Effect of Mutant and Engineered High-Acetate-Producing Saccharomyces cerevisiae var. boulardii Strains in Dextran Sodium Sulphate-Induced Colitis
Abstract
1. Introduction
2. Materials and Methods
3. Results
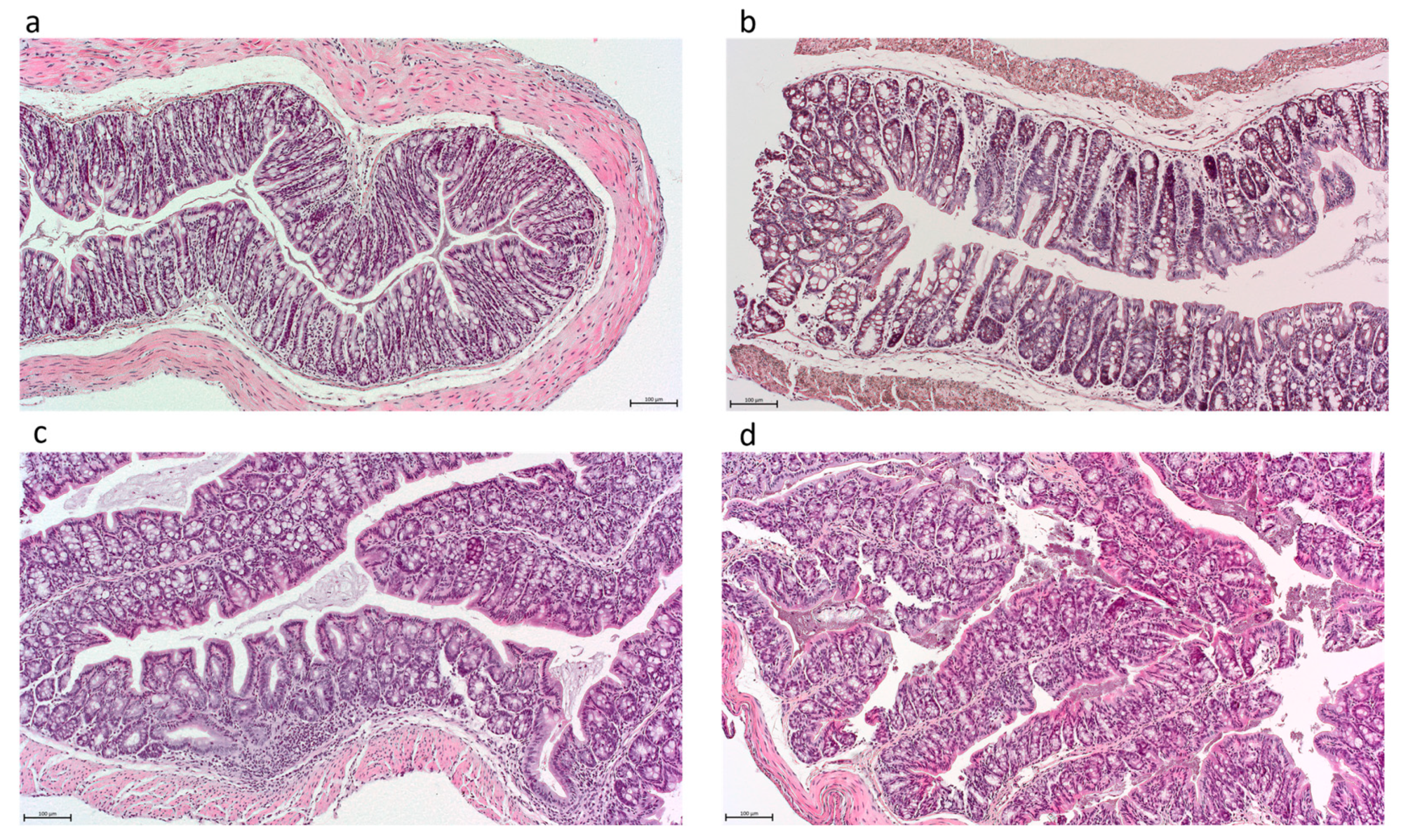
3.1. High-Acetate-Producing Saccharomyces cerevisiae var. boulardii Strains Attenuate Inflammation and Show Dose-Dependent Histologic Improvement
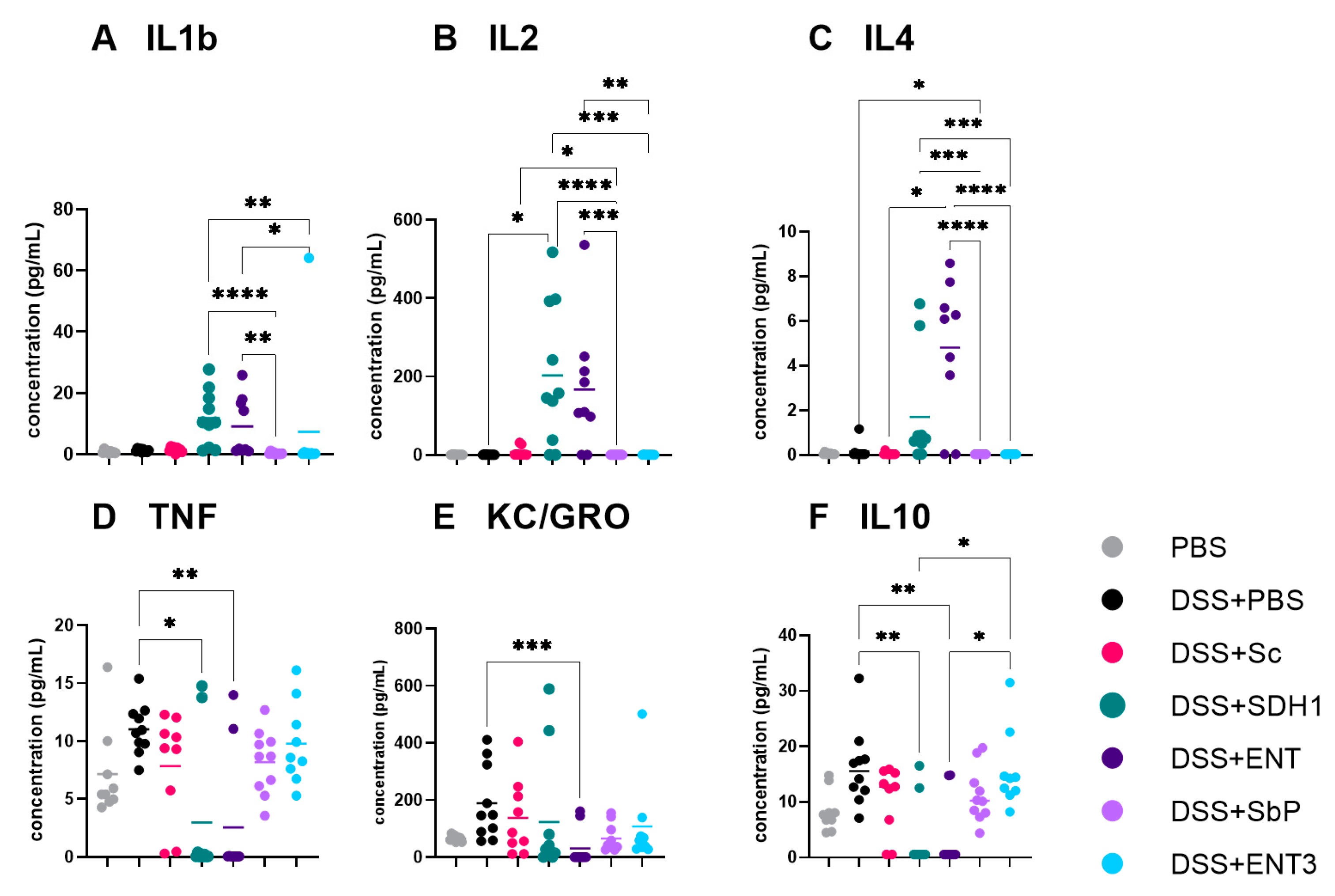
3.2. Serum Cytokines Showed Mixed Profiles across Different Treatment Groups
3.3. The Highest-Acetate-Producing Strain Exhibits Anti-Inflammatory Effects on Gene Expression
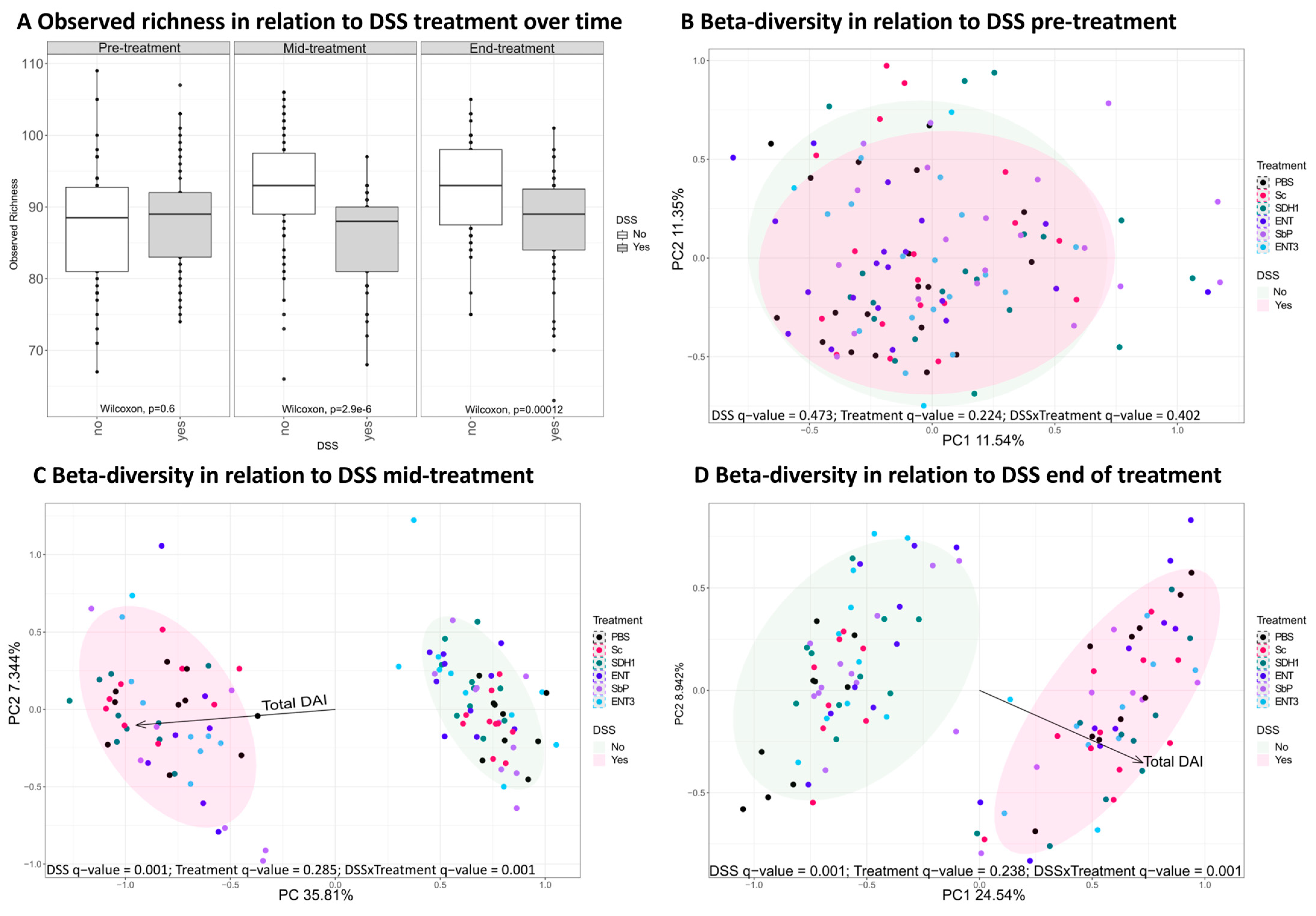
3.4. Induction of Colitis by DSS Administration Perturbs the Gut Microbiome
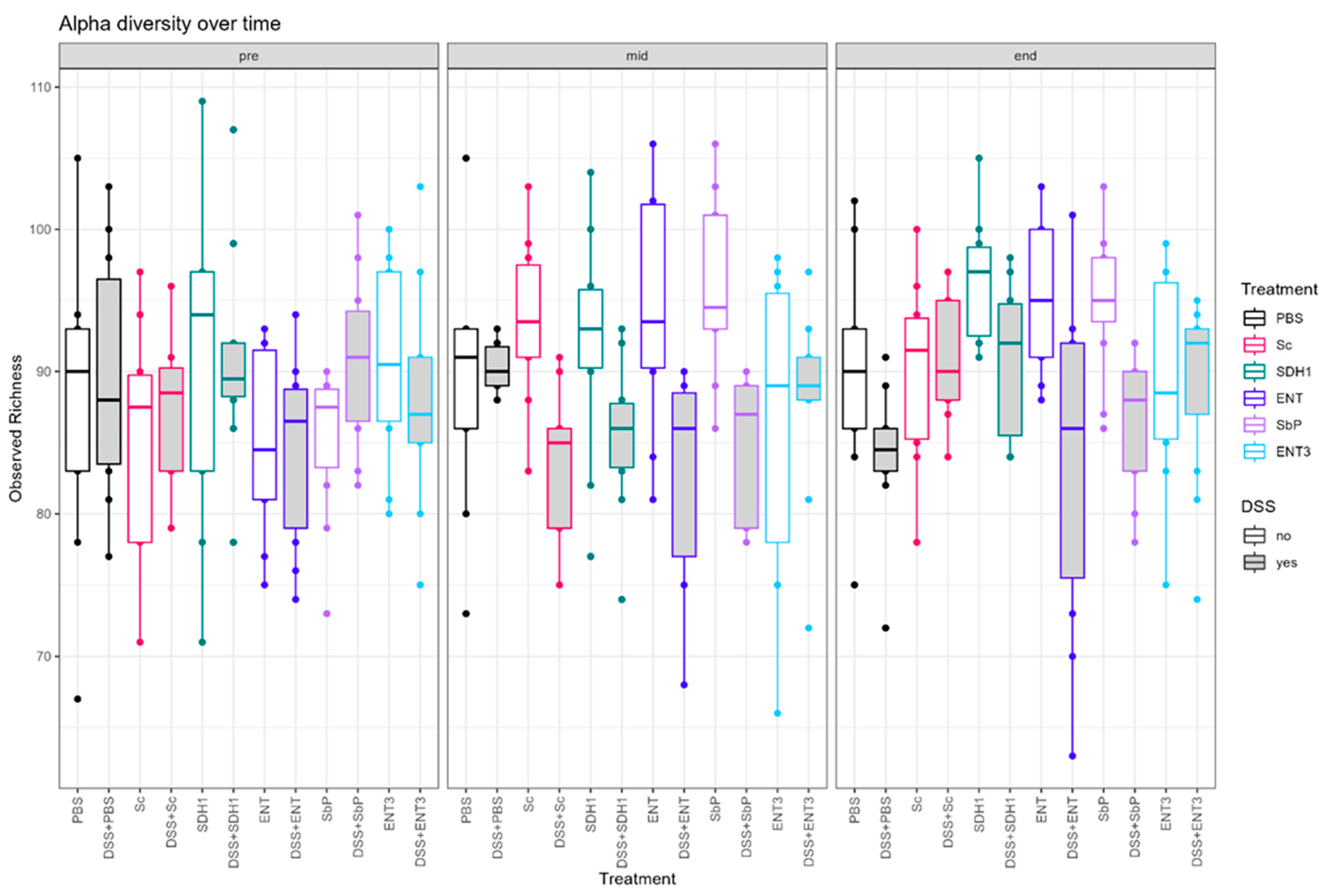
3.5. Gut Microbial Species Richness Remains Consistently Preserved during Inflammation upon Treatment with the Highest-Acetate-Producing Saccharomyces cerevisiae var. boulardii Strains
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CD | Crohn’s Disease |
| DSS | Dextran sodium sulphate |
| ENT | Enterol-Saccharomyces cerevisiae var. boulardii strain |
| ENT3 | Extra-high-acetate-producing Saccharomyces cerevisiae var. boulardii strain |
| IBD | Inflammatory bowel disease |
| PBS | Phosphate buffered saline |
| Sb | Saccharomyces cerevisiae var. boulardii |
| Sc | Saccharomyces cerevisiae |
| Sb.P | High-acetate-producing Saccharomyces cerevisiae var. boulardii strain |
| SCFA | Short chain fatty acids |
| SDH1 | Non-acetate-producing Saccharomyces cerevisiae var. boulardii strain |
| UC | Ulcerative colitis |
References
- Barko, P.C.; McMichael, M.A.; Swanson, K.S.; Williams, D.A. The Gastrointestinal Microbiome: A Review. J. Vet. Intern. Med. 2018, 32, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Hold, G.L.; Smith, M.; Grange, C.; Watt, E.R.; El-Omar, E.M.; Mukhopadhya, I. Role of the Gut Microbiota in Inflammatory Bowel Disease Pathogenesis: What Have We Learnt in the Past 10 Years? World J. Gastroenterol. 2014, 20, 1192–1210. [Google Scholar] [CrossRef] [PubMed]
