Cardiometabolic Risk Factors Associated with Magnesium and Vitamin D Nutrients during Pregnancy—A Narrative Review
Abstract
1. Introduction
2. Pathophysiology of HDPs
3. Overview of the Prevalence of Magnesium and Vitamin D Deficiencies during Pregnancy
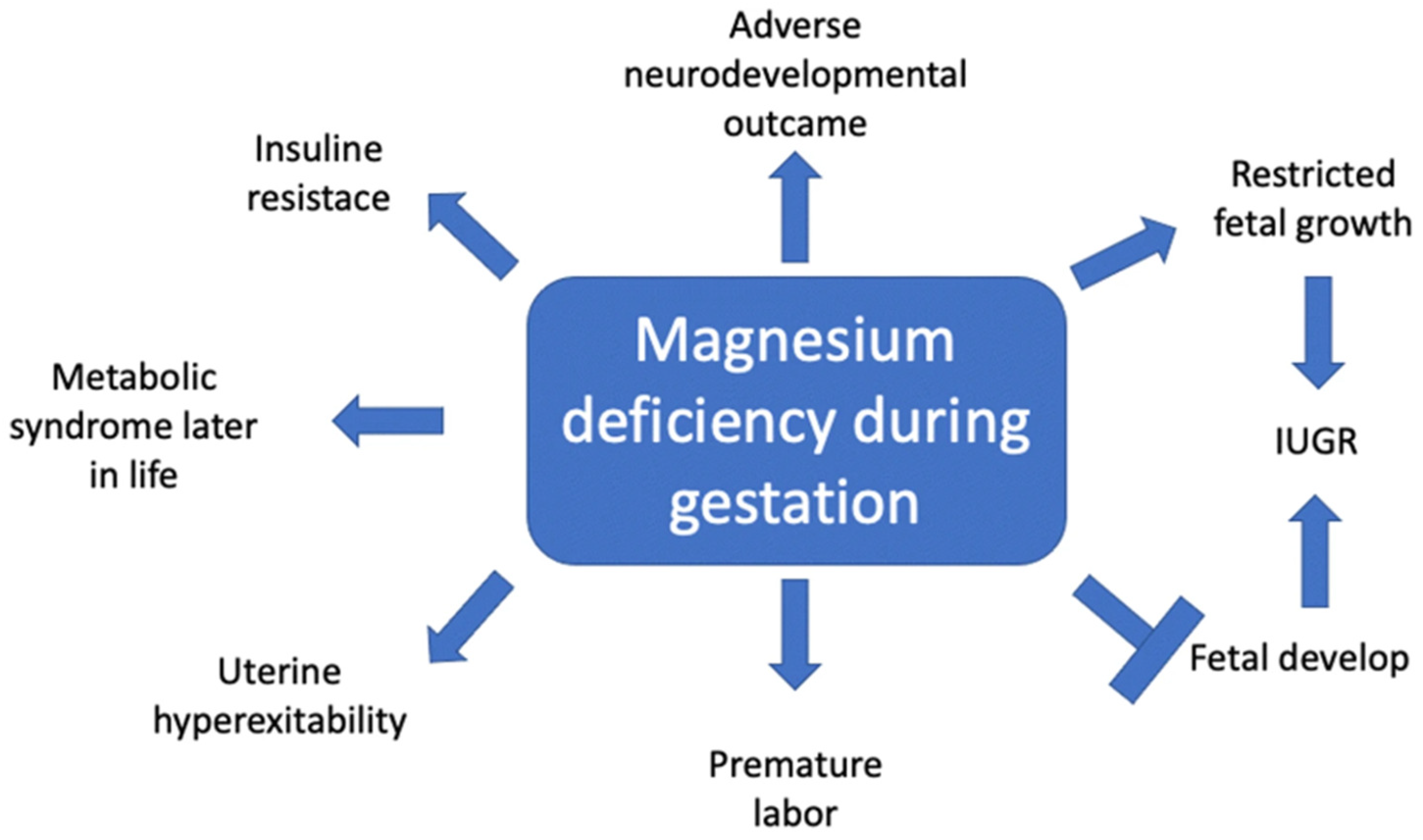
4. In-Depth Exploration of the Physiological Roles of Magnesium during Gestation
4.1. Impact of Magnesium Deficiency on HDPs
4.2. Association between Magnesium Deficiency and Glucose Tolerance
4.3. Impact of Magnesium on Fetal Development
5. In-Depth Exploration of the Functional Roles of Vitamin D during Gestation
5.1. Examination of the Relationship of Vitamin D Deficiency and HDP
5.2. Vitamin D Functions in Glucose Homeostasis and Insulin Sensitivity during Gestation
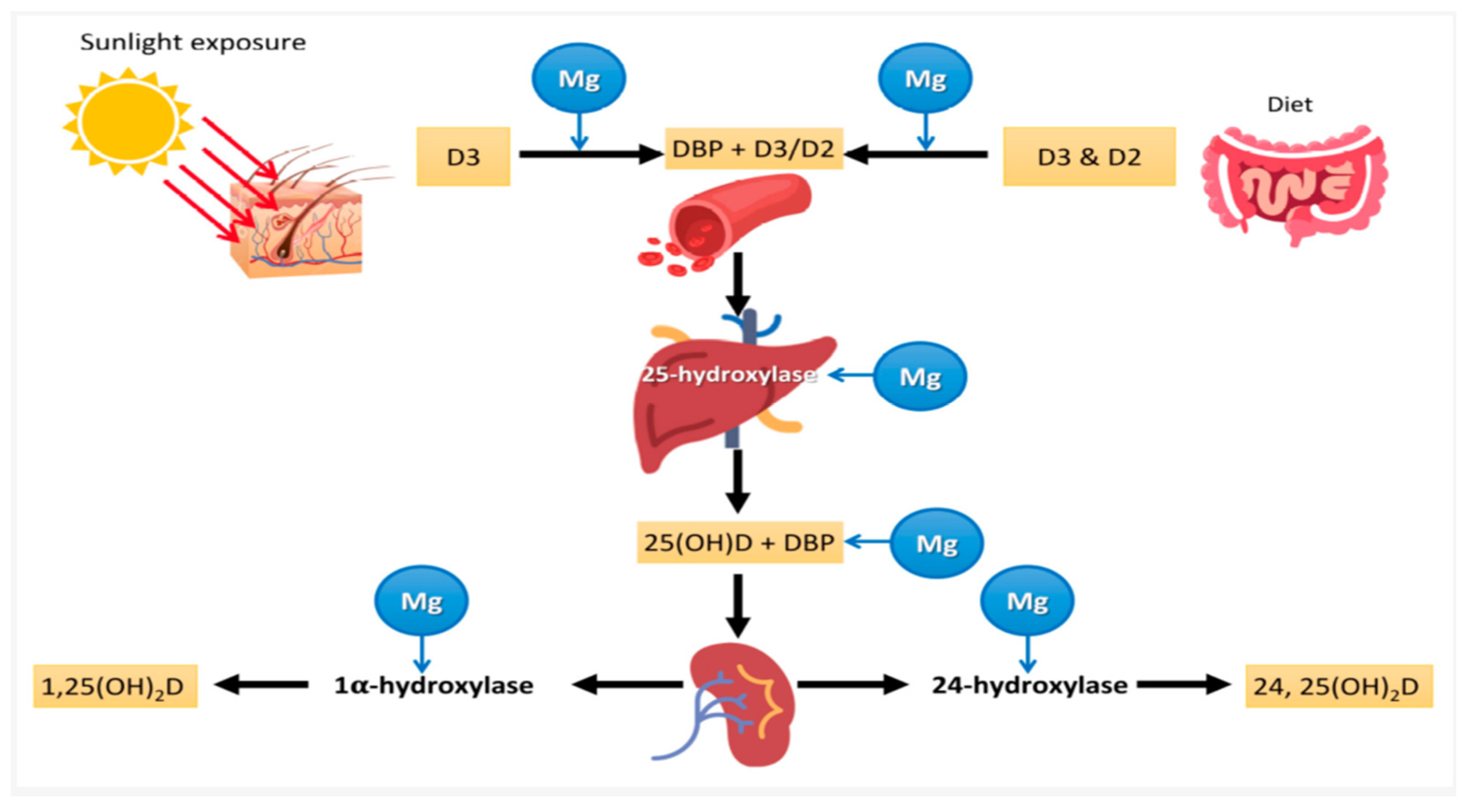
6. Interplay Connecting Magnesium and Vitamin D
In-Depth Exploration of the Interconnected Physiological Process Influenced by Both Magnesium and Vitamin D
7. Summary and Research Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Lin, R.; Feng, W.; Yang, Y.; Xu, J.; Yang, H.; Wu, J.; Li, J.; Qin, G.; Yu, Y.; Chen, J. Association of dietary calcium with mortality from all causes, cardiovascular disease and cancer in people with hypertension. J. Clin. Hypertens. 2023, 25, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Fritzen, R.; Davies, A.; Veenhuizen, M.; Campbell, M.; Pitt, S.J.; Ajjan, R.A.; Stewart, A.J. Magnesium Deficiency and Cardiometabolic Disease. Nutrients 2023, 15, 2355. [Google Scholar] [CrossRef]
- Wang, W.; Xie, X.; Yuan, T.; Wang, Y.; Zhao, F.; Zhou, Z.; Zhang, H. Epidemiological trends of maternal hypertensive disorders of pregnancy at the global, regional, and national levels: A population-based study. BMC Pregnancy Childbirth 2021, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ford, N.D.; Cox, S.; Ko, J.Y.; Ouyang, L.; Romero, L.; Colarusso, T.; Ferre, C.D.; Kroelinger, C.D.; Hayes, D.K.; Barfield, W.D. Hypertensive Disorders in Pregnancy and Mortality at Delivery Hospitalization—United States, 2017–2019. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Magee, L.A.; von Dadelszen, P. State-of-the-Art Diagnosis and Treatment of Hypertension in Pregnancy [Internet]. In Mayo Clinic Proceedings; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 1664–1677. Available online: https://pubmed.ncbi.nlm.nih.gov/30392546/ (accessed on 18 May 2024).
