272, 125768. [Google Scholar] [CrossRef]
- Schakelaar, M.Y.; Kemperman, H.; Schoneveld, A.H.; Hoefer, I.E.; Tiel Groenestege, W.M. Analysis of C-reactive protein from finger stick dried blood spot to predict high risk of cardiovascular disease. Sci. Rep. 2023, 13, 2515. [Google Scholar] [CrossRef]
- Anderson, L.; Razavi, M.; Pope, M.E.; Pope, M.E.; Yip, R.; Cameron, L.C.; Bassini-Cameron, A.; Pearson, T.W. Precision multiparameter tracking of inflammation on timescales of hours to years using serial dried blood spots. Bioanalysis 2020, 12, 937–955. [Google Scholar] [CrossRef]
- Ansdell, P.; Thomas, K.; Hicks, K.M.; Hunter, S.K.; Howatson, G.; Goodall, S. Physiological sex differences affect the integrative response to exercise: Acute and chronic implications. Exp. Physiol. 2020, 105, 2007–2021. [Google Scholar] [CrossRef]
- Kobayashi, R.; Shimomura, Y.; Murakami, T.; Nakai, N.; Fujitsuka, N.; Otsuka, M.; Arakawa, N.; Popov, K.M.; Harris, R.A. Gender difference in regulation of branched-chain amino acid catabolism. Biochem. J. 1997, 327, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, K.J.; Arieta, L.R.; Brewer, G.J.; Hoselton, A.L.; Gould, L.M.; Smith-Ryan, A.E. Sex differences and considerations for female specific nutritional strategies: A narrative review. J. Int. Soc. Sports Nutr. 2021, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.A.J.; Wang, G.; Lu, H.; Huang, X.; Liu, Y.; Zha, W.; Hao, H.; Zhang, Y.; Liu, L.; Gu, S.; et al. Metabolomic investigation into variation of endogenous metabolites in professional athletes subject to strength-endurance training. J. Appl. Physiol. 2009, 106, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Muniz-Santos, R.; França, A.; Jurisica, I.; Cameron, L.C. From Microcosm to Macrocosm: The -Omics, Multiomics, and Sportomics Approaches in Exercise and Sports. OMICS 2023, 27, 499–518. [Google Scholar] [CrossRef]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Li, X.; Guo, X. Factors affecting the protection of data rights in sports events: A configurational analysis. Sci. Rep. 2024, 14, 5353. [Google Scholar] [CrossRef]
- Testoni, D.; Hornik, C.P.; Smith, P.B.; Benjamin, D.K.; McKinney, R.E. Sports medicine and ethics. Am. J. Bioeth. 2013, 13, 4–12. [Google Scholar] [CrossRef]
- Lin, C.Y.; Casey, E.; Herman, D.C.; Katz, N.; Tenforde, A.S. Sex Differences in Common Sports Injuries. PMR 2018, 10, 1073–1082. [Google Scholar] [CrossRef]
- Martínez-Fortuny, N.; Alonso-Calvete, A.; Da Cuña-Carrera, I.; Abalo-Núñez, R. Menstrual Cycle and Sport Injuries: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 3264. [Google Scholar] [CrossRef]
- Factsheet-Women in the Olympic Movement. International Olympic Committee. Available online: https://stillmed.olympics.com/media/Documents/Olympic-Movement/Factsheets/Women-in-the-Olympic-Movement.pdf (accessed on 15 June 2024).
- Hakimi, O.; Cameron, L.C. Effect of Exercise on Ovulation: A Systematic Review. Sports Med. 2017, 47, 1555–1567. [Google Scholar] [CrossRef]
- National Health Council. Resolution No. 466 of December 12, 2012. Brasília: Official Gazette of the Federative Republic of Brazil. 2013. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/cns/2013/res0466_12_12_2012.html (accessed on 15 June 2024).
- Wong, M.H. Grpandplot, Version 1.0.0; GitHub: San Francisco, CA, USA, 2023. Available online: https://github.com/manhowong/grpandplot/releases/tag/v1.0.0 (accessed on 29 December 2023).