- Pandey, H.; Jain, D.; Tang, D.W.T.; Wong, S.H.; Lal, D. Gut microbiota in pathophysiology, diagnosis, and therapeutics of inflammatory bowel disease. Intest. Res. 2024, 22, 15–43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vieira-Silva, S.; Sabino, J.; Valles-Colomer, M.; Falony, G.; Kathagen, G.; Caenepeel, C.; Cleynen, I.; van der Merwe, S.; Vermeire, S.; Raes, J. Quantitative Microbiome Profiling Disentangles Inflammation- and Bile Duct Obstruction-Associated Microbiota Alterations across PSC/IBD Diagnoses. Nat. Microbiol. 2019, 4, 1826–1831. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, J.; Chang, E.B. The Gut Microbiota and Inflammatory Bowel Diseases. Transl. Res. 2017, 179, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Roy, B.; Khan, S.; Septer, S.; Umar, S. Microbiome, Metabolome and Inflammatory Bowel Disease. Microorganisms 2016, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Serban, D.E. Microbiota in Inflammatory Bowel Disease Pathogenesis and Therapy: Is It All about Diet? Nutr. Clin. Pract. 2015, 30, 760–779. [Google Scholar] [CrossRef]
- Santana, P.T.; Rosas, S.L.B.; Ribeiro, B.E.; Marinho, Y.; de Souza, H.S.P. Dysbiosis in Inflammatory Bowel Disease: Pathogenic Role and Potential Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 3464. [Google Scholar] [CrossRef]
- McIlroy, J.; Ianiro, G.; Mukhopadhya, I.; Hansen, R.; Hold, G.L. Review Article: The Gut Microbiome in Inflammatory Bowel Disease—Avenues for Microbial Management. Aliment. Pharmacol. Ther. 2018, 47, 26–42. [Google Scholar] [CrossRef]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients with Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109.e6. [Google Scholar] [CrossRef]
- Costello, S.P.; Hughes, P.A.; Waters, O.; Bryant, R.V.; Vincent, A.D.; Blatchford, P.; Katsikeros, R.; Makanyanga, J.; Campaniello, M.A.; Mavrangelos, C.; et al. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients with Ulcerative Colitis: A Randomized Clinical Trial. J. Am. Med. Assoc. 2019, 321, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Paramsothy, S.; Paramsothy, R.; Rubin, D.T.; Kamm, M.A.; Kaakoush, N.O.; Mitchell, H.M.; Castaño-Rodríguez, N. Faecal Microbiota Transplantation for Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J. Crohns Colitis 2017, 11, 1180–1199. [Google Scholar] [CrossRef] [PubMed]
