Postprandial Blood Glucose and Insulin Response in Healthy Adults When Lentils Replace High-Glycemic Index Food Ingredients in Muffins, Chilies and Soups
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participant Recruitment and Screening
2.3. Study Treatments
2.4. Study Visit Preparation
2.5. Anthropometric Measurements
2.6. Blood Collection and Analysis
2.7. Data and Statistical Analysis
3. Results
3.1. Participant Flow and Characteristics
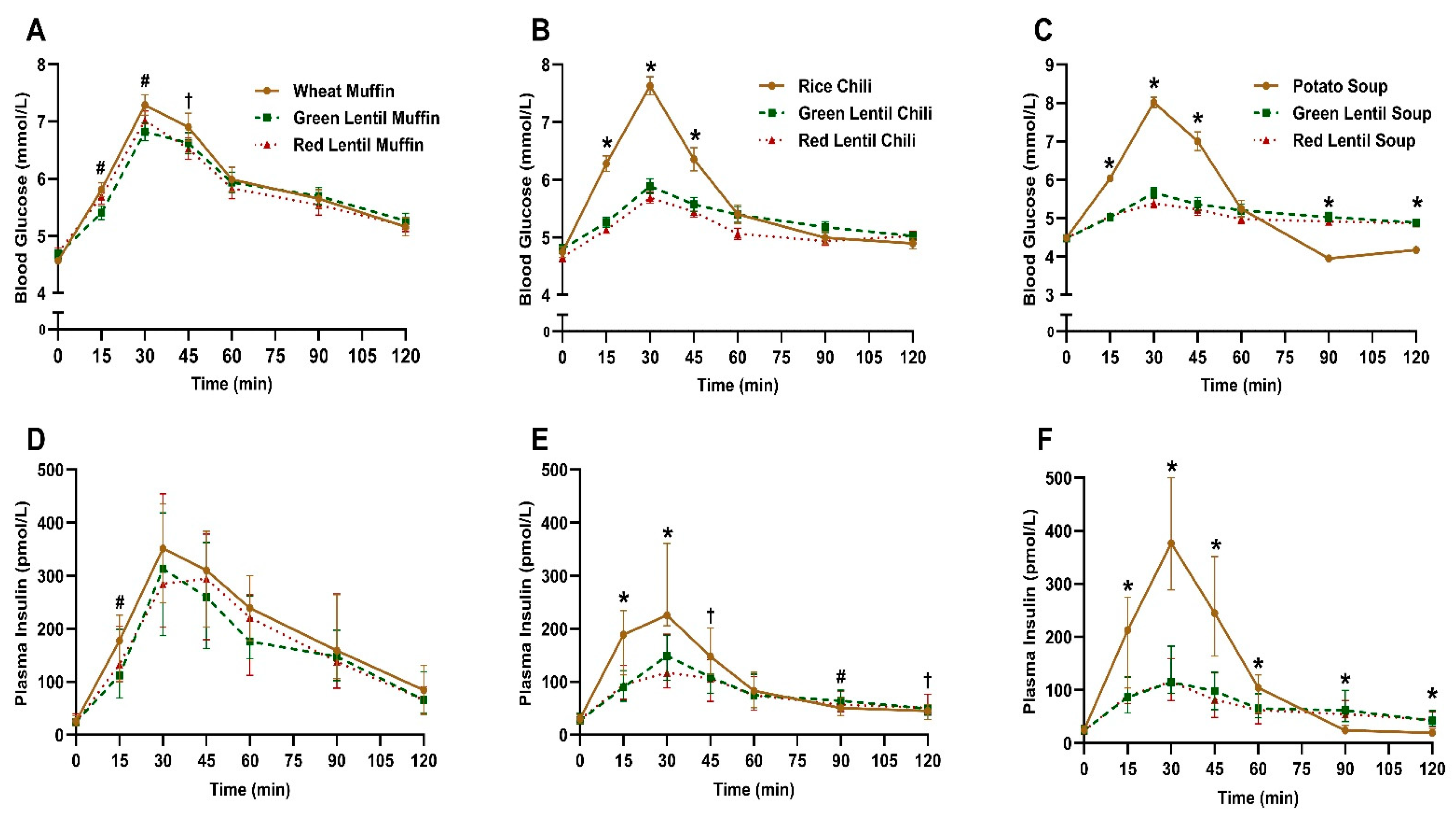
3.2. Postprandial Blood Glucose and Insulin Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Available carbohydrates |
| BMI | Body mass index |
| CMAX | Maximum concentration |
| GI | Glycemic index |
| HNRU | Human Nutraceutical Research Unit |
| iAUC | Incremental area under the curve |
| PBGR | Postprandial blood glucose response |
| RGR | Relative Glycemic Response |
References
- Hall, C.; Hillen, C.; Garden Robinson, J. Composition, Nutritional Value, and Health Benefits of Pulses. Cereal Chem. 2017, 94, 11–31. [Google Scholar] [CrossRef]
- Ramdath, D.D.; Wolever, T.M.S.; Siow, Y.C.; Ryland, D.; Hawke, A.; Taylor, C.; Zahradka, P.; Aliani, M. Effect of Processing on Postprandial Glycemic Response and Consumer Acceptability of Lentil-Containing Food Items. Foods 2018, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Villegas, R.; Gao, Y.T.; Yang, G.; Li, H.L.; Elasy, T.A.; Zheng, W.; Shu, X.O. Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women’s Health Study. Am. J. Clin. Nutr. 2008, 87, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Buil-Cosiales, P.; Corella, D.; Estruch, R.; Santos-Lozano, J.M.; Portoles, O.; Casas, R.; Medina-Renom, A.; Baena, J.M.; Duaso, I.; García, Y.; et al. Legume consumption is inversely associated with type 2 diabetes incidence in adults: A prospective assessment from the PREDIMED study. Clin. Nutr. 2018, 37, 906–913. [Google Scholar] [CrossRef]
