A Mixture of Soybean Oil and Lard Alleviates Postpartum Cognitive Impairment via Regulating the Brain Fatty Acid Composition and SCFA/ERK(1/2)/CREB/BDNF Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Behavioral Tests
2.3. The Staining of Hematoxylin and Eosin (H&E) and Nissl
2.4. Analysis of Fatty Acid Composition
2.5. Inflammatory Cytokines Analysis
2.6. Immunofluorescence Staining
2.7. Western Blotting
2.8. Gut Microbiota Analysis
2.9. Detection of SCFAs in Feces
2.10. The Transmission Electron Micrograph in the Hippocampus
2.11. Statistical Analysis
3. Results
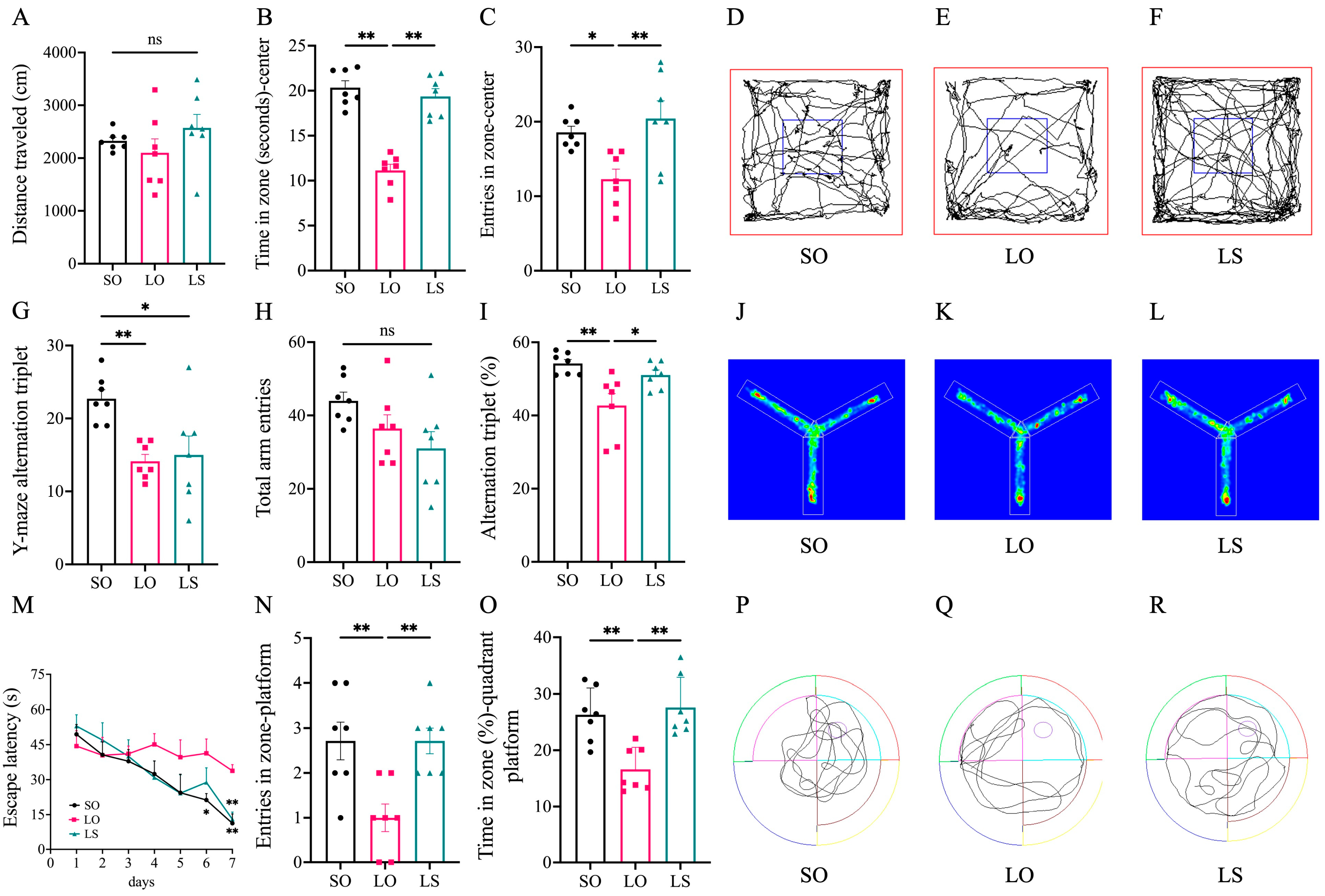
3.1. Effect of a Mixture of Soybean Oil and Lard on Postpartum Cognitive Function
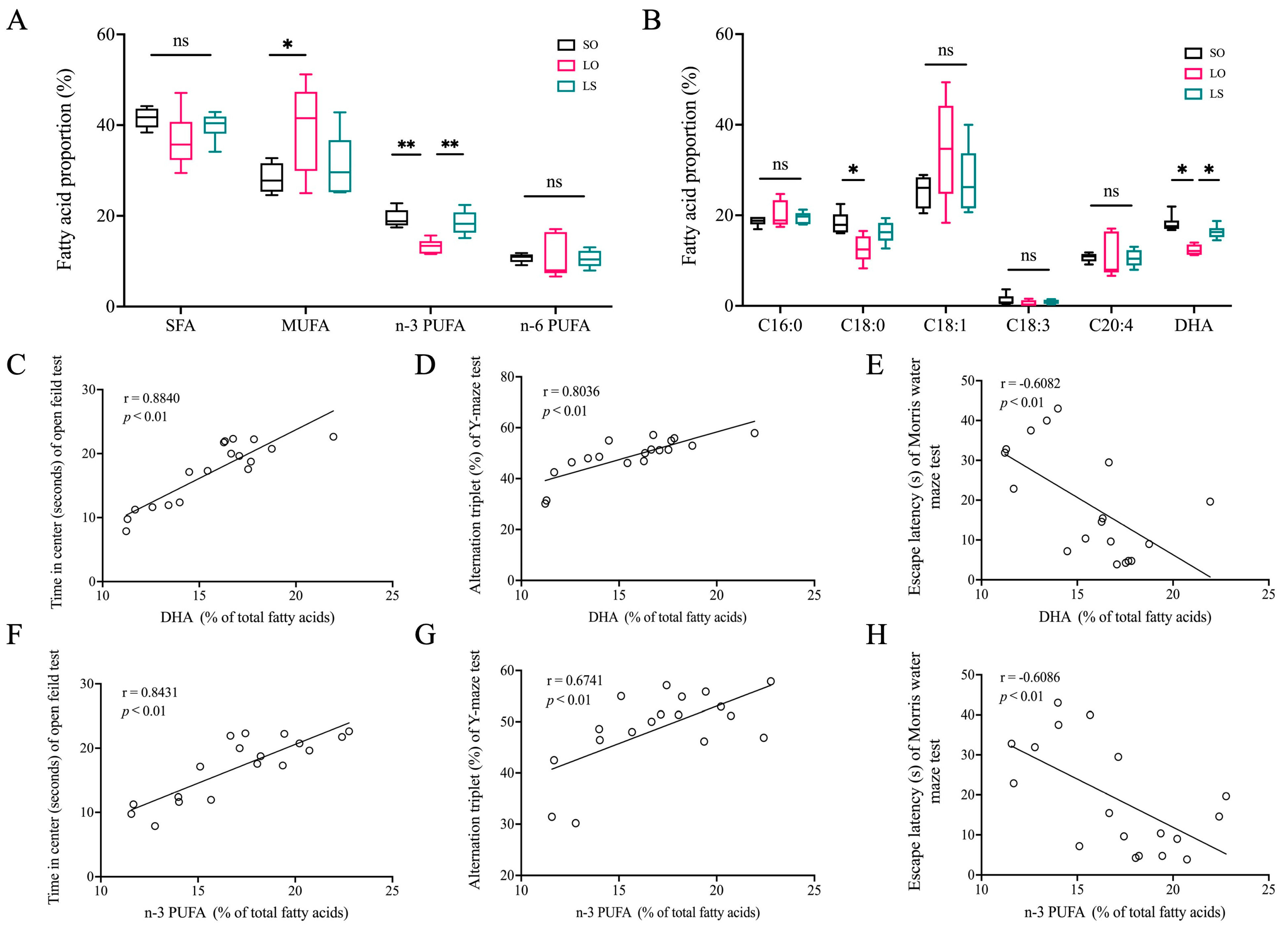
3.2. Effect of a Mixture of Soybean Oil and Lard on Brain Fatty Acid Composition and Its Correlation with Behavioral Testing Outcome Indicators
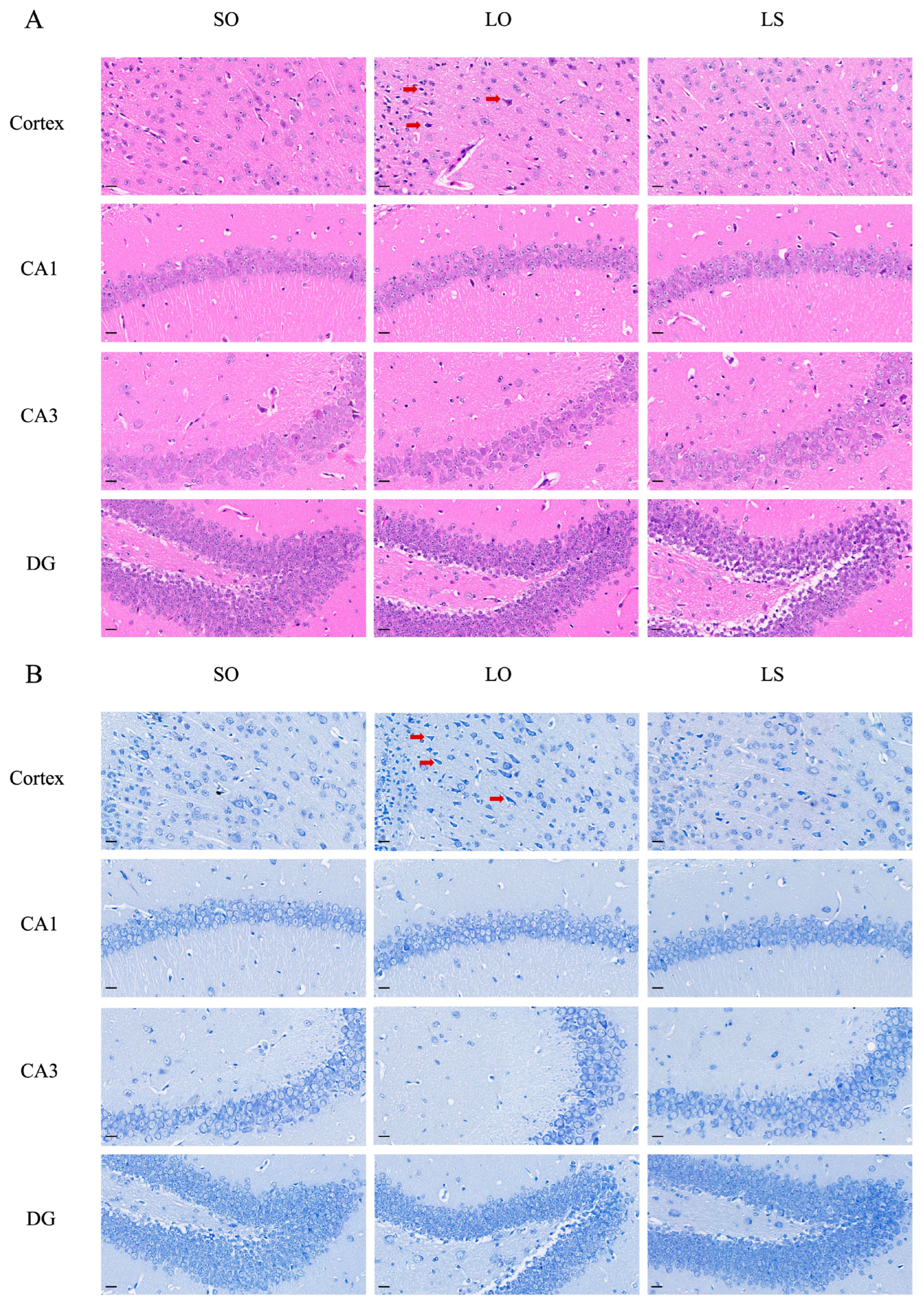
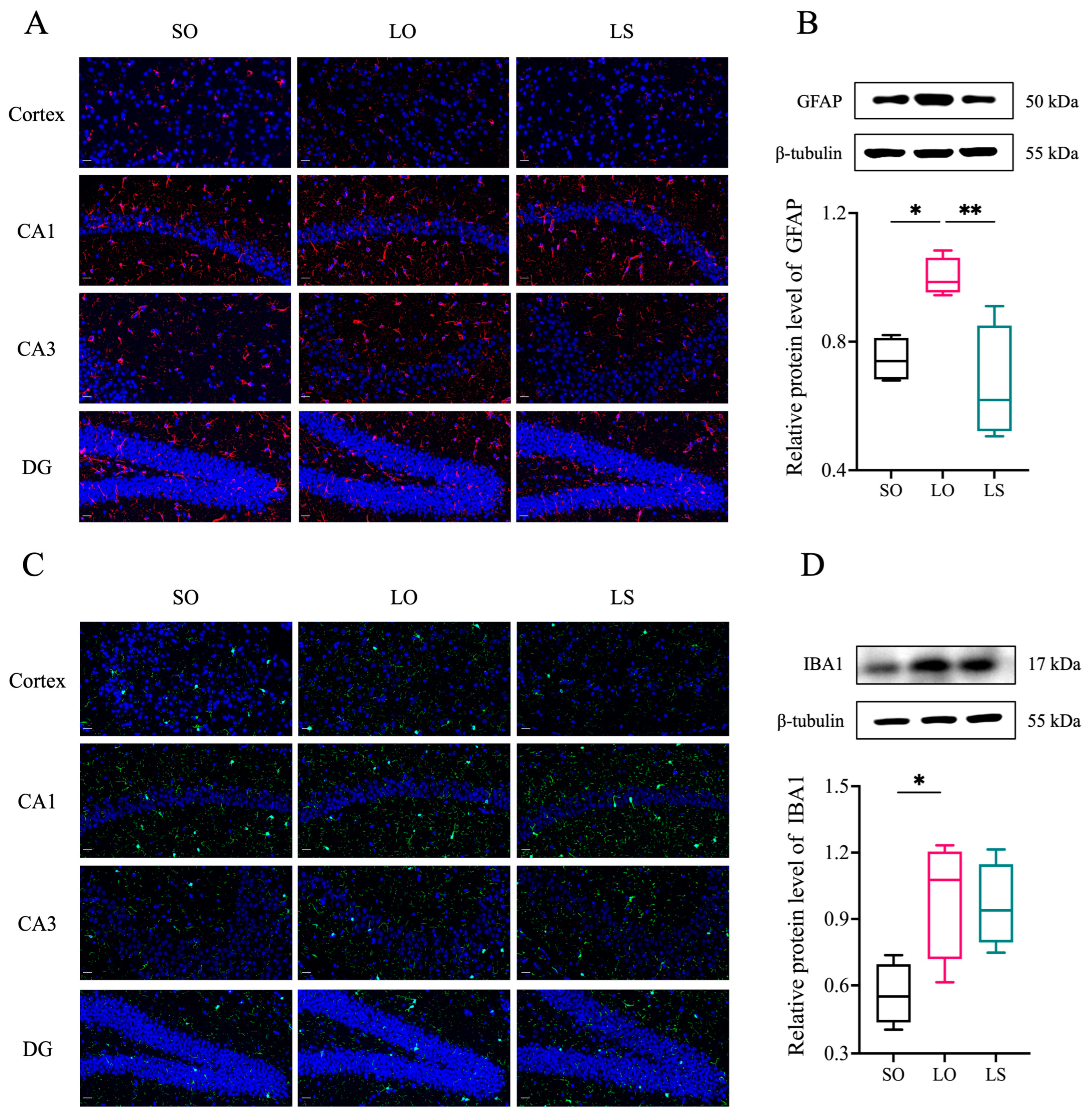
3.3. Effect of a Mixture of Soybean Oil and Lard on Brain Histopathology and the Activation of Neuroglial Cells
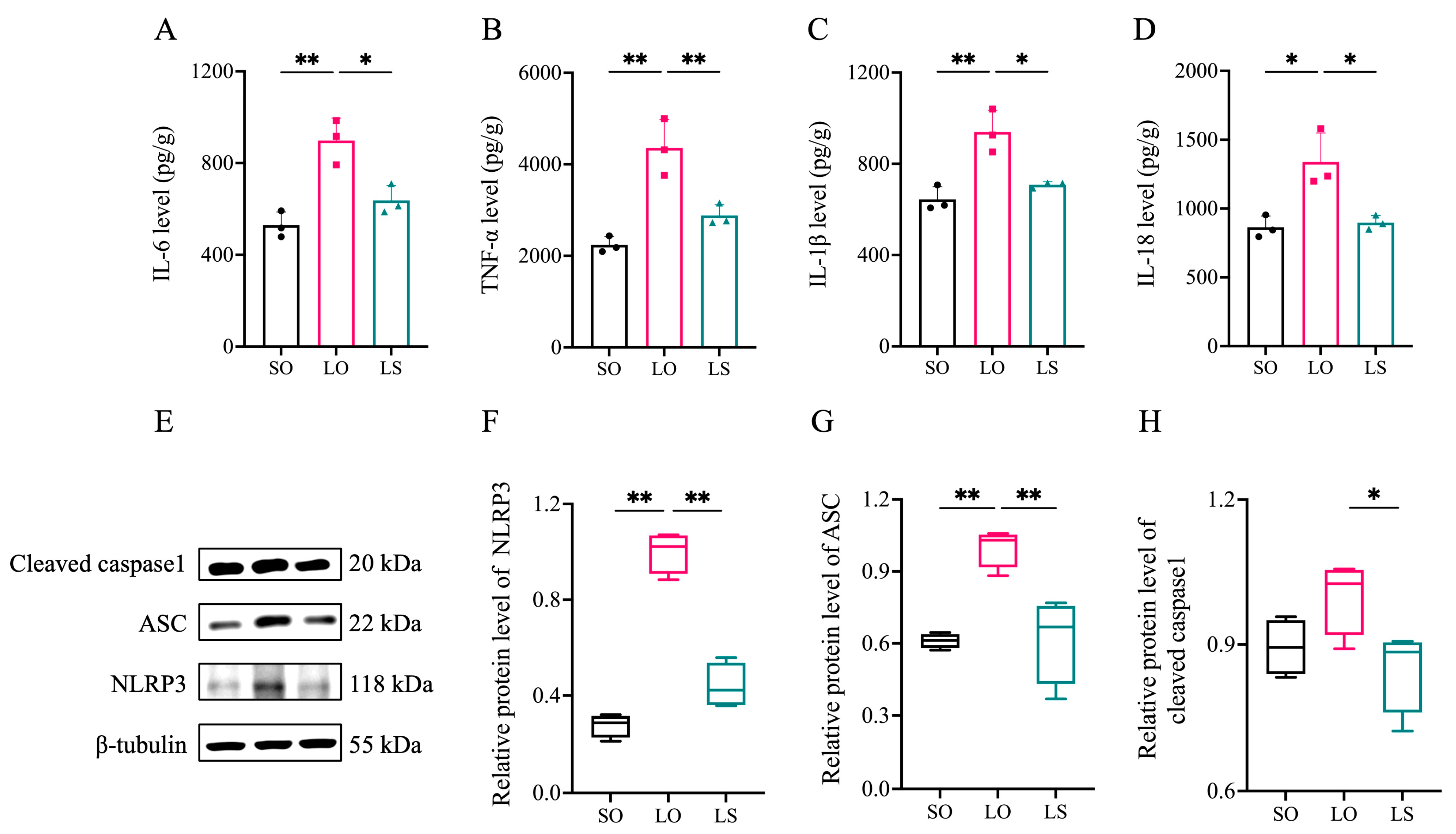
3.4. Effect of a Mixture of Soybean Oil and Lard on Brain Neuroinflammation
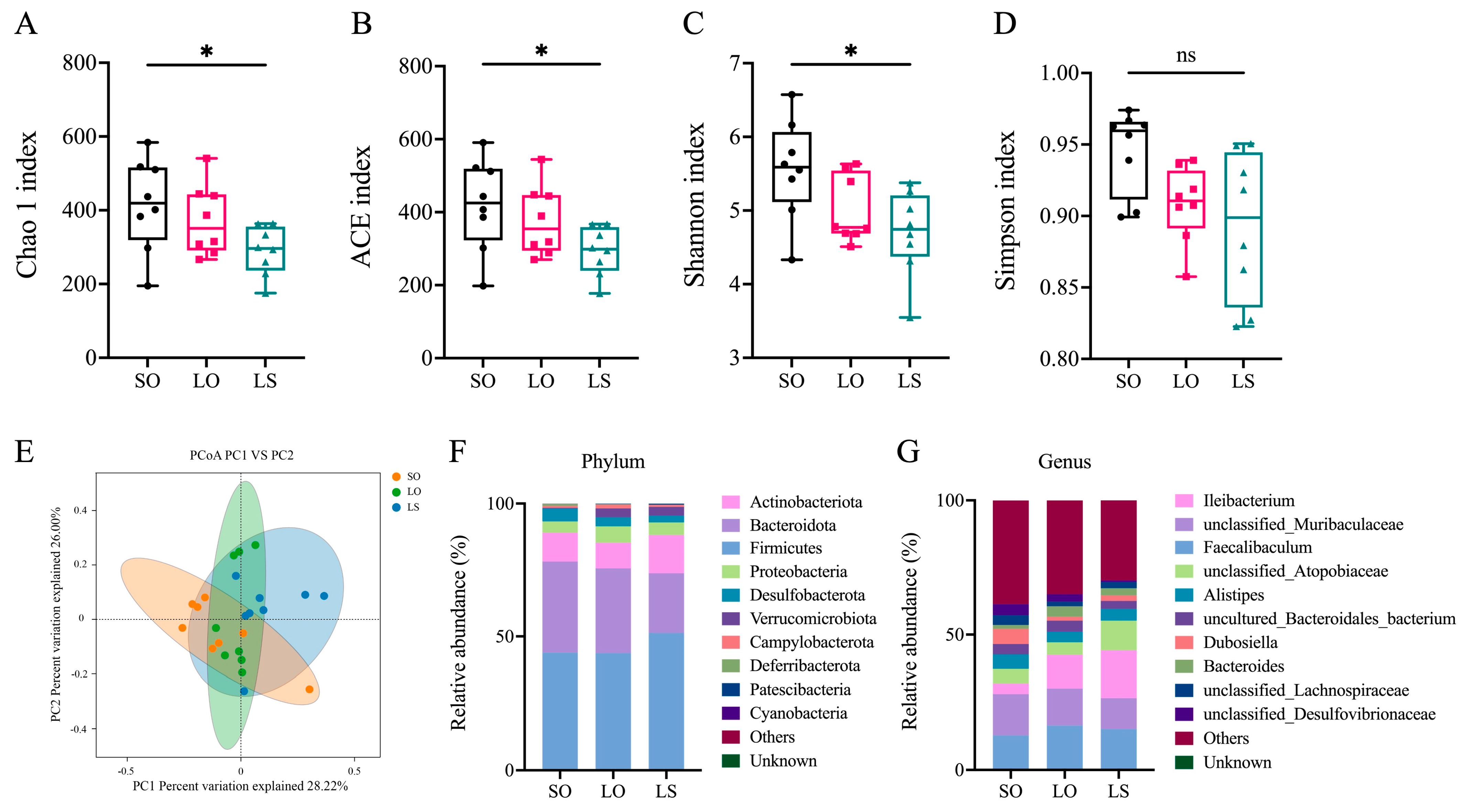
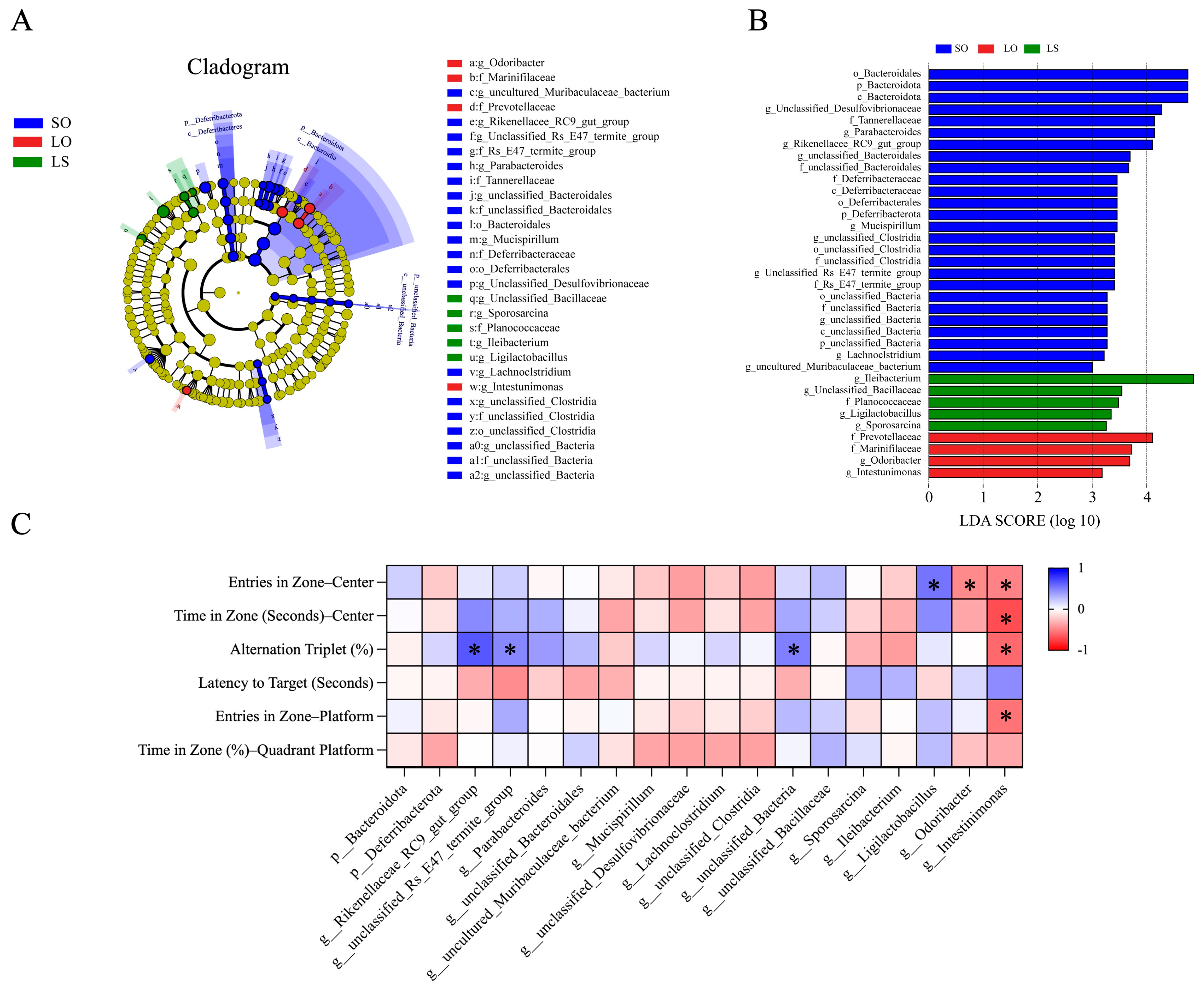
3.5. Effect of a Mixture of Soybean Oil and Lard on the Gut Microbiota Composition
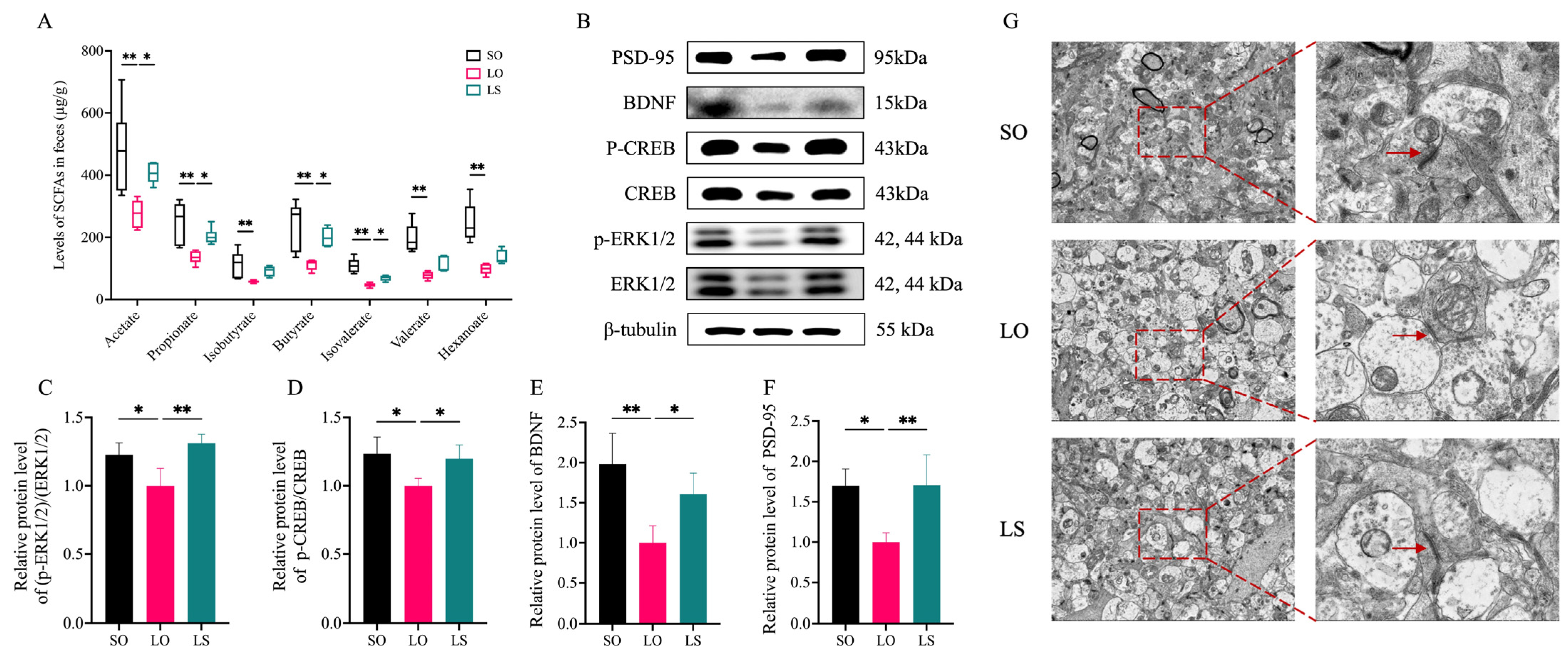
3.6. Effect of a Mixture of Soybean Oil and Lard on the SCFA and ERK(1/2)/CREB/BDNF Pathway-Related Protein Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Orchard, E.R.; Rutherford, H.J.V.; Holmes, A.J.; Jamadar, S.D. Matrescence: Lifetime impact of motherhood on cognition and the brain. Trends Cogn. Sci. 2023, 27, 302–316. [Google Scholar] [CrossRef]
- Hoekzema, E.; Barba-Müller, E.; Pozzobon, C.; Picado, M.; Lucco, F.; García-García, D.; Soliva, J.C.; Tobeña, A.; Desco, M.; Crone, E.A.; et al. Pregnancy leads to long-lasting changes in human brain structure. Nat. Neurosci. 2017, 20, 287–296. [Google Scholar] [CrossRef]
