Exploring Diet-Based Treatments for Atrial Fibrillation: Patient Empowerment and Citizen Science as a Model for Quality-of-Life-Centered Solutions
Abstract
1. Introduction
2. Reducing AF Risk Factors through Diet
2.1. The Mediterranean Diet
2.2. Whole Foods Plant-Based Diet
3. Modifiable Risk Factors and the Effect of a Plant-Based Diet
3.1. Hypertension
3.2. Diabetes and Obesity
3.3. Inflammation
3.4. Heart Failure and Coronary Artery Disease
3.5. Epicardial Adipose Tissue
4. Patient Empowerment as a Model for Quality-of-Life-Centered Approaches
4.1. L-Glutamine and AF
4.2. Electrolytes and AF
5. Patient-Tailored AF Solutions through Citizen Science
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brundel, B.; Ai, X.; Hills, M.T.; Kuipers, M.F.; Lip, G.Y.H.; de Groot, N.M.S. Atrial fibrillation. Nat. Rev. Dis. Prim. 2022, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Linz, D.; Gawalko, M.; Betz, K.; Hendriks, J.M.; Lip, G.Y.H.; Vinter, N.; Guo, Y.; Johnsen, S. Atrial fibrillation: Epidemiology, screening and digital health. Lancet Reg. Health Eur. 2024, 37, 100786. [Google Scholar] [CrossRef] [PubMed]
- Morseth, B.; Geelhoed, B.; Linneberg, A.; Johansson, L.; Kuulasmaa, K.; Salomaa, V.; Iacoviello, L.; Costanzo, S.; Soderberg, S.; Niiranen, T.J.; et al. Age-specific atrial fibrillation incidence, attributable risk factors and risk of stroke and mortality: Results from the MORGAM Consortium. Open Heart 2021, 8, e001624. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2020, 42, 373–498. [Google Scholar] [CrossRef]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, A.; Scott, S.S.; Greenlee, A.N.; Binda, N.; Noor, A.; Webb, A.; Guo, S.; Purdy, N.; Pennza, N.; Habib, A.; et al. Atrial fibrillation in cancer, anticancer therapies, and underlying mechanisms. J. Mol. Cell. Cardiol. 2024, 194, 118–132. [Google Scholar] [CrossRef]
- Lim, M.W.; Kalman, J.M. The impact of lifestyle factors on atrial fibrillation. J. Mol. Cell. Cardiol. 2024, 193, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Franczyk, B.; Rysz, J.; Olszewski, R.; Gluba-Sagr, A. Do Implantable Cardioverter-Defibrillators Prevent Sudden Cardiac Death in End-Stage Renal Disease Patients on Dialysis? J. Clin. Med. 2024, 13, 1176. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Kong, X.J.; Ji, Y.Y.; Fan, J.; Ji, C.C.; Chen, X.M.; Ma, Y.D.; Tang, A.L.; Cheng, Y.J.; Wu, S.H. Serum electrolyte concentrations and risk of atrial fibrillation: An observational and mendelian randomization study. BMC Genom. 2024, 25, 280. [Google Scholar] [CrossRef]
- Acharya, P.; Safarova, M.S.; Dalia, T.; Bharati, R.; Ranka, S.; Vindhyal, M.; Jiwani, S.; Barua, R.S. Effects of Vitamin D Supplementation and 25-Hydroxyvitamin D Levels on the Risk of Atrial Fibrillation. Am. J. Cardiol. 2022, 173, 56–63. [Google Scholar] [CrossRef]