- Traub, A.; Sharma, A.; Gongora, M.C. Hypertensive Disorders of Pregnancy: A Literature Review—Pathophysiology, Current Management, Future Perspectives, and Healthcare Disparities. US Cardiol. Rev. 2024, 18, e03. [Google Scholar] [CrossRef]
- Granger, J.P.; Alexander, B.T.; Llinas, M.T.; Bennett, W.A.; Khalil, R.A. Pathophysiology of Hypertension During Preeclampsia Linking Placental Ischemia With Endothelial Dysfunction. Hypertension 2001, 38, 718–722. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; O’Keefe, J.H.; Wilson, W. Subclinical magnesium deficiency: A principal driver of cardiovascular disease and a public health crisis. Open Heart 2018, 5, e000668. [Google Scholar] [CrossRef]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef]
- Razzaque, M.S. Magnesium: Are We Consuming Enough? Nutrients 2018, 10, 1863. [Google Scholar] [CrossRef]
- Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients 2021, 13, 1136. [Google Scholar] [CrossRef]
- Durlach, J. New Data on the Importance of Gestational Mg Deficiency. J. Am. Coll. Nutr. 2004, 23, 694S–700S. [Google Scholar] [CrossRef] [PubMed]
- Orlova, S.; Dikke, G.; Pickering, G.; Yaltseva, N.; Konchits, S.; Starostin, K.; Bevz, A. Risk factors and comorbidities associated with magnesium deficiency in pregnant women and women with hormone-related conditions: Analysis of a large real-world dataset. BMC Pregnancy Childbirth 2021, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.E.; Abrams, S.A.; Aloia, J.; Bergeron, G.; Bourassa, M.W.; Brown, K.H.; Calvo, M.S.; Cashman, K.D.; Combs, G.; De-Regil, L.M.; et al. Global prevalence and disease burden of vitamin D deficiency: A roadmap for action in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2018, 1430, 44–79. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D. Vitamin D Deficiency: Defining, Prevalence, Causes, and Strategies of Addressing. Calcif. Tissue Int. 2020, 106, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Benedik, E. Sources of vitamin D for humans. Int. J. Vitam. Nutr. Res. 2022, 92, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Brannon, P.M. Vitamin D and adverse pregnancy outcomes: Beyond bone health and growth. Proc. Nutr. Soc. 2012, 71, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Olmos-Ortiz, A.; Avila, E.; Durand-Carbajal, M.; Díaz, L. Regulation of Calcitriol Biosynthesis and Activity: Focus on Gestational Vitamin D Deficiency and Adverse Pregnancy Outcomes. Nutrients 2015, 7, 443–480. [Google Scholar] [CrossRef] [PubMed]
- Tamblyn, J.A.; Hewison, M.; Wagner, C.L.; Bulmer, J.N.; Kilby, M.D. Immunological role of vitamin D at the maternal–Fetal interface. J. Endocrinol. 2015, 224, R107–R121. [Google Scholar] [CrossRef] [PubMed]
- Saraf, R.; Morton, S.M.B.; Camargo, C.A., Jr.; Grant, C.C. Global summary of maternal and newborn vitamin D status–a systematic review. Matern. Child Nutr. 2016, 12, 647–668. [Google Scholar] [CrossRef]
- Jeyakumar, A.; Shinde, V.; Ravindran, R. Pooled estimate of vitamin D deficiency among pregnant women in India: A systematic review and meta-analysis. J. Health Popul. Nutr. 2021, 40, 28. [Google Scholar] [CrossRef]
- Jiang, Z.; Pu, R.; Li, N.; Chen, C.; Li, J.; Dai, W.; Wang, Y.; Hu, J.; Zhu, D.; Yu, Q.; et al. High prevalence of vitamin D deficiency in Asia: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2023, 63, 3602–3611. [Google Scholar] [CrossRef] [PubMed]