- Rossen, N.G.; Fuentes, S.; Van Der Spek, M.J.; Tijssen, J.G.; Hartman, J.H.A.; Duflou, A.; Löwenberg, M.; Van Den Brink, G.R.; Mathus-Vliegen, E.M.H.; De Vos, W.M.; et al. Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients with Ulcerative Colitis. Gastroenterology 2015, 149, 110–118.e4. [Google Scholar] [CrossRef]
- Danne, C.; Rolhion, N.; Sokol, H. Recipient Factors in Faecal Microbiota Transplantation: One Stool Does Not Fit All. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Pais, P.; Almeida, V.; Yılmaz, M.; Teixeira, M.C. Saccharomyces boulardii: What Makes It Tick as Successful Probiotic? J. Fungi 2020, 6, 78. [Google Scholar] [CrossRef]
- Bjarnason, I.; Sission, G.; Hayee, B.H. A Randomised, Double-Blind, Placebo-Controlled Trial of a Multi-Strain Probiotic in Patients with Asymptomatic Ulcerative Colitis and Crohn’s Disease. Inflammopharmacology 2019, 27, 465–473. [Google Scholar] [CrossRef]
- Lavelle, A.; Sokol, H. Gut Microbiota-Derived Metabolites as Key Actors in Inflammatory Bowel Disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef]
- Shin, Y.; Han, S.; Kwon, J.; Ju, S.; Choi, T.G.; Kang, I.; Kim, S.S. Roles of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Nutrients 2023, 15, 4466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Venegas, D.P.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs) Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Rhodes, J.M. Nutrition and Gut Health: The Impact of Specific Dietary Components—It’s Not Just Five-a-Day. Proc. Nutr. Soc. 2021, 80, 9–18. [Google Scholar] [CrossRef]
- Gill, P.A.; van Zelm, M.C.; Muir, J.G.; Gibson, P.R. Review Article: Short Chain Fatty Acids as Potential Therapeutic Agents in Human Gastrointestinal and Inflammatory Disorders. Aliment. Pharmacol. Ther. 2018, 48, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Offei, B.; Vandecruys, P.; de Graeve, S.; Foulquié-Moreno, M.R.; Thevelein, J.M. Unique Genetic Basis of the Distinct Antibiotic Potency of High Acetic Acid Production in the Probiotic Yeast Saccharomyces cerevisiae Var. Boulardii. Genome Res. 2019, 29, 1478–1494. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, B.T.; Subotić, A.; Vandecruys, P.; Deleu, S.; Vermeire, S.; Thevelein, J.M. Enhancing Probiotic Impact: Engineering Saccharomyces Boulardii for Optimal Acetic Acid Production and Gastric Passage Tolerance. Appl. Environ. Microbiol. 2024, 90, e0032524. [Google Scholar] [CrossRef] [PubMed]