- Yang, L.; Chung, M.K. Lifestyle changes in atrial fibrillation management and intervention. J. Cardiovasc. Electrophysiol. 2023, 34, 2163–2178. [Google Scholar] [CrossRef]
- Chung, M.K.; Eckhardt, L.L.; Chen, L.Y.; Ahmed, H.M.; Gopinathannair, R.; Joglar, J.A.; Noseworthy, P.A.; Pack, Q.R.; Sanders, P.; Trulock, K.M.; et al. Lifestyle and Risk Factor Modification for Reduction of Atrial Fibrillation: A Scientific Statement From the American Heart Association. Circulation 2020, 141, e750–e772. [Google Scholar] [CrossRef]
- Starreveld, R.; Ramos, K.S.; Muskens, A.; Brundel, B.; de Groot, N.M.S. Daily Supplementation of L-Glutamine in Atrial Fibrillation Patients: The Effect on Heat Shock Proteins and Metabolites. Cells 2020, 9, 1729. [Google Scholar] [CrossRef]
- Marcus, G.M.; Modrow, M.F.; Schmid, C.H.; Sigona, K.; Nah, G.; Yang, J.; Chu, T.C.; Joyce, S.; Gettabecha, S.; Ogomori, K.; et al. Individualized Studies of Triggers of Paroxysmal Atrial Fibrillation: The I-STOP-AFib Randomized Clinical Trial. JAMA Cardiol. 2022, 7, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Barrio-Lopez, M.T.; Ruiz-Canela, M.; Ramos, P.; Tercedor, L.; Ibanez Criado, J.L.; Ortiz, M.; Goni, L.; Ibanez Criado, A.; Macias-Ruiz, R.; Garcia-Bolao, I.; et al. PREvention of recurrent arrhythmias with Mediterranean diet (PREDIMAR) study in patients with atrial fibrillation: Rationale, design and methods. Am. Heart J. 2020, 220, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Barrio-Lopez, M.T.; Ruiz-Canela, M.; Goni, L.; Valiente, A.M.; Garcia, S.R.; de la, O.V.; Anton, B.D.; Fernandez-Friera, L.; Castellanos, E.; Martinez-Gonzalez, M.A.; et al. Mediterranean diet and epicardial adipose tissue in patients with atrial fibrillation treated with ablation: A substudy of the ‘PREDIMAR’ trial. Eur. J. Prev. Cardiol. 2024, 31, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Ioannidis, J.P.A. PREDIMED trial of Mediterranean diet: Retracted, republished, still trusted? BMJ 2019, 364, l341. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; Toledo, E.; Aros, F.; Fiol, M.; Corella, D.; Salas-Salvado, J.; Ros, E.; Covas, M.I.; Fernandez-Crehuet, J.; Lapetra, J.; et al. Extravirgin olive oil consumption reduces risk of atrial fibrillation: The PREDIMED (Prevencion con Dieta Mediterranea) trial. Circulation 2014, 130, 18–26. [Google Scholar] [CrossRef]
- Finicelli, M.; Di Salle, A.; Galderisi, U.; Peluso, G. The Mediterranean Diet: An Update of the Clinical Trials. Nutrients 2022, 14, 2956. [Google Scholar] [CrossRef]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet; a Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef]
- Pathak, R.K.; Middeldorp, M.E.; Meredith, M.; Mehta, A.B.; Mahajan, R.; Wong, C.X.; Twomey, D.; Elliott, A.D.; Kalman, J.M.; Abhayaratna, W.P.; et al. Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study (LEGACY). J. Am. Coll. Cardiol. 2015, 65, 2159–2169. [Google Scholar] [CrossRef]