- Fanni, D.; Gerosa, C.; Nurchi, V.M.; Manchia, M.; Saba, L.; Coghe, F.; Crisponi, G.; Gibo, Y.; Van Eyken, P.; Fanos, V.; et al. The Role of Magnesium in Pregnancy and in Fetal Programming of Adult Diseases. Biol. Trace Elem. Res. 2021, 199, 3647–3657. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.A.; Ismail, N.A. Magnesium: A Mineral Essential for Health Yet Generally Underestimated or Even Ignored. J. Nutr. Food Sci. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Lee, C.C.; Yang, P.K.; Chen, L.C.; Cheong, M.L.; Tsai, Y.L.; Tsai, M.S. Associations between gene expression of magnesium transporters and glucose metabolism in pregnancy. J. Formos. Med. Assoc. 2022, 121, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Wynn, A.; Wynn, M. Magnesium and other nutrient deficiencies as possible causes of hypertension and low birthweight. Nutr. Health 1988, 6, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Schoenaker, D.A.J.M.; Soedamah-Muthu, S.S.; Mishra, G.D. The association between dietary factors and gestational hypertension and pre-eclampsia: A systematic review and meta-analysis of observational studies. BMC Med. 2014, 12, 157. [Google Scholar] [CrossRef]
- Ren, Q.; Wang, H.; Zeng, Y.; Tan, X.; Cheng, X.; Zhou, T.; Huang, W.; Xu, Y. The Association Between Serum Magnesium Levels and Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Biol. Trace Elem. Res. 2023, 201, 5115–5125. [Google Scholar] [CrossRef]
- Agus, Z.S.; Morad, M. Modulation of cardiac ion channels by magnesium. Annu. Rev. Physiol. 1991, 53, 299–307. [Google Scholar] [CrossRef]
- White, R.E.; Hartzell, H.C. Magnesium ions in cardiac function. Regulator of ion channels and second messengers. Biochem. Pharmacol. 1989, 38, 859–867. [Google Scholar] [CrossRef]
- Eisner, D.A.; Caldwell, J.L.; Kistamás, K.; Trafford, A.W. Calcium and Excitation-Contraction Coupling in the Heart. Circ. Res. 2017, 121, 181. [Google Scholar] [CrossRef]
- Tangvoraphonkchai, K.; Davenport, A. Magnesium and Cardiovascular Disease. Adv. Chronic Kidney Dis. 2018, 25, 251–260. [Google Scholar] [CrossRef]
- Schlegel, R.N.; Cuffe, J.S.M.; Moritz, K.M.; Paravicini, T.M. Maternal hypomagnesemia causes placental abnormalities and fetal and postnatal mortality. Placenta. 2015, 36, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Kostov, K.; Halacheva, L. Role of magnesium deficiency in promoting atherosclerosis, endothelial dysfunction, and arterial stiffening as risk factors for hypertension. Int. J. Mol. Sci. 2018, 19, 1724. [Google Scholar] [CrossRef] [PubMed]
- Houston, M. The role of magnesium in hypertension and cardiovascular disease. J. Clin. Hypertens. 2011, 13, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Zarean, E.; Tarjan, A. Effect of Magnesium Supplement on Pregnancy Outcomes: A Randomized Control Trial. Adv. Biomed. Res. 2017, 6, 109. [Google Scholar]
- Standley, C.A.; Whitty, J.E.; Mason, B.A.; Cotton, D.B. Serum ionized magnesium levels in normal and preeclamptic gestation. Obstet. Gynecol. 1997, 89, 24–27. [Google Scholar] [CrossRef]
- Duley, L.; Gülmezoglu, A.M.; Henderson-Smart, D.J.; Chou, D. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst. Rev. 2010, 2010, CD000025. [Google Scholar] [CrossRef]
- Touyz, R.M. Role of magnesium in the pathogenesis of hypertension. Mol. Asp. Med. 2002, 24, 107–136. [Google Scholar] [CrossRef]
- Metzger, B.E. Summary and recommendations of the third international workshop-conference on gestational diabetes mellitus. Diabetes 1991, 40 (Suppl. 2), 197–201. [Google Scholar] [CrossRef]
- Seshadri, R. American diabetes association gestational diabetes mellitus. Diabetes Care 2002, 25, S94–S96. [Google Scholar]