- Brown, K.R.; Otasek, D.; Ali, M.; McGuffin, M.J.; Xie, W.; Devani, B.; van Toch, I.L.; Jurisica, I. NAViGaTOR: Network Analysis, Visualization and Graphing Toronto. Bioinformatics 2009, 25, 3327–3329. [Google Scholar] [CrossRef] [PubMed]
- Déglon, J.; Thomas, A.; Mangin, P.; Staub, C. Direct analysis of dried blood spots coupled with mass spectrometry: Concepts and biomedical applications. Anal. Bioanal. Chem. 2012, 402, 2485–2498. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.Y.; Yao, Y.M. The Clinical Significance and Potential Role of C-Reactive Protein in Chronic Inflammatory and Neurodegenerative Diseases. Front. Immunol. 2018, 9, 1302. [Google Scholar] [CrossRef]
- Rizo-Téllez, S.A.; Sekheri, M.; Filep, J.G. C-reactive protein: A target for therapy to reduce inflammation. Front. Immunol. 2023, 14, 1237729. [Google Scholar] [CrossRef]
- Lee, E.C.; Fragala, M.S.; Kavouras, S.A.; Queen, R.M.; Pryor, J.L.; Casa, D.J. Biomarkers in Sports and Exercise: Tracking Health, Performance, and Recovery in Athletes. J. Strength Cond. Res. 2017, 31, 2920–2937. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Tian, W.; Wang, T.; Jia, J.; Lai, R.; Wang, T.; Zhang, Z.; Song, L.; Ju, J.; et al. The effect of various types and doses of statins on C-reactive protein levels in patients with dyslipidemia or coronary heart disease: A systematic review and network meta-analysis. Front. Cardiovasc. Med. 2022, 9, 936817. [Google Scholar] [CrossRef]
- Albert, M.A.; Danielson, E.; Rifai, N.; Ridker, P.M.; Investigators, P. Effect of statin therapy on C-reactive protein levels: The pravastatin inflammation/CRP evaluation (PRINCE): A randomized trial and cohort study. JAMA 2001, 286, 64–70. [Google Scholar] [CrossRef]
- Lassale, C.; Batty, G.D.; Steptoe, A.; Cadar, D.; Akbaraly, T.N.; Kivimäki, M.; Zaninotto, P. Association of 10-Year C-Reactive Protein Trajectories With Markers of Healthy Aging: Findings From the English Longitudinal Study of Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 195–203. [Google Scholar] [CrossRef]
- Khera, A.; McGuire, D.K.; Murphy, S.A.; Stanek, H.G.; Das, S.R.; Vongpatanasin, W.; Wians, F.H., Jr.; Grundy, S.M.; de Lemos, J.A. Race and gender differences in C-reactive protein levels. J. Am. Coll. Cardiol. 2005, 46, 464–469. [Google Scholar] [CrossRef]
- Lakoski, S.G.; Cushman, M.; Criqui, M.; Rundek, T.; Blumenthal, R.S.; D’Agostino, R.B., Jr.; Herrington, D.M. Gender and C-reactive protein: Data from the Multiethnic Study of Atherosclerosis (MESA) cohort. Am. Heart J. 2006, 152, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Valentine, R.J.; Vieira, V.J.; Woods, J.A.; Evans, E.M. Stronger relationship between central adiposity and C-reactive protein in older women than men. Menopause 2009, 16, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.; Vega, G.L.; Das, S.R.; Ayers, C.; McGuire, D.K.; Grundy, S.M.; de Lemos, J.A. Sex differences in the relationship between C-reactive protein and body fat. J. Clin. Endocrinol. Metab. 2009, 94, 3251–3258. [Google Scholar] [CrossRef] [PubMed]
- Rogowski, O.; Zeltser, D.; Shapira, I.; Shapira, I.; Burke, M.; Zakut, V.; Mardi, T.; Ben-Assayag, E.; Serov, J.; Rozenblat, M.; et al. Gender difference in C-reactive protein concentrations in individuals with atherothrombotic risk factors and apparently healthy ones. Biomarkers 2004, 9, 85–92. [Google Scholar] [CrossRef]