- Abimosleh, S.M.; Lindsay, R.J.; Butler, R.N.; Cummins, A.G.; Howarth, G.S. Emu Oil Increases Colonic Crypt Depth in a Rat Model of Ulcerative Colitis. Dig. Dis. Sci. 2012, 57, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Walton, K.L.W.; Blue, R.E.; MacNaughton, K.; Magness, S.T.; Lund, P.K. Mucosal Healing and Fibrosis after Acute or Chronic Inflammation in Wild Type FVB-N Mice and C57BL6 Procollagen a1(I)-Promoter-GFP Reporter Mice. PLoS ONE 2012, 7, e42568. [Google Scholar] [CrossRef]
- de Wiele, T.V.; Boon, N.; Possemiers, S.; Jacobs, H.; Verstraete, W. Prebiotic effects of chicory inulin in the simulator of the human intestinal microbial ecosystem. FEMS Microbiol. Ecol. 2004, 51, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [PubMed]
- Tito, R.Y.; Cypers, H.; Joossens, M.; Varkas, G.; van Praet, L.; Glorieus, E.; van den Bosch, F.; de Vos, M.; Raes, J.; Elewaut, D. Brief Report: Dialister as a Microbial Marker of Disease Activity in Spondyloarthritis. Arthritis Rheumatol. 2017, 69, 114–121. [Google Scholar] [CrossRef]
- Hildebrand, F.; Tadeo, R.; Voigt, A.; Bork, P.; Raes, J. LotuS: An Efficient and User-Friendly OTU Processing Pipeline. Microbiome 2014, 2, 30. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.-H. Butyrate Producers, “The Sentinel of Gut”: Their Intestinal Significance with and beyond Butyrate, and Prospective Use as Microbial Therapeutics. Front. Microbiol. 2023, 13, 1103836. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Xue, W.; Lin, X.; Lv, M.; Luo, G.; Dai, Y.; Sun, H.; Liu, S.; Sun, C.; Hu, T.; et al. Butyribacter intestini Gen. Nov., Sp. Nov., a Butyric Acid-Producing Bacterium of the Family Lachnospiraceae Isolated from Human Faeces, and Reclassification of Acetivibrio Ethanolgignens as Acetanaerobacter Ethanolgignens Gen. Nov., Comb. Nov. Syst. Appl. Microbiol. 2021, 44, 126201. [Google Scholar] [CrossRef]