- Sievenpiper, J.L.; Kendall, C.W.C.; Esfahani, A.; Wong, J.M.W.; Carleton, A.J.; Jiang, H.Y.; Bazinet, R.P.; Vidgen, E.; Jenkins, D.J.A. Effect of non-oil-seed pulses on glycaemic control: A systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia 2009, 52, 1479–1495. [Google Scholar] [CrossRef] [PubMed]
- Bornet, F.R.; Costagliola, D.; Rizkalla, S.W.; Blayo, A.; Fontvieille, A.M.; Haardt, M.J.; Letanoux, M.; Tchobroutsky, G.; Slama, G. Insulinemic and glycemic indexes of six starch-rich foods taken alone and in a mixed meal by type 2 diabetics. Am. J. Clin. Nutr. 1987, 45, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.H.; Liu, Y.; Smith, C.E.; Liu, T.T.; Nunez, M.F.; Mollard, R.C.; Luhovyy, B.L. The acute effect of commercially available pulse powders on postprandial glycaemic response in healthy young men. Br. J. Nutr. 2014, 112, 1966–1973. [Google Scholar] [CrossRef] [PubMed]
- Mollard, R.C.; Zykus, A.; Luhovyy, B.L.; Nunez, M.F.; Wong, C.L.; Anderson, G.H. The acute effects of a pulse-containing meal on glycaemic responses and measures of satiety and satiation within and at a later meal. Br. J. Nutr. 2012, 108, 509–517. [Google Scholar] [CrossRef]
- Anguah, K.O.B.; Wonnell, B.S.; Campbell, W.W.; McCabe, G.P.; McCrory, M.A. A Blended- Rather Than Whole-Lentil Meal with or without α-Galactosidase Mildly Increases Healthy Adults’ Appetite but Not Their Glycemic Response. J. Nutr. 2014, 144, 1963–1969. [Google Scholar] [CrossRef][Green Version]
- Mollard, R.C.; Wong, C.L.; Luhovyy, B.L.; Anderson, G.H. First and second meal effects of pulses on blood glucose, appetite, and food intake at a later meal. Appl. Physiol. Nutr. Metab. = Physiol. Appl. Nutr. Metab. 2011, 36, 634–642. [Google Scholar] [CrossRef]
- Fujiwara, N.; Hall, C.; Jenkins, A.L. Development of Low Glycemic Index (GI) Foods by Incorporating Pulse Ingredients into Cereal-Based Products: Use of In Vitro Screening and In Vivo Methodologies. Cereal Chem. 2017, 94, 110–116. [Google Scholar] [CrossRef]