- Scharhag, J.; Meyer, T.; Gabriel, H.H.; Schlick, B.; Faude, O.; Kindermann, W. Does prolonged cycling of moderate intensity affect immune cell function? Br. J. Sports Med. 2005, 39, 171–177. [Google Scholar] [CrossRef]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise-A Systematic Review. Front. Physiol. 2019, 10, 1550. [Google Scholar] [CrossRef]
- Ha, E.K.; Kim, J.H.; Yon, D.K.; Lee, S.W.; Kim, M.A.; Lee, K.S.; Sung, M.; Jee, H.M.; Shin, Y.H.; Han, M.Y.; et al. Association of serum lipopolysaccharide-binding protein level with sensitization to food allergens in children. Sci. Rep. 2021, 11, 2143. [Google Scholar] [CrossRef]
- Pastor Rojo, O.; López San Román, A.; Albéniz Arbizu, E.; de la Hera Martínez, A.; Ripoll Sevillano, E.; Albillos Martínez, A. Serum lipopolysaccharide-binding protein in endotoxemic patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2007, 13, 269–277. [Google Scholar] [CrossRef]
- Costa, R.J.S.; Snipe, R.M.J.; Kitic, C.M.; Gibson, P.R. Systematic review: Exercise-induced gastrointestinal syndrome-implications for health and intestinal disease. Aliment Pharmacol. Ther. 2017, 46, 246–265. [Google Scholar] [CrossRef]
- Clauss, M.; Gérard, P.; Mosca, A.; Leclerc, M. Interplay Between Exercise and Gut Microbiome in the Context of Human Health and Performance. Front. Nutr. 2021, 8, 637010. [Google Scholar] [CrossRef]
- Chantler, S.; Griffiths, A.; Matu, J.; Davison, G.; Jones, B.; Deighton, K. The Effects of Exercise on Indirect Markers of Gut Damage and Permeability: A Systematic Review and Meta-analysis. Sports Med. 2021, 51, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Marriott, I.; Bost, K.L.; Huet-Hudson, Y.M. Sexual dimorphism in expression of receptors for bacterial lipopolysaccharides in murine macrophages: A possible mechanism for gender-based differences in endotoxic shock susceptibility. J. Reprod. Immunol. 2006, 71, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Vallelian, F.; Buehler, P.W.; Schaer, D.J. Hemolysis, free hemoglobin toxicity, and scavenger protein therapeutics. Blood 2022, 140, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Sanchis-Gomar, F. Epidemiological, biological and clinical update on exercise-induced hemolysis. Ann. Transl. Med. 2019, 7, 270. [Google Scholar] [CrossRef]
- Lee, P.L.; Lee, K.Y.; Cheng, T.M.; Chuang, H.C.; Wu, S.M.; Feng, P.H.; Liu, W.T.; Chen, K.Y.; Ho, S.C. Relationships of Haptoglobin Phenotypes with Systemic Inflammation and the Severity of Chronic Obstructive Pulmonary Disease. Sci. Rep. 2019, 9, 189. [Google Scholar] [CrossRef]
- Wan, B.N.; Zhou, S.G.; Wang, M.; Zhang, X.; Ji, G. Progress on haptoglobin and metabolic diseases. World J. Diabetes 2021, 12, 206–214. [Google Scholar] [CrossRef]
- Bessa, A.; Nissenbaum, M.; Monteiro, A.; Gandra, P.G.; Nunes, L.S.; Bassini-Cameron, A.; Werneck-de-Castro, J.P.S.; Vaz de Macedo, D.; Cameron, L.C. High-intensity ultraendurance promotes early release of muscle injury markers. Br. J. Sports Med. 2008, 42, 889–893. [Google Scholar] [CrossRef]
- Morozov, V.I.; Pryatkin, S.A.; Kalinski, M.I.; Rogozkin, V.A. Effect of exercise to exhaustion on myeloperoxidase and lysozyme release from blood neutrophils. Eur. J. Appl. Physiol. 2003, 89, 257–262. [Google Scholar] [CrossRef]
