Impact of Probiotics on the Glycemic Control of Pediatric and Adolescent Individuals with Type 1 Diabetes: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.2. Literature Search Strategy
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Quality Assessment of Included Studies
2.6. Data Collection Process
2.7. Data Synthesis
2.8. Statistical Analysis
3. Results
3.1. Studies Included in the Meta-Analysis
3.2. Quality Assessment
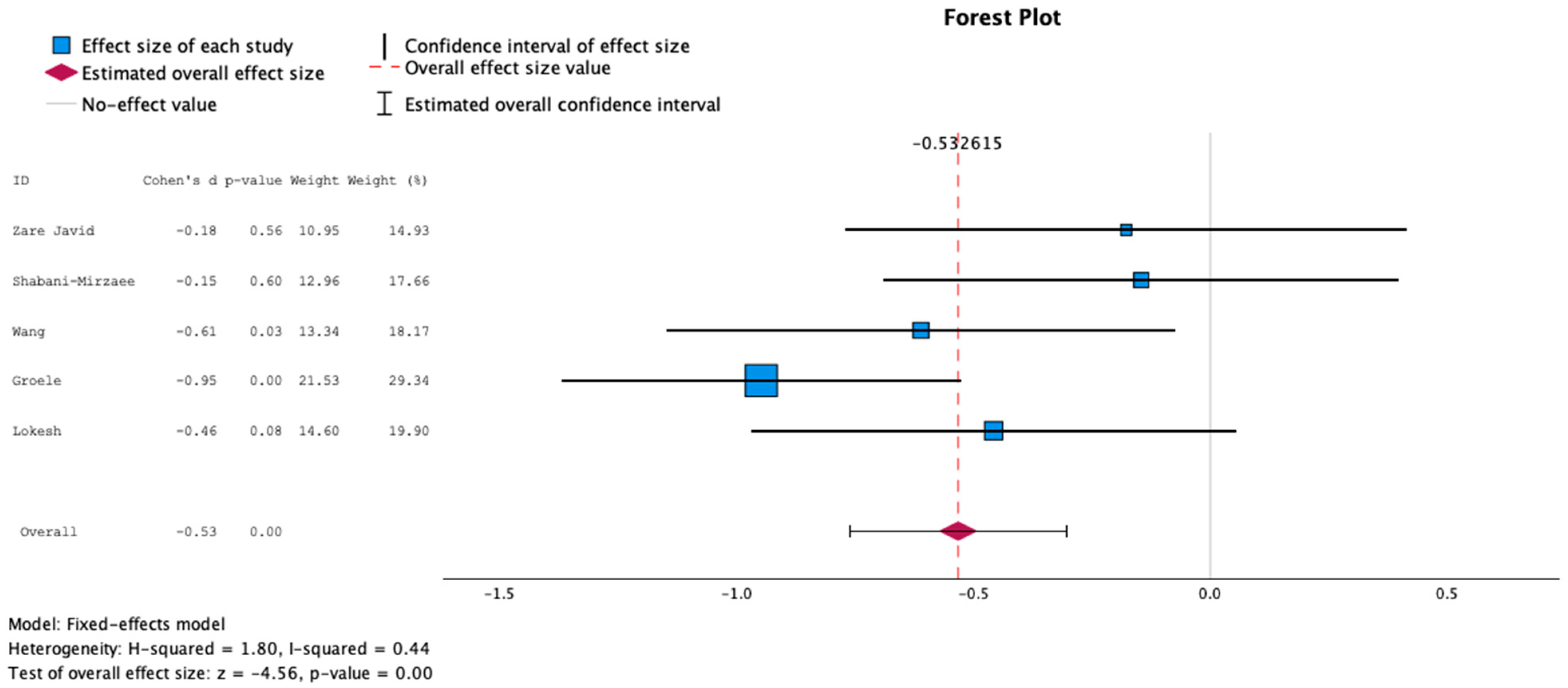
3.3. Main Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Adolfsson, P.; Hanas, R.; Zaharieva, D.P.; Dovc, K.; Jendle, J. Automated Insulin Delivery Systems in Pediatric Type 1 Diabetes: A Narrative Review. J. Diabetes Sci. Technol. 2024, 19322968241248404. [Google Scholar] [CrossRef] [PubMed]
- Sherr, J.L.; Schoelwer, M.; Dos Santos, T.J.; Reddy, L.; Biester, T.; Galderisi, A.; van Dyk, J.C.; Hilliard, M.E.; Berget, C.; DiMeglio, L.A. ISPAD Clinical Practice Consensus Guidelines 2022: Diabetes technologies: Insulin delivery. Pediatr. Diabetes 2022, 23, 1406–1431. [Google Scholar] [CrossRef] [PubMed]
- Sjoholm, A. Using adjuvant pharmacotherapy in the treatment of type 1 diabetes. Expert. Opin. Pharmacother. 2021, 22, 2143–2148. [Google Scholar] [CrossRef] [PubMed]
- Vitale, R.J.; Laffel, L.M. Sodium-Glucose Transporter Inhibition in Adult and Pediatric Patients with Type 1 Diabetes Mellitus. Adv. Chronic Kidney Dis. 2021, 28, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Bombaci, B.; Passanisi, S.; Pecoraro, M.; Sorrenti, L.; Papa, M.; Salzano, G.; Lombardo, F. Use of teplizumab in children and adolescents at risk of type 1 diabetes: Perspectives of parents and caregivers from an Italian Pediatric Diabetes Center. Acta Diabetol. 2024, 61, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.L.; Dayan, C.M.; Chatenoud, L.; Sumnik, Z.; Simmons, K.M.; Szypowska, A.; Gitelman, S.E.; Knecht, L.A.; Niemoeller, E.; Tian, W.; et al. Teplizumab and beta-Cell Function in Newly Diagnosed Type 1 Diabetes. N. Engl. J. Med. 2023, 389, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Herold, K.C.; Bundy, B.N.; Long, S.A.; Bluestone, J.A.; DiMeglio, L.A.; Dufort, M.J.; Gitelman, S.E.; Gottlieb, P.A.; Krischer, J.P.; Linsley, P.S.; et al. An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N. Engl. J. Med. 2019, 381, 603–613. [Google Scholar] [CrossRef]
- Sherwood, J.S.; Russell, S.J.; Putman, M.S. New and Emerging Technologies in Type 1 Diabetes. Endocrinol. Metab. Clin. N. Am. 2020, 49, 667–678. [Google Scholar] [CrossRef]
- Quarta, A.; Guarino, M.; Tripodi, R.; Giannini, C.; Chiarelli, F.; Blasetti, A. Diet and Glycemic Index in Children with Type 1 Diabetes. Nutrients 2023, 15, 3507. [Google Scholar] [CrossRef]