- Davies, S.J.; Lum, J.A.; Skouteris, H.; Byrne, L.K.; Hayden, M.J. Cognitive impairment during pregnancy: A meta-analysis. Med. J. Aust. 2018, 208, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Falah-Hassani, K.; Shiri, R.; Dennis, C.L. The prevalence of antenatal and postnatal co-morbid anxiety and depression: A meta-analysis. Psychol. Med. 2017, 47, 2041–2053. [Google Scholar] [CrossRef]
- Wang, J.; Yan, S.; Xiao, H.; Zhou, H.; Liu, S.; Zeng, Y.; Liu, B.; Li, R.; Yuan, Z.; Wu, J.; et al. Anti-obesity effect of a traditional Chinese dietary habit-blending lard with vegetable oil while cooking. Sci. Rep. 2017, 7, 14689. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, B.S. The Effect of Short-Term Feeding of a High-Coconut Oil or High-Fat Diet on Neuroinflammation and the Performance of an Object-Place Task in Rats. Neurochem. Res. 2021, 46, 287–298. [Google Scholar] [CrossRef]
- Nakajima, S.; Fukasawa, K.; Gotoh, M.; Murakami-Murofushi, K.; Kunugi, H. Saturated fatty acid is a principal cause of anxiety-like behavior in diet-induced obese rats in relation to serum lysophosphatidyl choline level. Int. J. Obes. 2020, 44, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yuan, J.; Wen, P.; Guo, X.; Wen, H.; Guo, Y.; Li, D. Effect of lard plus soybean oil on blood pressure and other cardiometabolic risk factors in healthy subjects: A randomized controlled-feeding trial. Food Funct. 2023, 14, 7117–7129. [Google Scholar] [CrossRef]
- Liu, Z.; Yuan, J.; Wen, P.; Guo, X.; Li, K.; Wang, Y.; Liu, R.; Guo, Y.; Li, D. Effect of Lard or Plus Soybean Oil on Markers of Liver Function in Healthy Subjects: A Randomized Controlled-Feeding Trial. Foods 2023, 12, 1894. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Macpherson, A.J.; Pachnis, V.; Prinz, M. Boundaries and integration between microbiota, the nervous system, and immunity. Immunity 2023, 56, 1712–1726. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.D.; Tung, T.H.; Teng, C.Y.; Chang, C.H.; Chen, Y.C.; Huang, H.Y.; Lee, H.C.; Huang, S.Y. Fish oil ameliorates neuropsychiatric behaviors and gut dysbiosis by elevating selected microbiota-derived metabolites and tissue tight junctions in rats under chronic sleep deprivation. Food Funct. 2022, 13, 2662–2680. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, Y.; Zhao, F.; Song, S.; Li, Y.; Xu, X.; Zhou, G.; Li, C. Fish oil, lard and soybean oil differentially shape gut microbiota of middle-aged rats. Sci. Rep. 2017, 7, 826. [Google Scholar] [CrossRef]