- Bury, T.B.; Pirnay, F. Effect of prolonged exercise on neutrophil myeloperoxidase secretion. Int. J. Sports Med. 1995, 16, 410–412. [Google Scholar] [CrossRef]
- Morozov, V.I.; Tsyplenkov, P.V.; Golberg, N.D.; Kalinski, M.I. The effects of high-intensity exercise on skeletal muscle neutrophil myeloperoxidase in untrained and trained rats. Eur. J. Appl. Physiol. 2006, 97, 716–722. [Google Scholar] [CrossRef]
- Ytting, H.; Christensen, I.J.; Thiel, S.; Jensenius, J.C.; Svendsen, M.N.; Nielsen, L.; Lottenburger, T.; Nielsen, H.J. Biological variation in circulating levels of mannan-binding lectin (MBL) and MBL-associated serine protease-2 and the influence of age, gender and physical exercise. Scand. J. Immunol. 2007, 66, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Aicale, R.; Tarantino, D.; Maffulli, N. Overuse injuries in sport: A comprehensive overview. J. Orthop. Surg. Res. 2018, 13, 309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Takahashi, M.; Noguchi, Y.; Sugimoto, T.; Kimura, T.; Okumura, A.; Ishikawa, T.; Kakumu, S. Plasma amino acid profiles applied for diagnosis of advanced liver fibrosis in patients with chronic hepatitis C infection. Hepatol. Res. 2006, 34, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Felig, P.; Marliss, E.; Ohman, J.L.; Cahill, C.F. Plasma amino acid levels in diabetic ketoacidosis. Diabetes 1970, 19, 727–728. [Google Scholar] [CrossRef]
- Felig, P.; Marliss, E.; Cahill, G.F. Plasma amino acid levels and insulin secretion in obesity. N. Engl. J. Med. 1969, 281, 811–816. [Google Scholar] [CrossRef]
- Park, J.G.; Tak, W.Y.; Park, S.Y.; Kweon, Y.O.; Chung, W.J.; Jang, B.K.; Bae, S.H.; Lee, H.J.; Jang, J.Y.; Suk, K.T.; et al. Effects of Branched-Chain Amino Acid (BCAA) Supplementation on the Progression of Advanced Liver Disease: A Korean Nationwide, Multicenter, Prospective, Observational, Cohort Study. Nutrients 2020, 12, 1429. [Google Scholar] [CrossRef]
- Chen, Q.; Liang, X.; Wu, T.; Jiang, J.; Jiang, Y.; Zhang, S.; Ruan, Y.; Zhang, H.; Zhang, C.; Chen, P. Integrative analysis of metabolomics and proteomics reveals amino acid metabolism disorder in sepsis. J. Transl. Med. 2022, 20, 123. [Google Scholar] [CrossRef]
- Muniz-Santos, R.; Lucieri-Costa, G.; de Almeida, M.A.P.; Moraes-de-Souza, I.; Dos Santos Mascarenhas Brito, M.A.; Ribeiro Silva, A.; Gonçalves-de-Albuquerque, C.F. Lipid oxidation dysregulation: An emerging player in the pathophysiology of sepsis. Front. Immunol. 2023, 14, 1224335. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, X.; Cheng, C.; Yu, W.; Yi, P. Metabolism of Amino Acids in Cancer. Front. Cell Dev. Biol. 2020, 8, 603837. [Google Scholar] [CrossRef]
- Begum, S.; Johnson, B.Z.; Morillon, A.C.; Yang, R.; Bong, S.H.; Whiley, L.; Gray, N.; Fear, V.S.; Cuttle, L.; Holland, A.J.A. Systemic long-term metabolic effects of acute non-severe paediatric burn injury. Sci. Rep. 2022, 12, 13043. [Google Scholar] [CrossRef]