- Pothoulakis, C. Review Article: Anti-Inflammatory Mechanisms of Action of Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2009, 30, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, H.; Shi, L.; Li, R.; Luo, Y.; Deng, Y.; Li, S.; Li, R.; Liu, Z. Saccharomyces boulardii Alleviates DSS-Induced Intestinal Barrier Dysfunction and Inflammation in Humanized Mice. Food Funct. 2022, 13, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Vezza, T.; Utrilla, M.P.; Chueca, N.; García, F.; Rodríguez-Cabezas, M.E.; Gálvez, J. Intestinal Anti-Inflammatory Effect of the Probiotic Saccharomyces boulardii in DSS-Induced Colitis in Mice: Impact on MicroRNAs Expression and Gut Microbiota Composition. J. Nutr. Biochem. 2018, 61, 129–139. [Google Scholar] [CrossRef]
- Cochran, K.E.; Lamson, N.G.; Whitehead, K.A. Expanding the Utility of the Dextran Sulfate Sodium (DSS) Mouse Model to Induce a Clinically Relevant Loss of Intestinal Barrier Function. PeerJ 2020, 8, e8681. [Google Scholar] [CrossRef]
- Kawashima, K.; Onizawa, M.; Fujiwara, T.; Gunji, N.; Imamura, H.; Katakura, K.; Ohira, H. Evaluation of the Relationship between the Spleen Volume and the Disease Activity in Ulcerative Colitis and Crohn Disease. Medicine 2022, 101, E28515. [Google Scholar] [CrossRef]
- Fang, K.; Bruce, M.; Pattillo, C.B.; Zhang, S.; Stone, R.; Clifford, J.; Kevil, C.G. Temporal Genomewide Expression Profiling of DSS Colitis Reveals Novel Inflammatory and Angiogenesis Genes Similar to Ulcerative Colitis. Physiol. Genom. 2011, 43, 43–56. [Google Scholar] [CrossRef]
- Caenepeel, C.; Sadat Seyed Tabib, N.; Vieira-Silva, S.; Vermeire, S. Review Article: How the Intestinal Microbiota May Reflect Disease Activity and Influence Therapeutic Outcome in Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2020, 52, 1453–1468. [Google Scholar] [CrossRef]
- Forster, S.C.; Clare, S.; Beresford-Jones, B.S.; Harcourt, K.; Notley, G.; Stares, M.D.; Kumar, N.; Soderholm, A.T.; Adoum, A.; Wong, H.; et al. Identification of Gut Microbial Species Linked with Disease Variability in a Widely Used Mouse Model of Colitis. Nat. Microbiol. 2022, 7, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Kim, W.-K.; Han, D.H.; Lee, K.; Ko, G. Lactobacillus Fermentum Species Ameliorate Dextran Sulfate Sodium-Induced Colitis by Regulating the Immune Response and Altering Gut Microbiota. Gut Microbes 2019, 10, 696–711. [Google Scholar] [CrossRef]