- Barbagallo, M.; Dominguez, L.J. Magnesium and type 2 diabetes. World J. Diabetes 2015, 6, 1152. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Melo, S.R.; dos Santos, L.R.; da Cunha Soares, T.; Cardoso, B.E.P.; da Silva Dias, T.M.; Morais, J.B.S.; de Paiva Sousa, M.; de Sousa, D.G.V.; da Silva, N.C.; da Silva, L.D.; et al. Participation of Magnesium in the Secretion and Signaling Pathways of Insulin: An Updated Review. Biol. Trace Elem. Res. 2022, 200, 3545–3553. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Maeda, K.; Suto, M.; Kaji, T.; Morine, M.; Kinoshita, T.; Yasui, T.; Irahara, M. Differences in insulin sensitivity in pregnant women with overweight and gestational diabetes mellitus. Gynecol. Endocrinol. 2006, 22, 343–349. [Google Scholar] [CrossRef]
- Di Cianni, G.; Miccoli, R.; Volpe, L.; Lencioni, C.; Del Prato, S. Intermediate metabolism in normal pregnancy and in gestational diabetes. Diabetes Metab. Res. Rev. 2003, 19, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Naderifar, M.; Samimi, M.; Mirhosseini, N.; Amirani, E.; Aghadavod, E.; Asemi, Z. The effects of magnesium supplementation on gene expression related to inflammatory markers, vascular endothelial growth factor, and pregnancy outcomes in patients with gestational diabetes. Magnes. Res. 2018, 31. [Google Scholar]
- Ryan, B.A.; Kovacs, C.S. Maternal and fetal vitamin D and their roles in mineral homeostasis and fetal bone development. J. Endocrinol. Investig. 2021, 44, 643–659. [Google Scholar] [CrossRef] [PubMed]
- Yamagami, R.; Huang, R.; Bevilacqua, P.C. Cellular Concentrations of Nucleotide Diphosphate-Chelated Magnesium Ions Accelerate Catalysis by RNA and DNA Enzymes. Biochemistry 2019, 58, 3971. [Google Scholar] [CrossRef] [PubMed]
- McCollum, E.V.; Pitz, W.; Simmonds, N.; Becker, J.E.; Shipley, P.G.; Bunting, R.W. The effect of additions of fluorine to the diet of the rat on the quality of the teeth. 1925. Studies on experimental rickets. XXI. An experimental demonstration of the existence of a vitamin which promotes calcium deposition. 1922. The effect of additions of fluorine to the diet of the rat on the quality of the teeth. 1925. J. Biol. Chem. 2002, 277, E8. [Google Scholar] [PubMed]
- Alvarez, J.A.; Ashraf, A. Role of vitamin D in insulin secretion and insulin sensitivity for glucose homeostasis. Int. J. Endocrinol. 2010, 2010, 351385. [Google Scholar] [CrossRef]
- Contreras-Bolívar, V.; García-Fontana, B.; García-Fontana, C.; Muñoz-Torres, M. Mechanisms Involved in the Relationship between Vitamin D and Insulin Resistance: Impact on Clinical Practice. Nutrients 2021, 13, 3491. [Google Scholar] [CrossRef]
- Vaidya, A.; Forman, J.P. Vitamin D and Hypertension. Hypertension 2010, 56, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Suliman KH, A.N.; Falak ZE, B.; Shoaib, M.; Ghulam NA, B.I.; Ijaz, U.L.; Kang, X.U.; Hui, L.I. Selected micronutrients: An option to boost immunity against COVID-19 and prevent adverse pregnancy outcomes in pregnant women: A narrative review. Iran. J. Public Health 2020, 49, 2032–2043. [Google Scholar]
- Karamali, M.; Bahramimoghadam, S.; Sharifzadeh, F.; Asemi, Z. Magnesium–zinc–calcium–vitamin D co-supplementation improves glycemic control and markers of cardiometabolic risk in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Appl. Physiol. Nutr. Metab. 2018, 43, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Berisha, A.T.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498. [Google Scholar] [CrossRef]
- Mansur, J.L.; Oliveri, B.; Giacoia, E.; Fusaro, D.; Costanzo, P.R. Vitamin D: Before, during and after Pregnancy: Effect on Neonates and Children. Nutrients 2022, 14, 1900. [Google Scholar] [CrossRef]