- Vanweert, F.; Boone, S.C.; Brouwers, B.; Mook-Kanamori, D.O.; de Mutsert, R.; Rosendaal, F.R.; Lamb, H.J.; Schrauwen-Hinderling, V.B.; Schrauwen, P.; Hesselink, M.K.C.; et al. The effect of physical activity level and exercise training on the association between plasma branched-chain amino acids and intrahepatic lipid content in participants with obesity. Int. J. Obes. 2021, 45, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Mach, N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: A systematic review for athletes. J. Int. Soc. Sports Nutr. 2016, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Bassini, A.; Cameron, L.C. Sportomics: Building a new concept in metabolic studies and exercise science. Biochem. Biophys. Res. Commun. 2014, 445, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Ishikura, K.; Ra, S.-G.; Ohmori, H. Exercise-induced changes in amino acid levels in skeletal muscle and plasma. J. Phys. Fit. Sports Med. 2013, 2, 301–310. [Google Scholar] [CrossRef]
- Pedersen, E.B.; Jørgensen, M.E.; Pedersen, M.B.; Siggaard, C.; Sørensen, T.B.; Mulvad, G.; Hansen, J.C.; Torstensen, A.M.; Aagaard, O.; Skjoldborg, H. Plasma amino acids in Greenlanders and Danes: Influence of seasons, residence, ethnicity, and diet. Am. J. Hum. Biol. 2006, 18, 99–111. [Google Scholar] [CrossRef]
- Kawase, T.; Nagasawa, M.; Ikeda, H.; Yasuo, S.; Koga, Y.; Furuse, M. Gut microbiota of mice putatively modifies amino acid metabolism in the host brain. Br. J. Nutr. 2017, 117, 775–783. [Google Scholar] [CrossRef]
- Vignoli, A.; Tenori, L.; Luchinat, C.; Saccenti, E. Age and Sex Effects on Plasma Metabolite Association Networks in Healthy Subjects. J. Proteome Res. 2018, 17, 97–107. [Google Scholar] [CrossRef]
- Andraos, S.; Lange, K.; Clifford, S.A.; Clifford, S.A.; Jones, B.; Thorstensen, E.B.; Wake, M.; Burgner, D.P.; Saffery, R.; O’Sullivan, J.M.; et al. Population epidemiology and concordance for plasma amino acids and precursors in 11-12-year-old children and their parents. Sci. Rep. 2021, 11, 3619. [Google Scholar] [CrossRef]
- Chekmeneva, E.; Dos Santos Correia, G.; Gómez-Romero, M.; Stamler, J.; Chan, Q.; Elliott, P.; Nicholson, J.K.; Holmes, E. Ultra-Performance Liquid Chromatography-High-Resolution Mass Spectrometry and Direct Infusion-High-Resolution Mass Spectrometry for Combined Exploratory and Targeted Metabolic Profiling of Human Urine. J. Proteome Res. 2018, 17, 3492–3502. [Google Scholar] [CrossRef]
- Lawton, K.A.; Berger, A.; Mitchell, M.; Milgram, K.E.; Evans, A.M.; Guo, L.; Hanson, R.W.; Kalhan, S.C.; Ryals, J.A.; Milburn, M.V.; et al. Analysis of the adult human plasma metabolome. Pharmacogenomics 2008, 9, 383–397. [Google Scholar] [CrossRef]
- Jové, M.; Maté, I.; Naudí, A.; Mota-Martorell, N.; Portero-Otín, M.; De la Fuente, M.; Pamplona, R. Human Aging Is a Metabolome-related Matter of Gender. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.E.; Siskos, A.P.; Maitre, L.; Robinson, O.; Athersuch, T.J.; Want, E.J.; Urquiza, J.; Casas, M.; Vafeiadi, M.; Roumeliotaki, T.; et al. Determinants of the urinary and serum metabolome in children from six European populations. BMC Med. 2018, 16, 202. [Google Scholar] [CrossRef] [PubMed]
- Bassini, A.; Magalhães-Neto, A.M.; Sweet, E.; Bottino, A.; Veiga, C.; Tozzi, M.B.; Pickard, M.B.; Cameron, L.C. Caffeine decreases systemic urea in elite soccer players during intermittent exercise. Med. Sci. Sports Exerc. 2013, 45, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.; Keul, J. Serum Alanine During Long-Lasting Physical Exercise. Int. J. Sports Med. 1980, 1, 199–202. [Google Scholar] [CrossRef]
- Henriksson, J. Effect of exercise on amino acid concentrations in skeletal muscle and plasma. J. Exp. Biol. 1991, 160, 149–165. [Google Scholar] [CrossRef]