- Liu, B.; Yang, L.; Wu, Y.; Zhao, X. Protective Effect of Limosilactobacillus Fermentum HFY06 on Dextran Sulfate Sodium-Induced Colitis in Mice. Front. Microbiol. 2022, 13, 935792. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Liu, H.; Yu, H.; Chen, M.; Yang, T.; Zeng, X.; Qiao, S. Core Altered Microorganisms in Colitis Mouse Model: A Comprehensive Time-Point and Fecal Microbiota Transplantation Analysis. Antibiotics 2021, 10, 643. [Google Scholar] [CrossRef] [PubMed]
- Deleu, S.; Arnauts, K.; Deprez, L.; Machiels, K.; Ferrante, M.; Huys, G.R.B.; Thevelein, J.M.; Raes, J.; Vermeire, S. High Acetate Concentration Protects Intestinal Barrier and Exerts Anti-Inflammatory Effects in Organoid-Derived Epithelial Monolayer Cultures from Patients with Ulcerative Colitis. Int. J. Mol. Sci. 2023, 24, 768. [Google Scholar] [CrossRef]
- Martyniak, A.; Medyńska-Przęczek, A.; Wędrychowicz, A.; Skoczeń, S.; Tomasik, P.J. Prebiotics, Probiotics, Synbiotics, Paraprobiotics and Postbiotic Compounds in IBD. Biomolecules 2021, 11, 1903. [Google Scholar] [CrossRef]
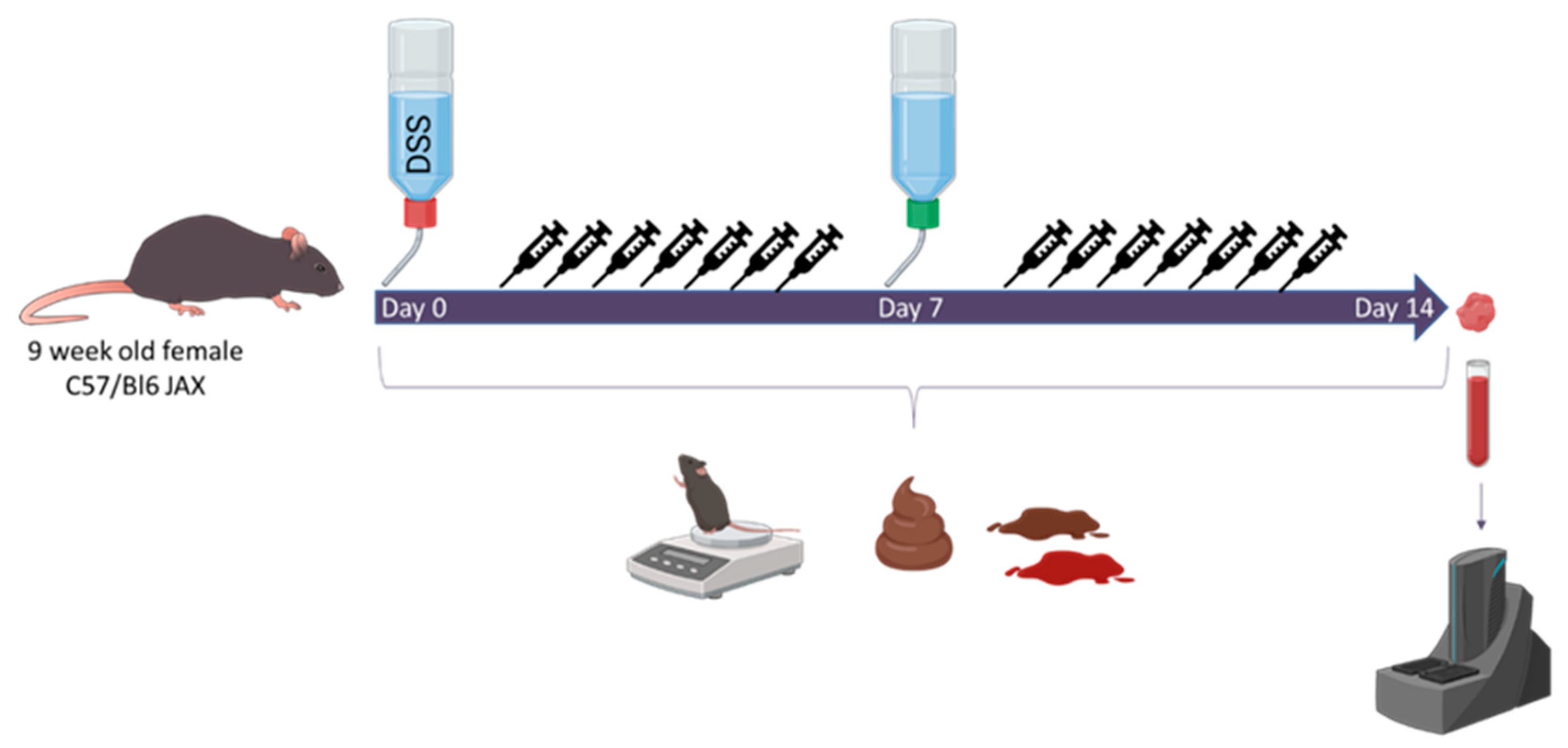
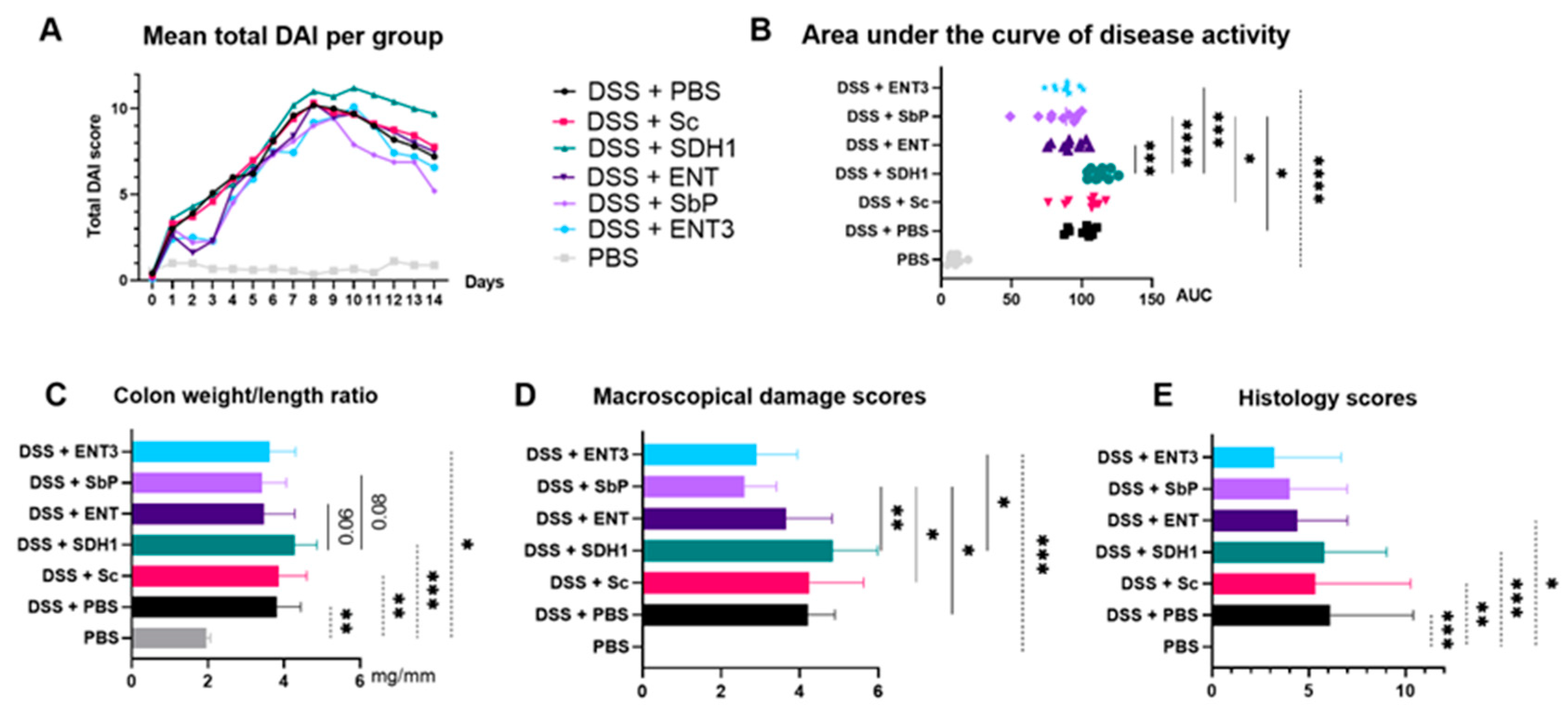
| Strain | Selection Criterion | Acetate Accumulation |
|---|---|---|
| PBS | Negative control | 0 g/L |
| S. cerevisiae [Sc] | Non-probiotic control | 0 g/L |
| S. cerevisiae var. boulardii SDH1 [SDH1] | No acetate production (engineered) | 0 g/L |
| S. cerevisiae var. boulardii Enterol [ENT] | Probiotic control | Transient with 1.8 g/L peak at 24 h, but 0 g/L after 48 h |
| S. cerevisiae var. boulardii Sb.P [Sb.P] | High acetate accumulation (natural mutation) | 5 g/L |
| S. cerevisiae var. boulardii ENT3 [ENT3] | Highest acetate accumulation (engineered) | 8.5 g/L |
| Gene | DSS + PBS | DSS + Sc | DSS + SDH1 | DSS + ENT | DSS + Sb.P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FC | Adj. p | FC | Adj. p | FC | Adj. p | FC | Adj. p | FC | Adj. p | ||
| Inflammatory genes | Tnf | 1.31 | <0.01 | 1.55 | <0.01 | 0.88 | 0.42 * | 0.48 | 0.74 | −0.29 | NA |
| Il1b | 2.49 | <0.01 | 4.6 | <0.01 | 1.77 | 0.18 * | 0.42 | 0.84 | 0.29 | NA | |
| Il2 | −0.55 | 0.36 | −0.74 | 0.26 | −0.71 | 0.58 | 0.25 | 0.86 | −0.65 | NA | |
| Il12a | 1.62 | <0.01 | 0.89 | 0.22 * | 0.005 | 0.99 | 0.24 | 0.89 | 0.01 | NA | |
| Barrier genes | Muc2 | −0.02 | 0.97 | 0.07 | 0.88 | −0.008 | 0.99 | 0.03 | 0.98 | 0.04 | 0.94 |