- Zheng, H.; Xu, P.; Jiang, Q.; Xu, Q.; Zheng, Y.; Yan, J.; Ji, H.; Ning, J.; Zhang, X.; Li, C.; et al. Depletion of acetate-producing bacteria from the gut microbiota facilitates cognitive impairment through the gut-brain neural mechanism in diabetic mice. Microbiome 2021, 9, 145. [Google Scholar] [CrossRef]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef] [PubMed]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef]
- Lv, J.; Lu, C.; Jiang, N.; Wang, H.; Huang, H.; Chen, Y.; Li, Y.; Liu, X. Protective effect of ginsenoside Rh2 on scopolamine-induced memory deficits through regulation of cholinergic transmission, oxidative stress and the ERK-CREB-BDNF signaling pathway. Phytother. Res. 2021, 35, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhou, M.; Zheng, R.; Ma, R.; Deng, J.; Hao, W.Z.; Wang, L.; Zhang, J.C.; Ho, C.T.; Huang, J.Q. Feruloylated oligosaccharides ameliorate MPTP-induced neurotoxicity in mice by activating ERK/CREB/BDNF/TrkB signalling pathway. Phytomedicine 2023, 108, 154512. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, B.Y.; Kim, J.I.; Lee, J.; Jeon, W.K. Mumefural Improves Recognition Memory and Alters ERK-CREB-BDNF Signaling in a Mouse Model of Chronic Cerebral Hypoperfusion. Nutrients 2023, 15, 3271. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, L.; Guo, X.; Li, S.; He, X.; Pei, S.; Li, D. N-3 polyunsaturated fatty acids prevent the d-galactose-induced cognitive impairment by up-regulating the levels of 5-hydroxymethylcytosine in the mouse brain. Food Funct. 2022, 13, 4101–4113. [Google Scholar] [CrossRef]
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Chumak, T.; Lecuyer, M.J.; Nilsson, A.K.; Faustino, J.; Ardalan, M.; Svedin, P.; Sjobom, U.; Ek, J.; Obenaus, A.; Vexler, Z.S.; et al. Maternal n-3 Polyunsaturated Fatty Acid Enriched Diet Commands Fatty Acid Composition in Postnatal Brain and Protects from Neonatal Arterial Focal Stroke. Transl. Stroke Res. 2022, 13, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Zhao, Y.; Shi, H.; Wang, C.; Zhang, T.; Wang, Y.; Xue, C. Short-term supplementation of DHA as phospholipids rather than triglycerides improve cognitive deficits induced by maternal omega-3 PUFA deficiency during the late postnatal stage. Food Funct. 