- Castell, L.M.; Newsholme, E.A. Glutamine and the effects of exhaustive exercise upon the immune response. Can. J. Physiol. Pharmacol. 1998, 76, 524–532. [Google Scholar] [CrossRef]
- Essen, P.; Wernerman, J.; Sonnenfeld, T.; Thunell, S.; Vinnars, E. Free amino acids in plasma and muscle during 24 hours post-operatively-a descriptive study. Clin. Physiol. 1992, 12, 163–177. [Google Scholar] [CrossRef]
- Parry-Billings, M.; Budgett, R.; Koutedakis, Y.; Blomstrand, E.; Brooks, S.; Williams, C.; Calder, P.C.; Pilling, S.; Baigrie, R.; Newsholme, E.A. Plasma amino acid concentrations in the overtraining syndrome: Possible effects on the immune system. Med. Sci. Sports Exerc. 1992, 24, 1353–1358. [Google Scholar] [CrossRef]
- Mourtzakis, M.; Graham, T.E. Glutamate ingestion and its effects at rest and during exercise in humans. J. Appl. Physiol. 2002, 93, 1251–1259. [Google Scholar] [CrossRef]
- Resende, N.M.; de Magalhães Neto, A.M.; Bachini, F.; de Castro, L.E.; Bassini, A.; Cameron, L.C. Metabolic changes during a field experiment in a world-class windsurfing athlete: A trial with multivariate analyses. OMICS 2011, 15, 695–704. [Google Scholar] [CrossRef]
- Blomstrand, E.; Celsing, F.; Newsholme, E.A. Changes in plasma concentrations of aromatic and branched-chain amino acids during sustained exercise in man and their possible role in fatigue. Acta Physiol. Scand. 1988, 133, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Gumus Balikcioglu, P.; Ramaker, M.E.; Mason, K.A.; Huffman, K.M.; Johnson, J.L.; Ilkayeva, O.; Muehlbauer, M.J.; Freemark, M.; Kraus, W.E. Branched-Chain Amino Acid Catabolism and Cardiopulmonary Function Following Acute Maximal Exercise Testing in Adolescents. Front. Cardiovasc. Med. 2021, 8, 721354. [Google Scholar] [CrossRef] [PubMed]
- Margolis, L.M.; Karl, J.P.; Wilson, M.A.; Coleman, J.L.; Whitney, C.C.; Pasiakos, S.M. Serum Branched-Chain Amino Acid Metabolites Increase in Males When Aerobic Exercise Is Initiated with Low Muscle Glycogen. Metabolites 2021, 11, 828. [Google Scholar] [CrossRef] [PubMed]
- Margolis, L.M.; Pasiakos, S.M.; Karl, J.P.; Rood, J.C.; Cable, S.J.; Williams, K.W.; Young, A.J.; McClung, J.P. Differential effects of military training on fat-free mass and plasma amino acid adaptations in men and women. Nutrients 2012, 4, 2035–2046. [Google Scholar] [CrossRef]
- Okamura, K.; Matsubara, F.; Yoshioka, Y.; Kikuchi, N.; Kikuchi, Y.; Kohri, H. Exercise-induced changes in branched chain amino acid/aromatic amino acid ratio in the rat brain and plasma. Jpn J. Pharmacol. 1987, 45, 243–248. [Google Scholar] [CrossRef]
- Miyazaki, T.; Matsuzaki, Y.; Karube, M.; Bouscarel, B.; Miyakawa, S.; Tanaka, N. Amino acid ratios in plasma and tissues in a rat model of liver cirrhosis before and after exercise. Hepatol. Res. 2003, 27, 230–237. [Google Scholar] [CrossRef]
- Cotel, F.; Exley, R.; Cragg, S.J.; Perrier, J.F. Serotonin spillover onto the axon initial segment of motoneurons induces central fatigue by inhibiting action potential initiation. Proc. Natl. Acad. Sci. USA 2013, 110, 4774–4779. [Google Scholar] [CrossRef]
- Maciejak, P.; Szyndler, J.; Turzyńska, D.; Sobolewska, A.; Kołosowska, K.; Krząścik, P.; Płaźnik, A. Is the interaction between fatty acids and tryptophan responsible for the efficacy of a ketogenic diet in epilepsy? The new hypothesis of action. Neuroscience 2016, 313, 30–48. [Google Scholar] [CrossRef]