- Tu, S.J.; Gallagher, C.; Elliott, A.D.; Bradbury, K.E.; Marcus, G.M.; Linz, D.; Pitman, B.M.; Middeldorp, M.E.; Hendriks, J.M.; Lau, D.H.; et al. Associations of dietary patterns, ultra-processed food and nutrient intake with incident atrial fibrillation. Heart 2023, 109, 1683–1689. [Google Scholar] [CrossRef]
- Garg, P.K.; Wilson, N.; Levitan, E.B.; Shikany, J.M.; Howard, V.J.; Newby, P.K.; Judd, S.; Howard, G.; Cushman, M.; Soliman, E.Z. Associations of dietary patterns with risk of incident atrial fibrillation in the REasons for Geographic And Racial Differences in Stroke (REGARDS). Eur. J. Nutr. 2023, 62, 2441–2448. [Google Scholar] [CrossRef] [PubMed]
- Tonstad, S.; Stewart, K.; Oda, K.; Batech, M.; Herring, R.P.; Fraser, G.E. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 292–299. [Google Scholar] [CrossRef]
- Tonstad, S.; Nathan, E.; Oda, K.; Fraser, G.E. Prevalence of hyperthyroidism according to type of vegetarian diet. Public Health Nutr. 2015, 18, 1482–1487. [Google Scholar] [CrossRef] [PubMed]
- Koebnick, C.; Leitzmann, R.; Garcia, A.L.; Heins, U.A.; Heuer, T.; Golf, S.; Katz, N.; Hoffmann, I.; Leitzmann, C. Long-term effect of a plant-based diet on magnesium status during pregnancy. Eur. J. Clin. Nutr. 2005, 59, 219–225. [Google Scholar] [CrossRef]
- Kristensen, N.B.; Madsen, M.L.; Hansen, T.H.; Allin, K.H.; Hoppe, C.; Fagt, S.; Lausten, M.S.; Gobel, R.J.; Vestergaard, H.; Hansen, T.; et al. Intake of macro- and micronutrients in Danish vegans. Nutr. J. 2015, 14, 115. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J. Health effects of vegan diets. Am. J. Clin. Nutr. 2009, 89, 1627S–1633S. [Google Scholar] [CrossRef]
- Storz, M.A.; Helle, P. Atrial fibrillation risk factor management with a plant-based diet: A review. J. Arrhythmia 2019, 35, 781–788. [Google Scholar] [CrossRef]
- Brandes, A.; Smit, M.D.; Nguyen, B.O.; Rienstra, M.; Van Gelder, I.C. Risk Factor Management in Atrial Fibrillation. Arrhythmia Electrophysiol. Rev. 2018, 7, 118–127. [Google Scholar] [CrossRef]
- Alexander, S.; Ostfeld, R.J.; Allen, K.; Williams, K.A. A plant-based diet and hypertension. J. Geriatr. Cardiol. 2017, 14, 327–330. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Nishimura, K.; Barnard, N.D.; Takegami, M.; Watanabe, M.; Sekikawa, A.; Okamura, T.; Miyamoto, Y. Vegetarian diets and blood pressure: A meta-analysis. JAMA Intern. Med. 2014, 174, 577–587. [Google Scholar] [CrossRef]
- Appleby, P.N.; Key, T.J. The long-term health of vegetarians and vegans. Proc. Nutr. Soc. 2016, 75, 287–293. [Google Scholar] [CrossRef]