- Huber, H.; Schieren, A.; Holst, J.J.; Simon, M.C. Dietary impact on fasting and stimulated GLP-1 secretion in different metabolic conditions—A narrative review. Am. J. Clin. Nutr. 2024, 119, 599–627. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wu, Y.; Zhang, Q.; Xiao, X. Crosstalk between glucagon-like peptide 1 and gut microbiota in metabolic diseases. mBio 2024, 15, e0203223. [Google Scholar] [CrossRef] [PubMed]
- Stefanaki, C.; Michos, A.; Mastorakos, G.; Mantzou, A.; Landis, G.; Zosi, P.; Bacopoulou, F. Probiotics in Adolescent Prediabetes: A Pilot RCT on Glycemic Control and Intestinal Bacteriome. J. Clin. Med. 2019, 8, 1743. [Google Scholar] [CrossRef] [PubMed]
- Stefanaki, C.; Bacopoulou, F.; Michos, A. The impact of probiotics’ administration on glycemic control, body composition, gut microbiome, mitochondria, and other hormonal signals in adolescents with prediabetes—A randomized, controlled trial study protocol. Contemp. Clin. Trials Commun. 2018, 11, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Stefanaki, C.; Peppa, M.; Mastorakos, G.; Chrousos, G.P. Examining the gut bacteriome, virome, and mycobiome in glucose metabolism disorders: Are we on the right track? Metabolism 2017, 73, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Callaway, L.K.; McIntyre, H.D.; Barrett, H.L.; Foxcroft, K.; Tremellen, A.; Lingwood, B.E.; Tobin, J.M.; Wilkinson, S.; Kothari, A.; Morrison, M.; et al. Probiotics for the Prevention of Gestational Diabetes Mellitus in Overweight and Obese Women: Findings From the SPRING Double-Blind Randomized Controlled Trial. Diabetes Care 2019, 42, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Nabrdalik, K.; Drozdz, K.; Kwiendacz, H.; Skonieczna-Zydecka, K.; Loniewski, I.; Kaczmarczyk, M.; Wijata, A.M.; Nalepa, J.; Holleman, F.; Nieuwdorp, M.; et al. Clinical Trial: Probiotics in Metformin Intolerant Patients with Type 2 Diabetes (ProGasMet). Biomed. Pharmacother. 2023, 168, 115650. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, M.; Salari Sharif, A.; Sangouni, A.A.; Emtiazi, H.; Mozaffari-Khosravi, H. Effect of probiotic yogurt consumption on glycemic control and lipid profile in patients with type 2 diabetes mellitus: A randomized controlled trial. Clin. Nutr. ESPEN 2023, 54, 144–149. [Google Scholar] [CrossRef]
- Dandona, P.; Ghanim, H. Macronutrient intake and oxidative stress/inflammation in type 1 diabetes. J. Diabetes Complicat. 2018, 32, 247–248. [Google Scholar] [CrossRef]
- Neubauer-Geryk, J.; Wielicka, M.; Mysliwiec, M.; Zorena, K.; Bieniaszewski, L. The Impact of Metabolic Memory on Immune Profile in Young Patients with Uncomplicated Type 1 Diabetes. Int. J. Mol. Sci. 2024, 25, 3190. [Google Scholar] [CrossRef]