2021, 12, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, S.; Sawada, N.; Matsuoka, Y.J.; Shikimoto, R.; Mimura, M.; Tsugane, S. Association Between Dietary Fish and PUFA Intake in Midlife and Dementia in Later Life: The JPHC Saku Mental Health Study. J. Alzheimers Dis. 2021, 79, 1091–1104. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Gao, X.; Shi, B.; Chen, S.; Zhou, X.; Li, Z.; Gan, Y.; Cui, L.; Kang, J.X.; Li, W.; et al. Enriched endogenous n-3 polyunsaturated fatty acids alleviate cognitive and behavioral deficits in a mice model of Alzheimer’s disease. Neuroscience 2016, 333, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Du, L.; Shi, H.H.; Ding, L.; Yanagita, T.; Xue, C.H.; Wang, Y.M.; Zhang, T.T. Dietary EPA-Enriched Phospholipids Alleviate Chronic Stress and LPS-Induced Depression- and Anxiety-Like Behavior by Regulating Immunity and Neuroinflammation. Mol. Nutr. Food Res. 2021, 65, e2100009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.T.; Du, X.M.; Ma, X.J.; Zong, Y.; Chen, J.K.; Yu, C.L.; Liu, Y.G.; Chen, Y.C.; Zhao, L.J.; Lu, G.C. Activation of the NLRP3 inflammasome in lipopolysaccharide-induced mouse fatigue and its relevance to chronic fatigue syndrome. J. Neuroinflammation 2016, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, I.; Salem, N., Jr. Omega-3 fatty acids and rodent behavior. Prostaglandins Leukot. Essent. Fatty Acids 2006, 75, 271–289. [Google Scholar] [CrossRef]
- Layé, S. Polyunsaturated fatty acids, neuroinflammation and well being. Prostaglandins Leukot. Essent. Fatty Acids 2010, 82, 295–303. [Google Scholar] [CrossRef]
- Xavier, S.; Soch, A.; Younesi, S.; Malik, S.; Spencer, S.J.; Sominsky, L. Maternal diet before and during pregnancy modulates microglial activation and neurogenesis in the postpartum rat brain. Brain Behav. Immun. 2021, 98, 185–197. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Farina, C. Astrocytes: Key Regulators of Neuroinflammation. Trends Immunol. 2016, 37, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Labrousse, V.F.; Nadjar, A.; Joffre, C.; Costes, L.; Aubert, A.; Grégoire, S.; Bretillon, L.; Layé, S. Short-term long chain omega3 diet protects from neuroinflammatory processes and memory impairment in aged mice. PLoS ONE 2012, 7, e36861. [Google Scholar] [CrossRef]