- Moravek, D.; Duncan, A.M.; VanderSluis, L.B.; Turkstra, S.J.; Rogers, E.J.; Wilson, J.M.; Hawke, A.; Ramdath, D.D. Carbohydrate Replacement of Rice or Potato with Lentils Reduces the Postprandial Glycemic Response in Healthy Adults in an Acute, Randomized, Crossover Trial. J. Nutr. 2018, 148, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Ramdath, D.; Renwick, S.; Duncan, A.M. The Role of Pulses in the Dietary Management of Diabetes. Can. J. Diabetes 2016, 40, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.C.M.S.R.D.; Lawrence, F.R.P.; Hartman, T.J.P.R.D.; Curran, J.M.P. Consumption of Dry Beans, Peas, and Lentils Could Improve Diet Quality in the US Population. J. Am. Diet. Assoc. 2009, 109, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Mudryj, A.N.; Yu, N.; Hartman, T.J.; Mitchell, D.C.; Lawrence, F.R.; Aukema, H.M. Pulse consumption in Canadian adults influences nutrient intakes. Br. J. Nutr. 2012, 108 (Suppl. S1), S27–S36. [Google Scholar] [CrossRef] [PubMed]
- Tovar, J.; Granfeldt, Y.; Björck, I.M. Effect of processing on blood glucose and insulin responses to starch in legumes. J. Agric. Food Chem. 1992, 40, 1846–1851. [Google Scholar] [CrossRef]
- Bhavadharini, B.; Mohan, V.; Dehghan, M.; Rangarajan, S.; Swaminathan, S.; Rosengren, A.; Wielgosz, A.; Avezum, A.; Lopez-Jaramillo, P.; Lanas, F.; et al. White Rice Intake and Incident Diabetes: A Study of 132,373 Participants in 21 Countries. Diabetes Care 2020, 43, 2643–2650. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.A.; Pan, A.; Malik, V.; Sun, Q. White rice consumption and risk of type 2 diabetes: Meta-analysis and systematic review. Bmj 2012, 344, e1454. [Google Scholar] [CrossRef]
- Public Health Agency of Canada. Reducing Health Disparities Related to Diabetes: Lessons Learned through the Canadian Diabetes Strategy Community-Based Program [Internet]; Report No.: HP5-111/2011E-PDF; Government of Canada: Ottawa, ON, Canada, 2011. Available online: https://publications.gc.ca/pub?id=9.694650&sl=0 (accessed on 11 June 2024).