- Al-Dubayee, M.; Babiker, A.; Alkewaibeen, A.; Alkhalifah, A.; Alanazi, T.; Nogoud, M.; Alotaibi, A.; Alotaibi, F.; Almetairi, F.; Alrowaily, M.A.; et al. Correlation analysis between cytokines’ profile, autoimmune antibodies and the duration of type 1 diabetes: A case control study in a specialized children’s centre in Riyadh. Int. J. Immunopathol. Pharmacol. 2023, 37, 3946320231209821. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Davoodvandi, A.; Marzban, H.; Goleij, P.; Sahebkar, A.; Morshedi, K.; Rezaei, S.; Mahjoubin-Tehran, M.; Tarrahimofrad, H.; Hamblin, M.R.; Mirzaei, H. Effects of therapeutic probiotics on modulation of microRNAs. Cell Commun. Signal 2021, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, M.; Korpela, R.; Ilonen, J.; Ludvigsson, J.; Vaarala, O. Probiotics for the prevention of beta cell autoimmunity in children at genetic risk of type 1 diabetes--the PRODIA study. Ann. N. Y Acad. Sci. 2006, 1079, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Zare Javid, A.; Aminzadeh, M.; Haghighi-Zadeh, M.H.; Jamalvandi, M. The Effects of Synbiotic Supplementation on Glycemic Status, Lipid Profile, and Biomarkers of Oxidative Stress in Type 1 Diabetic Patients. A Placebo-Controlled, Double-Blind, Randomized Clinical Trial. Diabetes Metab. Syndr. Obes. 2020, 13, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, R.; Rohilla, L.; Jacob, N.; Yadav, J.; Sachdeva, N. A high potency multi-strain probiotic improves glycemic control in children with new-onset type 1 diabetes mellitus: A randomized, double-blind, and placebo-controlled pilot study. Pediatr. Diabetes 2021, 22, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Groele, L.; Szajewska, H.; Szalecki, M.; Swiderska, J.; Wysocka-Mincewicz, M.; Ochocinska, A.; Stelmaszczyk-Emmel, A.; Demkow, U.; Szypowska, A. Lack of effect of Lactobacillus rhamnosus GG and Bifidobacterium lactis Bb12 on beta-cell function in children with newly diagnosed type 1 diabetes: A randomised controlled trial. BMJ Open Diabetes Res. Care 2021, 9, e001523. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Yen, H.R.; Lu, W.L.; Ho, H.H.; Lin, W.Y.; Kuo, Y.W.; Huang, Y.Y.; Tsai, S.Y.; Lin, H.C. Adjuvant Probiotics of Lactobacillus salivarius subsp. salicinius AP-32, L. johnsonii MH-68, and Bifidobacterium animalis subsp. lactis CP-9 Attenuate Glycemic Levels and Inflammatory Cytokines in Patients with Type 1 Diabetes Mellitus. Front. Endocrinol. 2022, 13, 754401. [Google Scholar] [CrossRef] [PubMed]
- Lokesh, M.N.; Kumar, R.; Jacob, N.; Sachdeva, N.; Rawat, A.; Yadav, J.; Dayal, D. Supplementation of High-Strength Oral Probiotics Improves Immune Regulation and Preserves Beta Cells among Children with New-Onset Type 1 Diabetes Mellitus: A Randomised, Double-Blind Placebo Control Trial. Indian J. Pediatr. 2024. [Google Scholar] [CrossRef]