- Appleby, P.N.; Davey, G.K.; Key, T.J. Hypertension and blood pressure among meat eaters, fish eaters, vegetarians and vegans in EPIC-Oxford. Public Health Nutr. 2002, 5, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Campbell, T. A plant-based diet and stroke. J. Geriatr. Cardiol. 2017, 14, 321–326. [Google Scholar] [CrossRef]
- Bazzano, L.A.; Green, T.; Harrison, T.N.; Reynolds, K. Dietary approaches to prevent hypertension. Curr. Hypertens. Rep. 2013, 15, 694–702. [Google Scholar] [CrossRef]
- Perrino, C.; Ferdinandy, P.; Botker, H.E.; Brundel, B.; Collins, P.; Davidson, S.M.; den Ruijter, H.M.; Engel, F.B.; Gerdts, E.; Girao, H.; et al. Improving translational research in sex-specific effects of comorbidities and risk factors in ischaemic heart disease and cardioprotection: Position paper and recommendations of the ESC Working Group on Cellular Biology of the Heart. Cardiovasc. Res. 2021, 117, 367–385. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Barnard, N.D.; Levin, S.M.; Watanabe, M. Vegetarian diets and glycemic control in diabetes: A systematic review and meta-analysis. Cardiovasc. Diagn. Ther. 2014, 4, 373–382. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, S.A.; Lee, I.K.; Kim, J.G.; Park, K.G.; Jeong, J.Y.; Jeon, J.H.; Shin, J.Y.; Lee, D.H. Effect of a Brown Rice Based Vegan Diet and Conventional Diabetic Diet on Glycemic Control of Patients with Type 2 Diabetes: A 12-Week Randomized Clinical Trial. PLoS ONE 2016, 11, e0155918. [Google Scholar] [CrossRef] [PubMed]
- Ramal, E.; Champlin, A.; Bahjri, K. Impact of a Plant-Based Diet and Support on Mitigating Type 2 Diabetes Mellitus in Latinos Living in Medically Underserved Areas. Am. J. Health Promot. 2018, 32, 753–762. [Google Scholar] [CrossRef]
- McMacken, M.; Shah, S. A plant-based diet for the prevention and treatment of type 2 diabetes. J. Geriatr. Cardiol. 2017, 14, 342–354. [Google Scholar] [CrossRef]
- Barnard, N.D.; Scialli, A.R.; Turner-McGrievy, G.; Lanou, A.J.; Glass, J. The effects of a low-fat, plant-based dietary intervention on body weight, metabolism, and insulin sensitivity. Am. J. Med. 2005, 118, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Estadella, D.; da Penha Oller do Nascimento, C.M.; Oyama, L.M.; Ribeiro, E.B.; Damaso, A.R.; de Piano, A. Lipotoxicity: Effects of dietary saturated and transfatty acids. Mediators Inflamm. 2013, 2013, 137579. [Google Scholar] [CrossRef]
- Barnard, N.D.; Levin, S.M.; Yokoyama, Y. A systematic review and meta-analysis of changes in body weight in clinical trials of vegetarian diets. J. Acad. Nutr. Diet 2015, 115, 954–969. [Google Scholar] [CrossRef]
- Newby, P.K.; Tucker, K.L.; Wolk, A. Risk of overweight and obesity among semivegetarian, lactovegetarian, and vegan women. Am. J. Clin. Nutr. 2005, 81, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Tonstad, S.; Butler, T.; Yan, R.; Fraser, G.E. Type of vegetarian diet, body weight, and prevalence of type 2 diabetes. Diabetes Care 2009, 32, 791–796. [Google Scholar] [CrossRef]