| Cldn1 | 1 | 0.03 | 0.75 | 0.16 * | 0.1 | 0.94 | 0.77 | 0.49 | 0.44 | NA | |
| Cldn2 | −0.25 | 0.43 | −0.4 | 0.22 * | −0.54 | 0.33 | −0.5 | 0.44 | −0.76 | 0.04 | |
| Cldn8 | 1.38 | <0.01 | 1.3 | <0.01 | 0.64 | 0.25 | 0.05 | 0.95 | −0.15 | 0.68 | |
| Tjp1~ZO1 | 0.13 | 0.63 | 0.34 | 0.2 | 0.31 | 0.54 | −0.07 | 0.91 | 0.56 | 0.06 | |
| Ocel1~OCLDN | 0.07 | 0.75 | −0.09 | 0.73 | 0.16 | 0.71 | 0.3 | 0.5 | 0.06 | 0.79 | |
| CAMs | Icam1 | 1.29 | <0.01 | 1 | <0.01 | 0.55 | 0.47 | 0.28 | 0.76 | 0.14 | 0.75 |
| Proliferation marker | Mki67 | 0.21 | 0.49 | 0.47 | 0.14 * | 0.09 | 0.91 | 0.2 | 0.8 | 0.07 | 0.86 |
| SCFA receptors | Ffar2 | 0.26 | 0.37 | 0.26 | 0.43 | 0.37 | 0.54 | 0.1 | 0.89 | −0.13 | 0.72 |
| Ffar3 | 0.32 | 0.33 | −0.08 | 0.85 | −0.06 | 0.94 | 0.21 | 0.81 | 0.13 | 0.76 | |
| Ffar4 | 0.16 | 0.67 | −0.1 | 0.81 | −0.33 | 0.65 | 0.14 | 0.87 | −0.64 | 0.1 | |
| Hcar2 | 0.46 | 0.3 | 1.02 | 0.02 | −0.12 | 0.92 | 0.24 | 0.84 | 0.22 | NA | |
| Model related genes | S100a8 | 4 | <0.01 | 5.57 | <0.01 | 2.67 | 0.18 * | 1.36 | 0.66 * | 0.88 | NA |
| S100a9 | 3.55 | <0.01 | 5.1 | <0.01 | 1.99 | 0.43 * | 0.68 | 0.83 | 0.88 | NA | |
| Lcn2 | 0.77 | 0.23 | 1.71 | 0.01 | 0.46 | 0.76 | 0.03 | 0.99 | −0.65 | NA | |
| Mid-Experiment | End of the Experiment | |||||
|---|---|---|---|---|---|---|
| SCFA | Controls | DSS Colitis | p (t-Test) | Controls | DSS Colitis | p (t-Test) |
| Acetate | 59.36 [24.63] | 75.28 [22.95] | <0.001 | 74.78 [20.25] | 78.25 [23.90] | 0.399 |
| Propionate | 4.60 [2.10] | 6.32 [1.92] | <0.001 | 6.90 [2.46] | 9.86 [4.02] | <0.001 |
| Butyrate | 6.64 [5.64] | 3.18 [1.61] | <0.001 | 9.36 [5.22] | 6.51 [5.20] | 0.004 |
| Comparison | Pre (p) | Mid (p) | End (p) |
|---|---|---|---|
| PBS vs. DSS + PBS | 1.00 | 1.00 | 0.049 |
| Sc vs. DSS + Sc | 0.47 | 0.0003 | 0.71 |
| SDH1 vs. DSS + SDH1 | 0.77 | 0.037 | 0.04 |
| ENT vs. DSS + ENT | 0.85 | 0.015 | 0.037 |
| Sb.P vs. DSS + Sb.P | 0.08 | 0.005 | 0.0048 |
| ENT3 vs. DSS + ENT3 | 0.41 | 0.93 | 0.8 |
| Acetate | Propionate | Butyrate | ||||
|---|---|---|---|---|---|---|
| Group | Mid | End | Mid | End | Mid | End |
| DSS + PBS | 77.19 [19.4] | 89.06 [38.7] | 6.78 [1.50] | 10.05 [5.24] | 3.84 [1.82] | 7.30 [6.65] |