- Forman, J.P.; Williams, J.S.; Fisher, N.D.L. Plasma 25-hydroxyvitamin D and regulation of the renin-angiotensin system in humans. Hypertension 2010, 55, 1283–1288. [Google Scholar] [CrossRef]
- Redman, C.W.; Jacobson, S.L.; Russell, R. Hypertension in Pregnancy. de Swiet’s Medical Disorders in Obstetric Practice; McMillan Company: Singapore, 2010. [Google Scholar]
- Tranquilli, A.L.; Brown, M.A.; Zeeman, G.G.; Dekker, G.; Sibai, B.M. The definition of severe and early-onset preeclampsia. Statements from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Pregnancy Hypertens Int. J. Women’s Cardiovasc. Health 2013, 3, 44–47. [Google Scholar] [CrossRef]
- Cakal, S.; Çakal, B.; Karaca, O. Association of vitamin D deficiency with arterial stiffness in newly diagnosed hypertension. Blood Press. Monit. 2021, 26, 113–117. [Google Scholar] [CrossRef]
- Dalan, R.; Liew, H.; Tan, W.K.A.; Chew, D.E.K.; Leow, M.K.S. Vitamin D and endothelium: Basic, translational and clinical research updates. IJC Metab. Endocr. 2014, 4, 4–17. [Google Scholar] [CrossRef]
- Kim, D.-H.; Meza, C.A.; Clarke, H.; Kim, J.-S.; Hickner, R.C. Vitamin D and endothelial function. Nutrients 2020, 12, 575. [Google Scholar] [CrossRef]
- Wei, S.; Audibert, F.; Hidiroglou, N.; Sarafin, K.; Julien, P.; Wu, Y.; Luo, Z.; Fraser, W. Longitudinal vitamin D status in pregnancy and the risk of pre-eclampsia. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; McDermid, J.M.; Al-Nimr, R.I.; Hakeem, R.; Moreschi, J.M.; Pari-Keener, M.; Stahnke, B.; Papoutsakis, C.; Handu, D.; Cheng, F.W. Vitamin D Supplementation during Pregnancy: An Evidence Analysis Center Systematic Review and Meta-Analysis. J. Acad. Nutr. Diet. 2020, 120, 898–924.e4. [Google Scholar] [CrossRef]
- Hollis, B.W.; Wagner, C.L. Vitamin D requirements and supplementation during pregnancy. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Marcinkevage, J.A.; Narayan, K.M.V. Gestational diabetes mellitus: Taking it to heart. Prim. Care Diabetes 2011, 5, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Sonagra, A.D.; Biradar, S.M.; Dattatreya, K.; DS, J.M. Normal pregnancy-a state of insulin resistance. J. Clin. Diagn. Res. JCDR 2014, 8, CC01. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.-Q.; Qi, H.-P.; Luo, Z.-C.; Fraser, W.D. Maternal vitamin D status and adverse pregnancy outcomes: A systematic review and meta-analysis. J. Matern. Neonatal Med. 2013, 26, 889–899. [Google Scholar] [CrossRef]
- Poel, Y.H.M.; Hummel, P.; Lips, P.; Stam, F.; Van Der Ploeg, T.; Simsek, S. Vitamin D and gestational diabetes: A systematic review and meta-analysis. Eur. J. Intern. Med. 2012, 23, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Borissova, A.; Tankova, T.; Kirilov, G.; Dakovska, L.; Kovacheva, R. The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int. J. Clin. Pract. 2003, 57, 258–261. [Google Scholar] [CrossRef]
- Pitocco, D.; Crinò, A.; Di Stasio, E.; Manfrini, S.; Guglielmi, C.; Spera, S.; Anguissola, G.B.; Visalli, N.; Suraci, C.; Matteoli, M.C.; et al. The effects of calcitriol and nicotinamide on residual pancreatic β-cell function in patients with recent-onset Type 1 diabetes (IMDIAB XI). Diabetes Med. 2006, 23, 920–923. [Google Scholar] [CrossRef]
- Baz-Hecht, M.; Goldfine, A.B. The impact of vitamin D deficiency on diabetes and cardiovascular risk. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 113–119. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell Physiol. 2019, 234, 8152–8161. [Google Scholar] [CrossRef]