- Harada, M.; Van Wagoner, D.R.; Nattel, S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ. J. 2015, 79, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Dudley, S.C., Jr. Evidence for Inflammation as a Driver of Atrial Fibrillation. Front. Cardiovasc. Med. 2020, 7, 62. [Google Scholar] [CrossRef]
- Haghighatdoost, F.; Bellissimo, N.; Totosy de Zepetnek, J.O.; Rouhani, M.H. Association of vegetarian diet with inflammatory biomarkers: A systematic review and meta-analysis of observational studies. Public Health Nutr. 2017, 20, 2713–2721. [Google Scholar] [CrossRef]
- Kahleova, H.; Matoulek, M.; Malinska, H.; Oliyarnik, O.; Kazdova, L.; Neskudla, T.; Skoch, A.; Hajek, M.; Hill, M.; Kahle, M.; et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with Type 2 diabetes. Diabet. Med. 2011, 28, 549–559. [Google Scholar] [CrossRef]
- Barnard, N.D.; Goldman, D.M.; Loomis, J.F.; Kahleova, H.; Levin, S.M.; Neabore, S.; Batts, T.C. Plant-Based Diets for Cardiovascular Safety and Performance in Endurance Sports. Nutrients 2019, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Esselstyn, C.B. A plant-based diet and coronary artery disease: A mandate for effective therapy. J. Geriatr. Cardiol. 2017, 14, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Ornish, D.; Scherwitz, L.W.; Billings, J.H.; Brown, S.E.; Gould, K.L.; Merritt, T.A.; Sparler, S.; Armstrong, W.T.; Ports, T.A.; Kirkeeide, R.L.; et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA 1998, 280, 2001–2007. [Google Scholar] [CrossRef] [PubMed]
- Tuso, P.; Stoll, S.R.; Li, W.W. A plant-based diet, atherogenesis, and coronary artery disease prevention. Perm. J. 2015, 19, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Goudis, C.A.; Vasileiadis, I.E.; Liu, T. Epicardial adipose tissue and atrial fibrillation: Pathophysiological mechanisms, clinical implications, and potential therapies. Curr. Med. Res. Opin. 2018, 34, 1933–1943. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.; Petraglia, L.; Cabaro, S.; Valerio, V.; Poggio, P.; Pilato, E.; Attena, E.; Russo, V.; Ferro, A.; Formisano, P.; et al. Epicardial Adipose Tissue and Cardiac Arrhythmias: Focus on Atrial Fibrillation. Front. Cardiovasc. Med. 2022, 9, 932262. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.R.; Lopez, F.L.; Folsom, A.R.; Agarwal, S.K.; Loehr, L.R.; Soliman, E.Z.; Maclehose, R.; Konety, S.; Alonso, A. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: The Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011, 123, 1501–1508. [Google Scholar] [CrossRef]
- Abed, H.S.; Wittert, G.A.; Leong, D.P.; Shirazi, M.G.; Bahrami, B.; Middeldorp, M.E.; Lorimer, M.F.; Lau, D.H.; Antic, N.A.; Brooks, A.G.; et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: A randomized clinical trial. JAMA 2013, 310, 2050–2060. [Google Scholar] [CrossRef]