- De Smedt-Peyrusse, V.; Sargueil, F.; Moranis, A.; Harizi, H.; Mongrand, S.; Layé, S. Docosahexaenoic acid prevents lipopolysaccharide-induced cytokine production in microglial cells by inhibiting lipopolysaccharide receptor presentation but not its membrane subdomain localization. J. Neurochem. 2008, 105, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Madore, C.; Nadjar, A.; Delpech, J.C.; Sere, A.; Aubert, A.; Portal, C.; Joffre, C.; Layé, S. Nutritional n-3 PUFAs deficiency during perinatal periods alters brain innate immune system and neuronal plasticity-associated genes. Brain Behav. Immun. 2014, 41, 22–31. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, W.; Zhou, R. NLRP3 inflammasome activation and cell death. Cell Mol. Immunol. 2021, 18, 2114–2127. [Google Scholar] [CrossRef]
- Lei, Q.; Yi, T.; Chen, C. NF-κB-Gasdermin D (GSDMD) Axis Couples Oxidative Stress and NACHT, LRR and PYD Domains-Containing Protein 3 (NLRP3) Inflammasome-Mediated Cardiomyocyte Pyroptosis Following Myocardial Infarction. Med. Sci. Monit. 2018, 24, 6044–6052. [Google Scholar] [CrossRef]
- Aziz, N.U.A.; Chiroma, S.M.; Moklas, M.A.M.; Adenan, M.I.; Ismail, A.; Basir, R.; Ali, R.M.; Bin Baharuldin, M.T.H. Menhaden fish oil attenuates postpartum depression in rat model via inhibition of NLRP3-inflammasome driven inflammatory pathway. J. Tradit. Complement. Med. 2021, 11, 419–426. [Google Scholar] [CrossRef]
- Agirman, G.; Hsiao, E.Y. SnapShot: The microbiota-gut-brain axis. Cell 2021, 184, 2524–2524.e2521. [Google Scholar] [CrossRef]
- Goyal, D.; Ali, S.A.; Singh, R.K. Emerging role of gut microbiota in modulation of neuroinflammation and neurodegeneration with emphasis on Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110112. [Google Scholar] [CrossRef]