- Shabani-Mirzaee, H.; Haghshenas, Z.; Malekiantaghi, A.; Vigeh, M.; Mahdavi, F.; Eftekhari, K. The effect of oral probiotics on glycated haemoglobin levels in children with type 1 diabetes mellitus—A randomized clinical trial. Pediatr. Endocrinol. Diabetes Metab. 2023, 29, 128–133. [Google Scholar] [CrossRef]
- Programme, C.A.S. CASP Randomised Controlled Trial Checklist. Available online: https://casp-uk.net/casp-tools-checklists/ (accessed on 7 July 2024).
- Mol, B.W.; Lai, S.; Rahim, A.; Bordewijk, E.M.; Wang, R.; van Eekelen, R.; Gurrin, L.C.; Thornton, J.G.; van Wely, M.; Li, W. Checklist to assess Trustworthiness in RAndomised Controlled Trials (TRACT checklist): Concept proposal and pilot. Res. Integr. Peer Rev. 2023, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- IBM Corp. IBM SPSS Statistics for Windows, Version 29.0.2.0. 2023. Available online: https://www.ibm.com/support/pages/ibm-spss-statistics-2902-documentation#en (accessed on 7 July 2024).
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- McFarland, L.V. Efficacy of Single-Strain Probiotics Versus Multi-Strain Mixtures: Systematic Review of Strain and Disease Specificity. Dig. Dis. Sci. 2021, 66, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Sniffen, J.C.; McFarland, L.V.; Evans, C.T.; Goldstein, E.J.C. Choosing an appropriate probiotic product for your patient: An evidence-based practical guide. PLoS ONE 2018, 13, e0209205. [Google Scholar] [CrossRef] [PubMed]
- Stefanaki, C.B.F.; Peppa, M. Prediabetes and Adolescence—Trends, Causes, Effects, and Screening. US Endocrinol. 2016, 12, 94–98. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, M.J. Effects of growth hormone on glucose metabolism and insulin resistance in human. Ann. Pediatr. Endocrinol. Metab. 2017, 22, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Kim-Dorner, S.J.; Sassmann, H.; Heidtmann, B.; Kapellen, T.M.; Kordonouri, O.; Nettelrodt, K.M.E.; Schweizer, R.; von Sengbusch, S.; Lange, K. Using person reported outcomes: Psychometric properties of the German diabetes treatment satisfaction questionnaire (DTSQ) for teens and parents. Heliyon 2024, 10, e27614. [Google Scholar] [CrossRef]
- Sassmann, H.; Kim-Dorner, S.J.; Framme, J.; Heidtmann, B.; Kapellen, T.; Kordonouri, O.; Krosta, K.M.E.; Pisarek, N.; Lange, K. Psychometric properties of the German teen and parent versions of the Problem Areas in Diabetes Scale (PAID). Psychol. Assess. 2023, 35, e31–e42. [Google Scholar] [CrossRef]
- Tsiouli, E.; Alexopoulos, E.C.; Stefanaki, C.; Darviri, C.; Chrousos, G.P. Effects of diabetes-related family stress on glycemic control in young patients with type 1 diabetes: Systematic review. Can. Fam. Physician 2013, 59, 143–149. [Google Scholar] [PubMed]