- James, S.L.; Castle, C.D.; Dingels, Z.V.; Fox, J.T.; Hamilton, E.B.; Liu, Z.; NL, S.R.; Sylte, D.O.; Henry, N.J.; LeGrand, K.E.; et al. Global injury morbidity and mortality from 1990 to 2017: Results from the Global Burden of Disease Study 2017. Inj. Prev. 2020, 26, i96–i114. [Google Scholar] [CrossRef]
- Craddock, J.C.; Neale, E.P.; Peoples, G.E.; Probst, Y.C. Vegetarian-Based Dietary Patterns and their Relation with Inflammatory and Immune Biomarkers: A Systematic Review and Meta-Analysis. Adv. Nutr. 2019, 10, 433–451. [Google Scholar] [CrossRef]
- Franco-de-Moraes, A.C.; de Almeida-Pititto, B.; da Rocha Fernandes, G.; Gomes, E.P.; da Costa Pereira, A.; Ferreira, S.R.G. Worse inflammatory profile in omnivores than in vegetarians associates with the gut microbiota composition. Diabetol. Metab. Syndr. 2017, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.Y.; Allen, K.; McDonnough, M.; Massera, D.; Ostfeld, R.J. A plant-based diet and heart failure: Case report and literature review. J. Geriatr. Cardiol. 2017, 14, 375–378. [Google Scholar] [CrossRef]
- Kerley, C.P. A Review of Plant-based Diets to Prevent and Treat Heart Failure. Card. Fail. Rev. 2018, 4, 54–61. [Google Scholar] [CrossRef]
- Zhang, S.; Marken, I.; Stubbendorff, A.; Ericson, U.; Qi, L.; Sonestedt, E.; Borne, Y. The EAT-Lancet Diet Index, Plasma Proteins, and Risk of Heart Failure in a Population-Based Cohort. JACC Heart Fail. 2024, 12, 1197–1208. [Google Scholar] [CrossRef]
- Iacobellis, G. Epicardial adipose tissue in contemporary cardiology. Nat. Rev. Cardiol. 2022, 19, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.; Austin, R.R.; Backonja, U.; Campos, H.; Chung, A.E.; Hekler, E.B.; Hsueh, P.S.; Kim, K.K.; Pho, A.; Salmi, L.; et al. Citizen science to further precision medicine: From vision to implementation. JAMIA Open 2020, 3, 2–8. [Google Scholar] [CrossRef]
- Brundel, B.J.J.M.; Shiroshita-Takeshita, A.; Qi, X.Y.; Yeh, Y.H.; Chartier, D.; Van Gelder, I.C.; Henning, R.H.; Kampinga, H.H.; Nattel, S. Induction of heat-shock response protects the heart against atrial fibrillation. Circ. Res. 2006, 99, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, J.; van Marion, D.M.S.; Zhang, D.; Brundel, B. Heat shock protein inducer GGA*-59 reverses contractile and structural remodeling via restoration of the microtubule network in experimental Atrial Fibrillation. J. Mol. Cell. Cardiol. 2019, 134, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Sakabe, M.; Shiroshita-Takeshita, A.; Maguy, A.; Brundel, B.; Fujiki, A.; Inoue, H.; Nattel, S. Effects of heat shock protein induction on Atrial Fibrillation caused by acute atrial ischemia. Cardiovasc. Res. 2008, 78, 63–70. [Google Scholar] [CrossRef]