- Liu, Z.; Li, L.; Ma, S.; Ye, J.; Zhang, H.; Li, Y.; Sair, A.T.; Pan, J.; Liu, X.; Li, X.; et al. High-Dietary Fiber Intake Alleviates Antenatal Obesity-Induced Postpartum Depression: Roles of Gut Microbiota and Microbial Metabolite Short-chain Fatty Acid Involved. J. Agric. Food Chem. 2020, 68, 13697–13710. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cao, J.; Gong, C.; Amakye, W.K.; Yao, M.; Ren, J. Exploring the microbiota-Alzheimer’s disease linkage using short-term antibiotic treatment followed by fecal microbiota transplantation. Brain Behav. Immun. 2021, 96, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Tian, P.; Zhu, H.; Zou, R.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Lactobacillus paracasei CCFM1229 and Lactobacillus rhamnosus CCFM1228 Alleviated Depression- and Anxiety-Related Symptoms of Chronic Stress-Induced Depression in Mice by Regulating Xanthine Oxidase Activity in the Brain. Nutrients 2022, 14, 1294. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Liu, Q.; Liu, W. Comparative Genomic Analysis Reveals Intestinal Habitat Adaptation of Ligilactobacillus equi Rich in Prophage and Degrading Cellulase. Molecules 2022, 27, 1867. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, L.; Zheng, Q.; Song, Y.; Huang, W.; Yang, L.; Xiong, Y.; Cai, Z.; Chen, Y.; Yuan, J. Kai-Xin-San improves cognitive impairment in D-gal and Aβ(25–35) induced AD rats by regulating gut microbiota and reducing neuronal damage. J. Ethnopharmacol. 2024, 329, 118161. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Jin, Y.; He, C.; Li, Z.; Yu, W.; Zhou, J.; Luo, R.; Chen, Q.; Wu, Y.; Wang, S.; et al. Danggui Shaoyao San: Comprehensive modulation of the microbiota-gut-brain axis for attenuating Alzheimer’s disease-related pathology. Front. Pharmacol. 2023, 14, 1338804. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Shen, X.; Chu, Q.; Zheng, X. Vaccinium bracteatum Thunb. fruit extract reduces high-fat diet-induced obesity with modulation of the gut microbiota in obese mice. J. Food Biochem. 2021, 45, e13808. [Google Scholar] [CrossRef] [PubMed]
- Mansuy-Aubert, V.; Ravussin, Y. Short chain fatty acids: The messengers from down below. Front. Neurosci. 2023, 17, 1197759. [Google Scholar] [CrossRef]