- Dinh Le, T.; Minh Bui, T.; Hien Vu, T.; Phi Thi Nguyen, N.; Thanh Thi Tran, H.; Nguyen, S.T.; Thi Nguyen, L.H.; Van Ngo, M.; Huy Duong, H.; Thanh Vu, B.; et al. Insulin Resistance in Gestational Diabetes Mellitus and Its Association with Anthropometric Fetal Indices. Clin. Med. Insights Endocrinol. Diabetes 2022, 15, 11795514221098403. [Google Scholar] [CrossRef]
- Ye, W.; Luo, C.; Huang, J.; Li, C.; Liu, Z.; Liu, F. Gestational diabetes mellitus and adverse pregnancy outcomes: Systematic review and meta-analysis. BMJ 2022, 377, e067946. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Kirwan, J.P.; Haugel-De Mouzon, S.; King, J. Gestational Diabetes and Insulin Resistance: Role in Short- and Long-Term Implications for Mother and Fetus. J. Nutr. 2003, 133, 1674S–1683S. [Google Scholar] [CrossRef] [PubMed]
- Kinshella, M.-L.W.; Omar, S.; Scherbinsky, K.; Vidler, M.; Magee, L.A.; von Dadelszen, P.; Moore, S.E.; Elango, R.; Poston, L.; Mistry, H.D.; et al. Maternal nutritional risk factors for pre-eclampsia incidence: Findings from a narrative scoping review. Reprod. Health 2022, 19, 188. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Veronese, N.; Guerrero-Romer, F.; Barbagallo, M. Magnesium in infectious diseases in older people. Nutrients 2021, 13, 180. [Google Scholar] [CrossRef]
- Uwitonze, A.M.; Razzaque, M.S. Role of magnesium in vitamin D activation and function. J. Osteopath. Med. 2018, 118, 181–189. [Google Scholar] [CrossRef]
- Rosanoff, A.; Dai, Q.; Shapses, S.A. Essential Nutrient Interactions: Does Low or Suboptimal Magnesium Status Interact with Vitamin D and/or Calcium Status? Adv. Nutr. 2016, 7, 25–43. [Google Scholar] [CrossRef]
- Rosanoff, A.; Weaver, C.M.; Rude, R.K. Suboptimal magnesium status in the United States: Are the health consequences underestimated? Nutr. Rev. 2012, 70, 153–164. [Google Scholar] [CrossRef]
- Rude, R.K.; Gruber, H.E.; Wei, L.Y.; Frausto, A.; Mills, B.G. Magnesium deficiency: Effect on bone and mineral metabolism in the mouse. Calcif. Tissue Int. 2003, 72, 32–41. [Google Scholar] [CrossRef]
- Khazai, N.; Judd, S.E.; Tangpricha, V. Calcium and vitamin D: Skeletal and extraskeletal health. Curr. Rheumatol. Rep. 2008, 10, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Aranow, C. Vitamin D and the immune system. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Ashique, S.; Kumar, S.; Hussain, A.; Mishra, N.; Garg, A.; Gowda, B.H.J.; Farid, A.; Gupta, G.; Dua, K.; Taghizadeh-Hesary, F. A narrative review on the role of magnesium in immune regulation, inflammation, infectious diseases, and cancer. J. Health Popul. Nutr. 2023, 42, 74. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, M.; Witkowska-Sędek, E.; Rumińska, M.; Stelmaszczyk-Emmel, A.; Sobol, M.; Majcher, A.; Pyrżak, B. Vitamin D effects on selected anti-inflammatory and pro-inflammatory markers of obesity-related chronic inflammation. Front. Endocrinol. 2022, 13, 920340. [Google Scholar] [CrossRef] [PubMed]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Wium-Andersen, M.K.; Ørsted, D.D.; Nielsen, S.F.; Nordestgaard, B.G. Elevated C-reactive protein levels, psychological distress, and depression in 73 131 individuals. JAMA Psychiatry 2013, 70, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, J.K. Magnesium: It’s Biologic Significance; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Quigley, M.; Rieger, S.; Capobianco, E.; Wang, Z.; Zhao, H.; Hewison, M.; Lisse, T.S. Vitamin D Modulation of Mitochondrial Oxidative Metabolism and mTOR Enforces Stress Adaptations and Anticancer Responses. JBMR Plus 2021, 6, e10572. [Google Scholar] [CrossRef] [PubMed]