- Rafaqat, S.; Afzal, S.; Khurshid, H.; Safdar, S.; Rafaqat, S.; Rafaqat, S. The Role of Major Inflammatory Biomarkers in the Pathogenesis of Atrial Fibrillation. J. Innov. Card. Rhythm. Manag. 2022, 13, 5265–5277. [Google Scholar] [CrossRef]
- Khan, A.M.; Lubitz, S.A.; Sullivan, L.M.; Sun, J.X.; Levy, D.; Vasan, R.S.; Magnani, J.W.; Ellinor, P.T.; Benjamin, E.J.; Wang, T.J. Low serum magnesium and the development of atrial fibrillation in the community: The Framingham Heart Study. Circulation 2013, 127, 33–38. [Google Scholar] [CrossRef]
- Curran, J.; Ross-White, A.; Sibley, S. Magnesium prophylaxis of new-onset atrial fibrillation: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0292974. [Google Scholar] [CrossRef] [PubMed]
- Kaisler, R.E.; Kulnik, S.T.; Klager, E.; Kletecka-Pulker, M.; Schaden, E.; Stainer-Hochgatterer, A. Introducing patient and public involvement practices to healthcare research in Austria: Strategies to promote change at multiple levels. BMJ Open 2021, 11, e045618. [Google Scholar] [CrossRef] [PubMed]
- Shahid, A.; Lalani, I.N.; Rosgen, B.K.; Sept, B.G.; Longmore, S.; Parsons Leigh, J.; Stelfox, H.T.; Fiest, K.M. A scoping review of methods to measure and evaluate citizen engagement in health research. Res. Involv. Engagem. 2022, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Haklay, M.; Fraisl, D.; Greshake Tzovaras, B.; Hecker, S.; Gold, M.; Hager, G.; Ceccaroni, L.; Kieslinger, B.; Wehn, U.; Woods, S.; et al. Contours of citizen science: A vignette study. R. Soc. Open Sci. 2021, 8, 202108. [Google Scholar] [CrossRef] [PubMed]
- Bryant, E.A.; Scott, A.M.; Greenwood, H.; Thomas, R. Patient and public involvement in the development of clinical practice guidelines: A scoping review. BMJ Open 2022, 12, e055428. [Google Scholar] [CrossRef]
- Babich, J.S.; McMacken, M.; Correa, L.; Polito-Moller, K.; Chen, K.; Adams, E.; Morgenstern, S.; Katz, M.; Long, T.G.; Joshi, S.; et al. Advancing Lifestyle Medicine in New York City’s Public Health Care System. Mayo Clin. Proc. Innov. Qual. Outcomes 2024, 8, 279–292. [Google Scholar] [CrossRef]
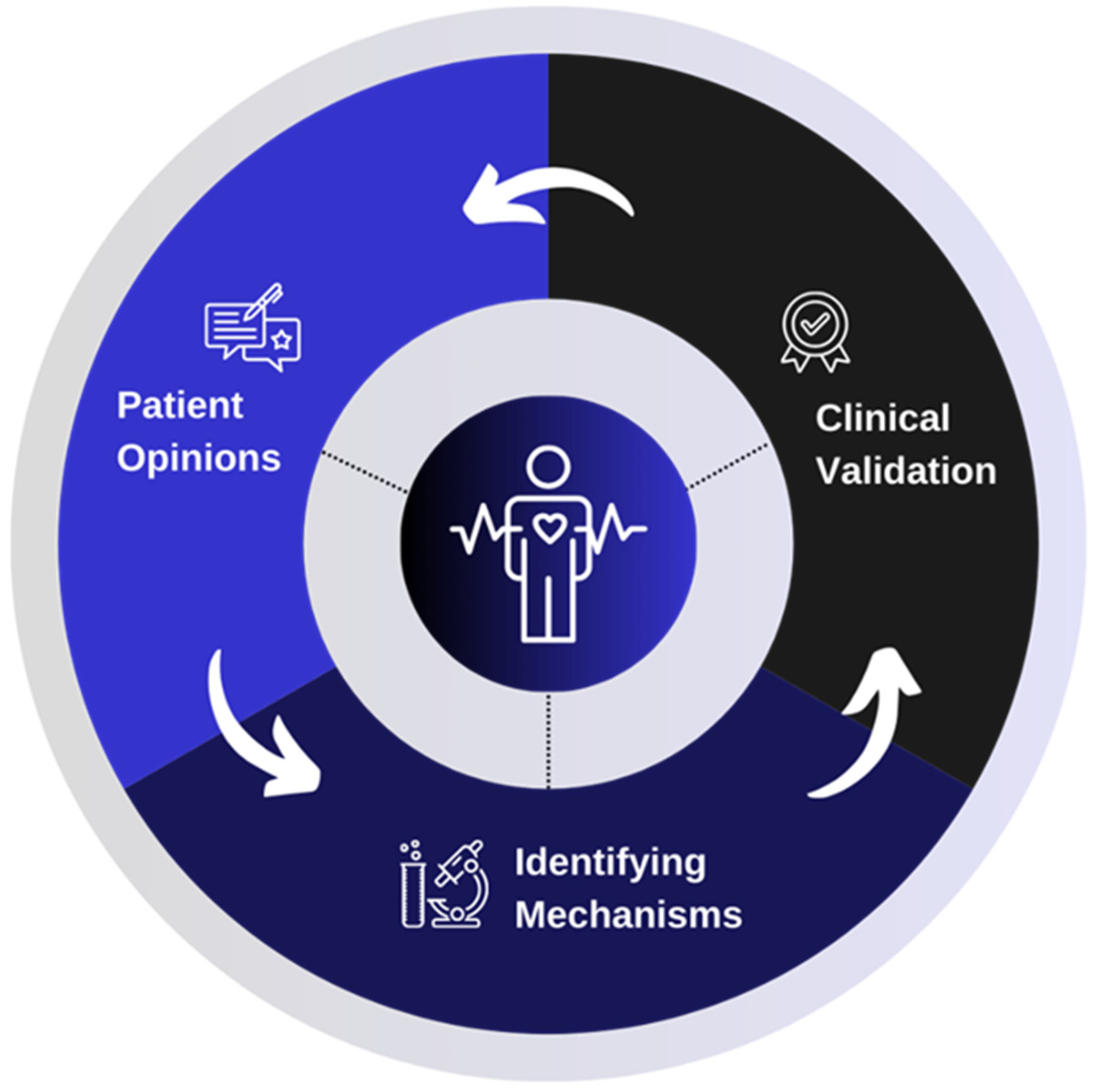
Main risk factors [4,5]:
|
| Type of Diet | Included | Excluded |
|---|---|---|
| Whole Foods Plant-Based Diet | Whole grains, fruits, vegetables (starchy and non-starchy), legumes (peas, beans and lentils), nuts and seeds (from Center for Nutrition studies). | The diet does not include dairy products, meat products, fish, and eggs. Also, minimal added oils, fats, sugar, and processed foods. |
| Mediterranean Diet | Poultry, fish, grains, seeds, and healthy oils like extra virgin olive oil. Also, low-fat dairy products. The diet recommends limiting poultry and dairy to 4 servings weekly [20]. | The diet recommends avoiding red meat, processed sugary foods, and high-fat foods. |
| Modifiable Risk Factor | Result of a Plant-Based Diet | Physiological Effects |