- Li-Geng, T.; Kilham, J.; McLeod, K.M. Cultural Influences on Dietary Self-Management of Type 2 Diabetes in East Asian Americans: A Mixed-Methods Systematic Review. Health Equity 2020, 4, 31–42. [Google Scholar] [CrossRef]
- Rai, A.; Misra, R.; Khan, H.; Shukla, S.; Patel, D.C.; Brown, A. Systematic review of the barriers and facilitators to dietary modification in people living with type 2 diabetes and pre-diabetes from South Asian ethnic populations. Diabet. Med. 2023, 40, e15132. [Google Scholar] [CrossRef]
- Creamer, J.; Attridge, M.; Ramsden, M.; Cannings-John, R.; Hawthorne, K. Culturally appropriate health education for Type 2 diabetes in ethnic minority groups: An updated Cochrane Review of randomized controlled trials. Diabet. Med. J. Br. Diabet. Assoc. 2016, 33, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Brummer, Y.; Kaviani, M.; Tosh, S.M. Structural and functional characteristics of dietary fibre in beans, lentils, peas and chickpeas. Food Res. Int. 2015, 67, 117–125. [Google Scholar] [CrossRef]
- Brouns, F.; Bjorck, I.; Frayn, K.N.; Gibbs, A.L.; Lang, V.; Slama, G.; Wolever, T.M. Glycaemic index methodology. Nutr. Res. Rev. 2005, 18, 145–171. [Google Scholar] [CrossRef]
- Sieri, S.; Krogh, V. Dietary glycemic index, glycemic load and cancer: An overview of the literature. Nutr. Metab. Cardiovasc. Dis. NMCD 2017, 27, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Muntoni, S.; Muntoni, S.; Draznin, B. Effects of chronic hyperinsulinemia in insulin-resistant patients. Curr. Diabetes Rep. 2008, 8, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Mollard, R.C.; Wong, C.L.; Luhovyy, B.L.; Cho, F.; Anderson, G.H. Second-meal effects of pulses on blood glucose and subjective appetite following a standardized meal 2 h later. Appl. Physiol. Nutr. Metab. = Physiol. Appl. Nutr. Metab. 2014, 39, 849–851. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Huot, P.S.P.; Lee, Y.T.; Vien, S.; Luhovyy, B.L.; Anderson, G.H. The effect of dairy and nondairy beverages consumed with high glycemic cereal on subjective appetite, food intake, and postprandial glycemia in young adults. Appl. Physiol. Nutr. Metab. 2017, 42, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Griffiths, C.; Krzeminska, K.; Lawrie, J.A.; Bennett, C.M.; Goff, D.V.; Sarson, D.L.; Bloom, S.R. Slow release dietary carbohydrate improves second meal tolerance. Am. J. Clin. Nutr. 1982, 35, 1339–1346. [Google Scholar] [CrossRef]
- Coulston, A.M.; Hollenbeck, C.B.; Liu, G.C.; Williams, R.A.; Starich, G.H.; Mazzaferri, E.L.; Reaven, G.M. Effect of source of dietary carbohydrate on plasma glucose, insulin, and gastric inhibitory polypeptide responses to test meals in subjects with noninsulin-dependent diabetes mellitus. Am. J. Clin. Nutr. 1984, 40, 965–970. [Google Scholar] [CrossRef]

| Muffin Treatments | Chili Treatments | Soup Treatments | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Wheat Control | Green Lentil | Red Lentil | Rice Control | Green Lentil | Red Lentil | Potato Control | Green Lentil | Red Lentil | |
| Serving * (g) | 128 | 204 | 178 | 237 | 305 | 283 | 471 | 361 | 339 |
| Energy † (kcal) | 384 | 505 | 458 | 194 | 314 | 287 | 190 | 276 | 245 |
| Protein ‡ (g) | 9.5 | 20.0 | 16.8 | 4.8 | 17.0 | 14.7 | 5.6 | 15.7 | 13.3 |