- Schutten, J.C.; Joris, P.J.; Groendijk, I.; Eelderink, C.; Groothof, D.; van der Veen, Y.; Westerhuis, R.; Goorman, F.; Danel, R.M.; de Borst, M.H.; et al. Effects of Magnesium Citrate, Magnesium Oxide, and Magnesium Sulfate Supplementation on Arterial Stiffness: A Randomized, Double-Blind, Placebo—Controlled Intervention Trial. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2022, 11, 21783. [Google Scholar] [CrossRef] [PubMed]
- Garrison, S.R.; Korownyk, C.S.; Kolber, M.R.; Allan, G.M.; Musini, V.M.; Sekhon, R.K.; Dugré, N. Magnesium for skeletal muscle cramps. Cochrane Database Syst. Rev. 2020, 9, CD009402. [Google Scholar] [CrossRef]
- Domitrz, I.; Cegielska, J. Magnesium as an Important Factor in the Pathogenesis and Treatment of Migraine—From Theory to Practice. Nutrients 2022, 14, 1089. [Google Scholar] [CrossRef]
- An, P.; Wan, S.; Luo, Y.; Luo, J.; Zhang, X.; Zhou, S.; Xu, T.; He, J.; Mechanick, J.I.; Wu, W.C.; et al. Micronutrient Supplementation to Reduce Cardiovascular Risk. J. Am. Coll. Cardiol. 2022, 80, 2269–2285. [Google Scholar] [CrossRef]
- Pelczyńska, M.; Moszak, M.; Bogdański, P. The Role of Magnesium in the Pathogenesis of Metabolic Disorders. Nutrients 2022, 14, 1714. [Google Scholar] [CrossRef]
- Rosanoff, A.; Costello, R.B.; Johnson, G.H. Effectively Prescribing Oral Magnesium Therapy for Hypertension: A Categorized Systematic Review of 49 Clinical Trials. Nutrients 2021, 13, 195. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, L.M.; Ohe, M.N.; Pallone, S.G.; Nacaguma, I.O.; Kunii, I.S.; da Silva RE, C.; Maeda, S.S.; Vieira JG, H.; Lazaretti-Castro, M. Levels of bioavailable, and free forms of 25(OH)D after supplementation with vitamin D3 in primary hyperparathyroidism. Endocrine 2023, 80, 183–190. [Google Scholar] [CrossRef]
- Zhu, A.; Kuznia, S.; Boakye, D.; Schöttker, B.; Brenner, H. Vitamin D-Binding Protein, Bioavailable, and Free 25(OH)D, and Mortality: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 3894. [Google Scholar] [CrossRef] [PubMed]
- Shamim, N.; Majid, H.; Khemani, S.; Salim, M.; Muneer, S.; Khan, A.H. Inappropriate supplementation of Vitamin D can result in toxicity: A crosssectional study of paediatrics population. JPMA J. Pak. Med. Assoc. 2023, 73, 500–504. [Google Scholar] [CrossRef] [PubMed]
| RDA Magnesium | |||
|---|---|---|---|
| Age | Female | Pregnancy | Lactation |
| 14–18 years | 360 mg | 400 mg | 360 mg |
| 19–30 years | 310 mg | 350 mg | 310 mg |
| 31–50 years | 320 mg | 360 mg | 320 mg |
| 51+ years | 420 mg | 320 mg | |
| RDA Vitamin D | |||
|---|---|---|---|
| Age | Female | Pregnancy | Lactation |
| 14–18 years | 15 mcg | 15 mcg | 15 mcg |
| 600 IU | 600 IU | 600 IU | |
| 19–50 years | 15 mcg | 15 mcg | 15 mcg |
| 600 IU | 600 IU | 600 IU | |
| 51–70 years | 15 mcg | ||
| 600 IU | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naowar, M.; Dickton, D.; Francis, J. Cardiometabolic Risk Factors Associated with Magnesium and Vitamin D Nutrients during Pregnancy—A Narrative Review. Nutrients 2024, 16, 2630. https://doi.org/10.3390/nu16162630
Naowar M, Dickton D, Francis J. Cardiometabolic Risk Factors Associated with Magnesium and Vitamin D Nutrients during Pregnancy—A Narrative Review. Nutrients. 2024; 16(16):2630. https://doi.org/10.3390/nu16162630
Chicago/Turabian StyleNaowar, Maisha, Darby Dickton, and Jimi Francis. 2024. "Cardiometabolic Risk Factors Associated with Magnesium and Vitamin D Nutrients during Pregnancy—A Narrative Review" Nutrients 16, no. 16: 2630. https://doi.org/10.3390/nu16162630
APA StyleNaowar, M., Dickton, D., & Francis, J. (2024). Cardiometabolic Risk Factors Associated with Magnesium and Vitamin D Nutrients during Pregnancy—A Narrative Review. Nutrients, 16(16), 2630. https://doi.org/10.3390/nu16162630