- la Marca, G.; Malvagia, S.; Toni, S.; Piccini, B.; Di Ciommo, V.; Bottazzo, G.F. Children who develop type 1 diabetes early in life show low levels of carnitine and amino acids at birth: Does this finding shed light on the etiopathogenesis of the disease? Nutr. Diabetes 2013, 3, e94. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; Chen, X.; Huo, D.; Arifuzzaman, M.; Qiao, S.; Jin, W.B.; Shi, H.; Li, X.V.; Consortium, J.R.I.L.C.B.; Iliev, I.D.; et al. Microbiota metabolism of intestinal amino acids impacts host nutrient homeostasis and physiology. Cell Host Microbe 2024, 32, 661–675.e610. [Google Scholar] [CrossRef] [PubMed]
- Zeighamy Alamdary, S.; Afifirad, R.; Asgharzadeh, S.; Asadollahi, P.; Mahdizade Ari, M.; Dashtibin, S.; Sabaghan, M.; Shokouhamiri, M.R.; Ghanavati, R.; Darbandi, A. The Influence of Probiotics Consumption on Management of Prediabetic State: A Systematic Review of Clinical Trials. Int. J. Clin. Pract. 2022, 2022, 5963679. [Google Scholar] [CrossRef]
- Baroni, I.; Fabrizi, D.; Luciani, M.; Magon, A.; Conte, G.; De Angeli, G.; Paglione, G.; Ausili, D.; Caruso, R. Probiotics and synbiotics for glycemic control in diabetes: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2024, 43, 1041–1061. [Google Scholar] [CrossRef]
- Taylor, B.L.; Woodfall, G.E.; Sheedy, K.E.; O’Riley, M.L.; Rainbow, K.A.; Bramwell, E.L.; Kellow, N.J. Effect of Probiotics on Metabolic Outcomes in Pregnant Women with Gestational Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2017, 9, 461. [Google Scholar] [CrossRef]




| Study | N (I/C) * | Age | Probiotic Type | Intervention Period | Dropouts | Meta-Analysis |
|---|---|---|---|---|---|---|
| Zare Javid et al., 2020 [25] | 44 (22/22) | 4–18 years | Single strain | 8 weeks | 6 | Included |
| Kumar et al., 2021 [26] | 90 (57/49) | 2–12 years | Multi-strain | 12 weeks | 6 | Excluded |
| Groele et al., 2021 [27] | 96 (48/48) | 8–17 years | Multi-strain | 24 weeks | 9 | Included |
| Wang et al., 2022 [28] | 56 (27/29) | 6–18 years | Multi-strain | 24 weeks | 3 | Included |
| Shabani-Mirzaee et al., 2023 [30] | 52 (26/26) | 2–16 years | Multi-strain | 12 weeks | 0 | Included |
| Lokesh et al., 2024 [29] | 50 (27/23) | 2–12 years | Multi-strain | 24 weeks | 10 | Included |
| CASP | Zare Javid et al., 2020 [25] | Kumar et al., 2021 [26] | Groele et al., 2021 [27] | Wang et al., 2021 [28] | Shabani-Mirzaee et al., 2023 [30] | Lokesh et al., 2024 [29] |
|---|---|---|---|---|---|---|
| 1. Did the study address a clearly focused research question? | Yes | Yes | Yes | Yes | Yes | Yes |
| 2. Was the assignment of participants to interventions randomized? | Yes | Yes | Yes | Yes | Yes | Yes |
| 3. Were all participants who entered the study accounted for at its conclusion? | No | Yes | No | Yes | Yes | Yes |
4.