- Xu, Q.; Sun, L.; Chen, Q.; Jiao, C.; Wang, Y.; Li, H.; Xie, J.; Zhu, F.; Wang, J.; Zhang, W.; et al. Gut microbiota dysbiosis contributes to depression-like behaviors via hippocampal NLRP3-mediated neuroinflammation in a postpartum depression mouse model. Brain Behav. Immun. 2024, 119, 220–235. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, Y.; Zhang, J.; Wang, H.; Ren, B. ERK in learning and memory: A review of recent research. Int. J. Mol. Sci. 2010, 11, 222–232. [Google Scholar] [CrossRef]
- Maher, P.; Akaishi, T.; Abe, K. Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proc. Natl. Acad. Sci. USA 2006, 103, 16568–16573. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.J.; Kogan, J.H.; Frankland, P.W.; Kida, S. CREB and memory. Annu. Rev. Neurosci. 1998, 21, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.; Li, H.; Zeng, W.; Qiao, M. Shuyu capsules relieve liver-qi depression by regulating ERK-CREB-BDNF signal pathway in central nervous system of rat. Exp. Ther. Med. 2017, 14, 4831–4838. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Ye, M.; Li, Z.; Bu, S.; Zhang, Y. Long-term supplementation of dehydroepiandrosterone improved depressive-like behaviors by increasing BDNF expression in the hippocampus in ovariectomized rats. Heliyon 2020, 6, e05180. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Z. Effects of puerarin on synaptic structural modification in hippocampus of ovariectomized mice. Planta Med. 2007, 73, 1047–1053. [Google Scholar] [CrossRef]
- Xu, Y.; Deng, C.; Zheng, Y.; Liu, N.; Fu, B. Applying vinpocetine to reverse synaptic ultrastructure by regulating BDNF-related PSD-95 in alleviating schizophrenia-like deficits in rat. Compr. Psychiatry 2019, 94, 152122. [Google Scholar] [CrossRef]

| SO | LO | LS | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| C4:0 | — | — | 0.21 | 0.44 | — | — |
| C6:0 | 0.48 | 0.72 | 0.52 | 0.91 | — | — |
| C8:0 | 0.14 | 0.20 | 0.03 | 0.05 | 0.35 | 0.32 |
| C10:0 | 0.07 | 0.07 | 0.03 | 0.05 | 0.11 | 0.24 |
| C11:0 | 0.09 | 0.20 | 0.09 | 0.19 | — | — |
| C12:0 | 0.01 | 0.01 | — | — | 0.02 | 0.03 |
| C13:0 | 0.16 | 0.35 | — | — | — | — |
| C14:0 | 0.05 | 0.07 | 0.73 | 0.46 | 0.09 | 0.13 |
| C15:0 | 0.08 | 0.11 | 1.04 | 2.15 | 0.89 | 1.80 |
| C16:0 | 18.70 | 0.91 | 20.16 | 2.48 | 19.50 | 1.18 |
| C18:0 | 18.34 | 2.28 | 12.61 | 2.55 | 16.26 | 2.14 |
| C20:0 | 0.34 | 0.75 | — | — | 0.06 | 0.13 |