| DSS + Sc | 78.65 [23.7] | 80.44 [16.1] | 6.84 [1.33] | 9.97 [3.99] | 3.66 [1.81] | 5.41 [1.83] |
| DSS + SDH1 | 76.03 [18.8] | 80.57 [22.0] | 6.37 [1.45] | 10.94 [2.89] | 2.60 [0.82] | 8.81 [8.00] |
| DSS + Sb.P | 67.45 [32.0] | 76.16 [17.1] | 5.87 [2.79] | 9.54 [3.98] | 3.49 [1.93] | 5.47 [2.95] |
| DSS + ENT | 73.93 [20.0] | 72.16 [23.4] | 6.12 [2.29] | 9.79 [5.46] | 2.60 [1.40] | 6.15 [5.73] |
| DSS + ENT3 | 78.39 [25.3] | 70.72 [19.4] | 5.95 [2.00] | 8.88 [2.71] | 2.88 [1.56] | 5.77 [3.53] |
| p | 0.9 | 0.58 | 0.81 | 0.93 | 0.33 | 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deleu, S.; Jacobs, I.; Vazquez Castellanos, J.F.; Verstockt, S.; Trindade de Carvalho, B.; Subotić, A.; Verstockt, B.; Arnauts, K.; Deprez, L.; Vissers, E.; et al. Effect of Mutant and Engineered High-Acetate-Producing Saccharomyces cerevisiae var. boulardii Strains in Dextran Sodium Sulphate-Induced Colitis. Nutrients 2024, 16, 2668. https://doi.org/10.3390/nu16162668
Deleu S, Jacobs I, Vazquez Castellanos JF, Verstockt S, Trindade de Carvalho B, Subotić A, Verstockt B, Arnauts K, Deprez L, Vissers E, et al. Effect of Mutant and Engineered High-Acetate-Producing Saccharomyces cerevisiae var. boulardii Strains in Dextran Sodium Sulphate-Induced Colitis. Nutrients. 2024; 16(16):2668. https://doi.org/10.3390/nu16162668
Chicago/Turabian StyleDeleu, Sara, Inge Jacobs, Jorge F. Vazquez Castellanos, Sare Verstockt, Bruna Trindade de Carvalho, Ana Subotić, Bram Verstockt, Kaline Arnauts, Lowie Deprez, Eva Vissers, and et al. 2024. "Effect of Mutant and Engineered High-Acetate-Producing Saccharomyces cerevisiae var. boulardii Strains in Dextran Sodium Sulphate-Induced Colitis" Nutrients 16, no. 16: 2668. https://doi.org/10.3390/nu16162668
APA StyleDeleu, S., Jacobs, I., Vazquez Castellanos, J. F., Verstockt, S., Trindade de Carvalho, B., Subotić, A., Verstockt, B., Arnauts, K., Deprez, L., Vissers, E., Lenfant, M., Vandermeulen, G., De Hertogh, G., Verbeke, K., Matteoli, G., Huys, G. R. B., Thevelein, J. M., Raes, J., & Vermeire, S. (2024). Effect of Mutant and Engineered High-Acetate-Producing Saccharomyces cerevisiae var. boulardii Strains in Dextran Sodium Sulphate-Induced Colitis. Nutrients, 16(16), 2668. https://doi.org/10.3390/nu16162668