| Cannot tell | Yes | Yes | Yes | Yes | Yes |
| Cannot tell | Yes | Cannot tell | Cannot tell | Yes | Yes |
| Cannot tell | Cannot tell | Cannot tell | Cannot tell | Yes | Yes |
| 5. Were the study groups similar at the start of the randomized controlled trial? | No | Yes | Yes | Yes | Yes | Yes |
| 6. Apart from the experimental intervention, did each group receive the same level of care (that is, were they treated equally)? | Yes | Yes | Cannot tell | Cannot tell | Yes | Cannot tell |
| 7. Were the effects of the intervention reported comprehensively? | Yes | No | Yes | Yes | Yes | Yes |
| 8. Was precision of the estimate of the intervention’s treatment effect reported? | Yes | No | No | No | No | No |
| 9. Do the benefits of the experimental intervention outweigh the harms and costs? | Cannot tell | Cannot tell | Cannot tell | Cannot tell | Cannot tell | Cannot tell |
| 10. Can the results be applied to your local population/in your context? | Yes | Yes | Cannot tell | Cannot tell | Cannot tell | Cannot tell |
| 11. Would the experimental intervention provide greater value to the people in your care than any of the existing interventions? | Cannot tell | Cannot tell | Cannot tell | Cannot tell | Cannot tell | Cannot tell |
| Study | Gover Nance | Author Group | Plausibility of Intervention Usage | Time Frame | Drop-Out Rates | Baseline Characteristics | Outcomes |
|---|---|---|---|---|---|---|---|
| Zare Javid et al., 2020 [25] | No concerns | Some concerns | No concerns | Major concerns | No concerns | No concerns | Major concerns |
| Some concerns | No concerns | Major concerns | Major concerns | Major concerns | No concerns | No concerns | |
| No concerns | No concerns | No concerns | No concerns | ||||
| No concerns | |||||||
| Kumar et al., 2021 [26] | No concerns | Some concerns | No concerns | Major concerns | No concerns | No concerns | No concerns |
| No concerns | No concerns | No concerns | No concerns | No concerns | No concerns | No concerns | |
| No concerns | No concerns | No concerns | No concerns | ||||
| No concerns | |||||||
| Groele et al., 2021 [27] | No concerns | Some concerns | No concerns | No concerns | Major concerns | No concerns | No concerns |
| Some concerns | No concerns | No concerns | No concerns | Major concerns | No concerns | No concerns | |
| No concerns | No concerns | No concerns | No concerns | ||||
| No concerns | |||||||
| Wang et al., 2021 [28] | No concerns | No concerns | No concerns | Major concerns | No concerns | Major concerns | Major concerns |
| No concerns | No concerns | Major concerns | Major concerns | No concerns | No concerns | ||
| No concerns | No concerns | No concerns | No concerns | ||||
| Major concerns | No concerns | ||||||
| Shabani-Mirzaee et al., 2023 [30] | No concerns | No concerns | No concerns | Some concerns | Major concerns | Some concerns | No concerns |
| Some concerns | No concerns | Major concerns | No concerns | No concerns | Some concerns | Some concerns | |
| No concerns | No concerns | Some concerns | No concerns | ||||
| No concerns | |||||||
| Lokesh et al., 2024 [29] | No concerns | No concerns | No concerns | No concerns | No concerns | No concerns | No concerns |
| No concerns | No concerns | Major concerns | No concerns | No concerns | No concerns | No concerns | |
| Some concerns | No concerns | No concerns | No concerns | ||||
| No concerns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanaki, C.; Rozou, P.; Efthymiou, V.; Xinias, I.; Mastorakos, G.; Bacopoulou, F.; Papagianni, M. Impact of Probiotics on the Glycemic Control of Pediatric and Adolescent Individuals with Type 1 Diabetes: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 2629. https://doi.org/10.3390/nu16162629
Stefanaki C, Rozou P, Efthymiou V, Xinias I, Mastorakos G, Bacopoulou F, Papagianni M. Impact of Probiotics on the Glycemic Control of Pediatric and Adolescent Individuals with Type 1 Diabetes: A Systematic Review and Meta-Analysis. Nutrients. 2024; 16(16):2629. https://doi.org/10.3390/nu16162629
Chicago/Turabian StyleStefanaki, Charikleia, Paraskevi Rozou, Vasiliki Efthymiou, Ioannis Xinias, George Mastorakos, Flora Bacopoulou, and Maria Papagianni. 2024. "Impact of Probiotics on the Glycemic Control of Pediatric and Adolescent Individuals with Type 1 Diabetes: A Systematic Review and Meta-Analysis" Nutrients 16, no. 16: 2629. https://doi.org/10.3390/nu16162629
APA StyleStefanaki, C., Rozou, P., Efthymiou, V., Xinias, I., Mastorakos, G., Bacopoulou, F., & Papagianni, M. (2024). Impact of Probiotics on the Glycemic Control of Pediatric and Adolescent Individuals with Type 1 Diabetes: A Systematic Review and Meta-Analysis. Nutrients, 16(16), 2629. https://doi.org/10.3390/nu16162629