- Hunter, S.K. The Relevance of Sex Differences in Performance Fatigability. Med. Sci. Sports Exerc. 2016, 48, 2247–2256. [Google Scholar] [CrossRef]
- Gomes, M.; Santos, P.; Correia, P.; Pezarat-Correia, P.; Mendonca, G.V. Sex differences in muscle fatigue following isokinetic muscle contractions. Sci. Rep. 2021, 11, 8141. [Google Scholar] [CrossRef]
- Tornero-Aguilera, J.F.; Jimenez-Morcillo, J.; Rubio-Zarapuz, A.; Clemente-Suárez, V.J. Central and Peripheral Fatigue in Physical Exercise Explained: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 3909. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.; Goubran, M.; Bilodeau, M. Sex differences in central and peripheral fatigue induced by sustained isometric ankle plantar flexion. J. Electromyogr. Kinesiol. 2022, 65, 102676. [Google Scholar] [CrossRef] [PubMed]
- Wijnands, K.A.; Castermans, T.M.; Hommen, M.P.; Meesters, D.M.; Poeze, M. Arginine and citrulline and the immune response in sepsis. Nutrients 2015, 7, 1426–1463. [Google Scholar] [CrossRef] [PubMed]
- Turunen, S.; Huhtakangas, J.; Nousiainen, T.; Valkealahti, M.; Melkko, J.; Risteli, J.; Lehenkari, P. Rheumatoid arthritis antigens homocitrulline and citrulline are generated by local myeloperoxidase and peptidyl arginine deiminases 2, 3 and 4 in rheumatoid nodule and synovial tissue. Arthritis Res. Ther. 2016, 18, 239. [Google Scholar] [CrossRef] [PubMed]
- Galijasevic, S.; Saed, G.M.; Diamond, M.P.; Abu-Soud, H.M. Myeloperoxidase up-regulates the catalytic activity of inducible nitric oxide synthase by preventing nitric oxide feedback inhibition. Proc. Natl. Acad. Sci. USA 2003, 100, 14766–14771. [Google Scholar] [CrossRef]
- Maiocchi, S.L.; Morris, J.C.; Rees, M.D.; Thomas, S.R. Regulation of the nitric oxide oxidase activity of myeloperoxidase by pharmacological agents. Biochem. Pharmacol. 2017, 135, 90–115. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muniz-Santos, R.; Bassini, A.; Falcão, J.; Prado, E.; Martin, L., III; Chandran, V.; Jurisica, I.; Cameron, L.C. Sportomics Analyses of the Exercise-Induced Impact on Amino Acid Metabolism and Acute-Phase Protein Kinetics in Female Olympic Athletes. Nutrients 2024, 16, 3538. https://doi.org/10.3390/nu16203538
Muniz-Santos R, Bassini A, Falcão J, Prado E, Martin L III, Chandran V, Jurisica I, Cameron LC. Sportomics Analyses of the Exercise-Induced Impact on Amino Acid Metabolism and Acute-Phase Protein Kinetics in Female Olympic Athletes. Nutrients. 2024; 16(20):3538. https://doi.org/10.3390/nu16203538
Chicago/Turabian StyleMuniz-Santos, Renan, Adriana Bassini, Jefferson Falcão, Eduardo Prado, LeRoy Martin, III, Vinod Chandran, Igor Jurisica, and L. C. Cameron. 2024. "Sportomics Analyses of the Exercise-Induced Impact on Amino Acid Metabolism and Acute-Phase Protein Kinetics in Female Olympic Athletes" Nutrients 16, no. 20: 3538. https://doi.org/10.3390/nu16203538
APA StyleMuniz-Santos, R., Bassini, A., Falcão, J., Prado, E., Martin, L., III, Chandran, V., Jurisica, I., & Cameron, L. C. (2024). Sportomics Analyses of the Exercise-Induced Impact on Amino Acid Metabolism and Acute-Phase Protein Kinetics in Female Olympic Athletes. Nutrients, 16(20), 3538. https://doi.org/10.3390/nu16203538