| Fat § (g) | 9.9 | 10.4 | 10.3 | 2.2 | 3.3 | 3.0 | 1.3 | 1.7 | 1.6 |
| Carbohydrates ║ (g) | 58.3 | 71.5 | 65.2 | 35.9 | 46.8 | 43.5 | 36.7 | 43.1 | 38.3 |
| Dietary Fiber ¶ (g) | 4.2 | 15.1 | 9.8 | 5.0 | 16.9 | 13.1 | 5.8 | 13.9 | 9.7 |
| Available Carbohydrates # (g) | 44.8 | 46.7 | 45.4 | 27.3 | 26.1 | 26.6 | 26.9 | 26.3 | 25.3 |
| Muffin (n = 24) | Chili (n = 24) | Soup (n = 20) | |
|---|---|---|---|
| Age (years) | 26.9 ± 1.3 | 26.6 ± 1.1 | 23.1 ± 0.7 |
| Sex (n male/female) | 9/15 | 10/14 | 9/11 |
| Body Weight (kg) | 69.5 ± 2.2 | 71.3 ± 2.2 | 69.3 ± 2.0 |
| BMI 2 (kg/m2) | 24.9 ± 0.4 | 24.2 ± 0.4 | 23.8 ± 0.5 |
| Waist Circumference (cm) | 80.4 ± 1.6 | 81.7 ± 1.5 | 80.2 ± 1.4 |
| Systolic Blood Pressure (mmHg) | 117 ± 3 | 117 ± 3 | 116 ± 3 |
| Diastolic Blood Pressure (mmHg) | 70 ± 2 | 69 ± 2 | 68 ± 2 |
| Muffin Study | Chili Study | Soup Study | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Wheat Control | Green Lentil | Red Lentil | Rice Control | Green Lentil | Red Lentil | Potato Control | Green Lentil | Red Lentil | |
| Postprandial blood glucose † mmol/L.min | 169.0 ± 12.8 a | 142.6 ± 14.9 a,b | 136.6 ± 13.2 b | 122.4 ± 11.0 a | 65.4 ± 7.4 b | 61.3 ± 10.0 b | 130.3 ± 9.8 a | 80.1 ± 9.3 b | 64.9 ± 9.1 b |
| CMAX, mmol/L | 5.1 ± 0.1 a | 5.1 ± 0.1 a | 5.1 ± 0.1 a | 7.7 ± 0.2 a | 6.0 ± 0.1 b | 5.7 ± 0.1 b | 8.0 ± 0.1 a | 5.8 ± 0.1 b | 5.6 ± 0.1 b |
| Postprandial plasma insulin ‡, nmol/L.min | 18.2 (15.1, 21.9) a | 15.1 (12.3, 18.5) b | 15.7 (13.0, 18.9) a,b | 8.4 (7.1, 9.9) a | 5.8 (4.7, 7.1) b | 4.9 (3.7, 6.4) b | 11.2 (9.1, 13.8) a | 4.4 (3.1, 6.2) b | 4.7 (3.8, 5.9) b |
| CMAX, nmol/L | 0.33 | 0.28 | 0.30 | 0.24 | 0.13 | 0.12 | 0.32 | 0.11 | 0.11 |
| (0.27, 0.41) a | (0.23, 0.35) a | (0.25, 0.35) a | (0.21, 0.28) a | (0.11, 0.16) b | (0.10, 0.15) b | (0.27, 0.39) a | (0.09, 0.13) b | (0.09, 0.13) b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamoun, D.; Duncan, A.M.; Lukus, P.K.; Loreto, M.D.; Pals-Horne, F.; Hawke, A.; Ramdath, D.D. Postprandial Blood Glucose and Insulin Response in Healthy Adults When Lentils Replace High-Glycemic Index Food Ingredients in Muffins, Chilies and Soups. Nutrients 2024, 16, 2669. https://doi.org/10.3390/nu16162669
Chamoun D, Duncan AM, Lukus PK, Loreto MD, Pals-Horne F, Hawke A, Ramdath DD. Postprandial Blood Glucose and Insulin Response in Healthy Adults When Lentils Replace High-Glycemic Index Food Ingredients in Muffins, Chilies and Soups. Nutrients. 2024; 16(16):2669. https://doi.org/10.3390/nu16162669
Chicago/Turabian StyleChamoun, Dita, Alison M. Duncan, Patricia K. Lukus, Michael D. Loreto, Frances Pals-Horne, Aileen Hawke, and D. Dan Ramdath. 2024. "Postprandial Blood Glucose and Insulin Response in Healthy Adults When Lentils Replace High-Glycemic Index Food Ingredients in Muffins, Chilies and Soups" Nutrients 16, no. 16: 2669. https://doi.org/10.3390/nu16162669
APA StyleChamoun, D., Duncan, A. M., Lukus, P. K., Loreto, M. D., Pals-Horne, F., Hawke, A., & Ramdath, D. D. (2024). Postprandial Blood Glucose and Insulin Response in Healthy Adults When Lentils Replace High-Glycemic Index Food Ingredients in Muffins, Chilies and Soups. Nutrients, 16(16), 2669. https://doi.org/10.3390/nu16162669