|---|---|---|
| Hypertension [12,30] | Reduced hypertension and risk of hypertension [31,32,33,34]. | Improved vasodilation [21,31]; increased potassium intake [35,36]; reduced blood viscosity [31,37]. |
| Diabetes [12,30] | Improved glycemic control and reduced insulin resistance [38,39,40]. | Reduced glycation end products and low saturated fat content; reduced lipotoxicity [41,42,43]. |
| Obesity and obstructive sleep apnea [12,30] | Reduced risk of obesity; suitable diet for weight loss [44,45,46]. | High fiber and low-fat content of plant foods. Reduced energy density and increased energy consumption [44]. |
| Systemic inflammation [12,47,48] | Decreased factors of the inflammatory response (hsCRP) [49]. | Increased in anti-inflammatory and antioxidant components [50]. Absence of pro-inflammatory fats [51]. |
| Coronary artery disease [12,30] | Prevention and gradual recovery from atherosclerosis and coronary heart disease [52,53]. | Prevention of vascular endothelial cell damage [52,54]. Prevention of LDL oxidation and oxidative inflammation through increased antioxidants [54]. |
| Epicardial adipose tissue (EAT) | Weigh loss reduced EAT [55,56]. | Reducing EAT can help maintain sinus rhythm and reduce AF burden [55]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuipers, M.F.; Laurila, R.; Remy, M.L.; van Oudheusden, M.; Hazlett, N.; Lipsky, S.; Reisner, L.L.; McCall, D.; de Groot, N.M.S.; Brundel, B.J.J.M. Exploring Diet-Based Treatments for Atrial Fibrillation: Patient Empowerment and Citizen Science as a Model for Quality-of-Life-Centered Solutions. Nutrients 2024, 16, 2672. https://doi.org/10.3390/nu16162672
Kuipers MF, Laurila R, Remy ML, van Oudheusden M, Hazlett N, Lipsky S, Reisner LL, McCall D, de Groot NMS, Brundel BJJM. Exploring Diet-Based Treatments for Atrial Fibrillation: Patient Empowerment and Citizen Science as a Model for Quality-of-Life-Centered Solutions. Nutrients. 2024; 16(16):2672. https://doi.org/10.3390/nu16162672
Chicago/Turabian StyleKuipers, Myrthe F., Ronja Laurila, Maurice L. Remy, Michiel van Oudheusden, Nedra Hazlett, Sally Lipsky, Lianna L. Reisner, Debbe McCall, Natasja M. S. de Groot, and Bianca J. J. M. Brundel. 2024. "Exploring Diet-Based Treatments for Atrial Fibrillation: Patient Empowerment and Citizen Science as a Model for Quality-of-Life-Centered Solutions" Nutrients 16, no. 16: 2672. https://doi.org/10.3390/nu16162672
APA StyleKuipers, M. F., Laurila, R., Remy, M. L., van Oudheusden, M., Hazlett, N., Lipsky, S., Reisner, L. L., McCall, D., de Groot, N. M. S., & Brundel, B. J. J. M. (2024). Exploring Diet-Based Treatments for Atrial Fibrillation: Patient Empowerment and Citizen Science as a Model for Quality-of-Life-Centered Solutions. Nutrients, 16(16), 2672. https://doi.org/10.3390/nu16162672