| C23:0 | 3.13 | 0.74 | 1.24 | 0.91 | 2.55 | 0.52 |
| SFA | 41.56 | 2.07 | 36.64 | 5.06 | 39.81 | 2.76 |
| C14:1 | — | — | 0.30 | 0.41 | — | — |
| C15:1 | 3.02 | 1.33 | 4.19 | 2.29 | 3.39 | 0.61 |
| C17:1 | — | — | 0.33 | 0.68 | — | — |
| C18:1 (C) | 25.27 | 3.17 | 34.39 | 9.32 | 27.76 | 6.51 |
| C20:1 | — | — | 0.27 | 0.57 | — | — |
| C22:1 | — | — | — | — | 0.03 | 0.07 |
| MUFA | 28.28 | 3.01 | 39.48 | 8.18 | 31.18 | 6.10 |
| C18:3 (n-3) | 1.25 | 1.15 | 0.56 | 0.55 | 0.85 | 0.41 |
| C20:3 (n-3) | — | — | 0.18 | 0.38 | 1.29 | 1.99 |
| C20:5 (EPA) | 0.06 | 0.13 | 0.19 | 0.38 | 0.03 | 0.06 |
| C22:6 (DHA) | 18.13 | 1.74 | 12.36 | 0.98 | 16.32 | 1.30 |
| n-3 PUFA | 19.44 | 1.83 | 13.29 | 1.34 | 18.49 | 2.44 |
| C20:4 | 10.72 | 0.90 | 10.59 | 3.99 | 10.52 | 1.68 |
| n-6 PUFA | 10.72 | 0.90 | 10.59 | 3.99 | 10.52 | 1.68 |
| n-3/n-6 | 1.82 | 0.09 | 1.47 | 0.51 | 1.77 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, R.; Tian, X.; Ji, A.; Zhang, T.; Xu, H.; Qi, Z.; Zhou, L.; Zhao, C.; Li, D. A Mixture of Soybean Oil and Lard Alleviates Postpartum Cognitive Impairment via Regulating the Brain Fatty Acid Composition and SCFA/ERK(1/2)/CREB/BDNF Pathway. Nutrients 2024, 16, 2641. https://doi.org/10.3390/nu16162641
Shi R, Tian X, Ji A, Zhang T, Xu H, Qi Z, Zhou L, Zhao C, Li D. A Mixture of Soybean Oil and Lard Alleviates Postpartum Cognitive Impairment via Regulating the Brain Fatty Acid Composition and SCFA/ERK(1/2)/CREB/BDNF Pathway. Nutrients. 2024; 16(16):2641. https://doi.org/10.3390/nu16162641
Chicago/Turabian StyleShi, Runjia, Xiaoying Tian, Andong Ji, Tianyu Zhang, Huina Xu, Zhongshi Qi, Liying Zhou, Chunhui Zhao, and Duo Li. 2024. "A Mixture of Soybean Oil and Lard Alleviates Postpartum Cognitive Impairment via Regulating the Brain Fatty Acid Composition and SCFA/ERK(1/2)/CREB/BDNF Pathway" Nutrients 16, no. 16: 2641. https://doi.org/10.3390/nu16162641
APA StyleShi, R., Tian, X., Ji, A., Zhang, T., Xu, H., Qi, Z., Zhou, L., Zhao, C., & Li, D. (2024). A Mixture of Soybean Oil and Lard Alleviates Postpartum Cognitive Impairment via Regulating the Brain Fatty Acid Composition and SCFA/ERK(1/2)/CREB/BDNF Pathway. Nutrients, 16(16), 2641. https://doi.org/10.3390/nu16162641







