Citrus limon var. pompia Camarda var. nova: A Comprehensive Review of Its Botanical Characteristics, Traditional Uses, Phytochemical Profile, and Potential Health Benefits
Abstract
1. Introduction
2. Taxonomic Classification
3. Pomological Characteristics
4. Genotypic Characterization
5. Traditional Uses of Pompia Fruit
6. Phytochemistry of Pompia
6.1. Peel Essential Oil
| Compounds | Fenu et al. Dec.2008 % | Fenu et al. Feb.2009 % | Fenu et al. Mar.2009 % | Flamini et al. Nov.2018 Peel (HD) % | Flamini et al. Nov.2019 Peel (CP) % | Flamini et al. Nov.2019 Leaf (HD) % |
|---|---|---|---|---|---|---|
| Limonene | 82.1 | 82.7 | 77.5 | 77.44 | 95.77 | 28.64 |
| Myrcene | 1.8 | 2.6 | 3.1 | 2.12 | 1.55 | 0.91 |
| Geranial | 1.7 | 1.8 | 3.7 | - | ||
| Neral | 1.4 | 1.6 | 3.2 | - | ||
| α-Pinene | 0.7 | 0.9 | 1.0 | 0.43 | 0.51 | |
| Geraniol | 1.5 | 0.8 | 1.6 | 1.46 | - | |
| Cis-β-ocimene | 1.0 | 0.8 | 2.6 | - | - | 0.46 |
| Trans-β-ocimene | 0.99 | 0.50 | 10.5 | |||
| Linalool | - | - | - | 1.13 | - | 0.56 |
| Nerol | - | - | - | - | 1.49 | |
| Monoterpene hydrocarbons | 81.12 | 98.38 | 42.01 | |||
| Oxygenated monoterpenes | 16.44 | - | 53.50 | |||
| Sesquiterpene hydrocarbons | 2.45 | 1.62 | 1.44 |
| Compound | Pompia [22] % | Citrus aurantium [26] % | Citrus limon [27] % | Citrus medica [28] % |
|---|---|---|---|---|
| Limonene | 82.1 | 77.53 | 53.9 | 46.9 |
| Myrcene | 1.8 | 2.76 | 2.7 | 1.5 |
| Neral | 1.4 | - | 1.7 | 2.8 |
| Geranial | 1.7 | - | 2.2 | 5.4 |
| Geraniol | 1.5 | - | 0.3 | 0.1 |
| a-Pinene | 0.7 | 2.98 | 0.7 | 2 |
| b-O-cimene | 1 | - | - | 0.8 |
6.2. Rind Extract
| Compound | Manconi et al., 2016 [31] µg/mg | Manconi et al., 2018 [38] µg/mg |
|---|---|---|
| Gallic acid | - | 128.3 |
| Eriocitrin | 0.09 | 40.4 |
| Neoeriocitrin | 46.53 | 42.5 |
| Naringin | 23.77 | 28 |
| Hesperidin | - | 16.9 |
| Neohesperidin | 44.57 | 76.5 |
| Myricetin-3-galactosyde | - | 29.3 |
| Ferulic acid | 1.03 | - |
| Quinic acid | 219.67 | - |
| Robinin | 1.08 | - |
| Sinapic acid | 30.13 | - |
| Rutin | 8.61 | - |
6.3. Leaves Essential Oil
6.4. Leaf Volatiles
6.5. Peel Volatiles
6.6. Juice
7. “Pompia Intrea” Candied Fruit Characterization
8. Potential Health Benefits: Antioxidant Activity of Pompia
9. Conclusions
10. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| CAT | Catalase |
| CP | Cold pressing |
| EO | Essential oils |
| GC-FID | Gas Chromatography-Flame Ionization Detection |
| GC-MS | Gas chromatography–mass spectrometry |
| HS-SPME-GC | Headspace solid-phase microextraction coupled to gas chromatography |
| HD | Hydro-distillation |
| HMF | Hydroxymethyl-furfural |
| HMG | 3-Hydroxy-3-MethylGlutaric acid |
| HPLC–MS | High-performance liquid chromatography–mass spectrometry |
| HS | Headspace |
| PI | Pompia Intrea |
| PLEO | Pompia leaves essential oil |
| PMF | Methoxylated flavonols |
| POD | Peroxidase |
| PPO | Polyphenol oxidase |
| SOD | Superoxide dismutase |
| TIC | Total Ion Chromatogram |
| TSS | Total Soluble Solids |
| VOCs | Volatile Organic Compounds |
References
- Moris, G.G. Flora Sardoa: Seu Historia Plantarum in Sardinia et Adjacentibus Insulis vel Sponte Nascentium vel ad Utilitatem Latius Excultarum ex Regio Typographeo; Ex Regio Typographeo; LuEsther T. Mertz Library: New York, NY, USA, 1859; Volume 3. [Google Scholar]
- Camarda, I.; Mazzola, P.; Brunu, A.; Fenu, G.; Lombardo, G.; Palla, F. Un agrume nella storia della Sardegna: Citrus limon var. pompia Camarda var. nova. Quad. Bot. Amb. Appl. 2013, 24, 109–118. [Google Scholar]
- Mignani, I.; Mulas, M.; Mantegazza, R.; Lovigu, N.; Spada, A.; Nicolosi, E.; Bassi, D. Characterization by Molecular Markers of ‘Pompia’, a Natural Citrus Hybrid Cultivated in Sardinia. Acta Hortic. 2015, 1065, 165–172. [Google Scholar] [CrossRef]
- Petretto, G.L.; Sarais, G.; Maldini, M.T.; Foddai, M.; Tirillini, B.; Rourke, J.P.; Chessa, M.; Pintore, G. Citrus monstruosa Discrimination among Several Citrus Species by Multivariate Analysis of Volatiles: A Metabolomic Approach. J. Food Process. Pres. 2016, 40, 950–957. [Google Scholar] [CrossRef]
- Curk, F.; Luro, F. Classificazione ed Evoluzione Degli Agrumi. In Workshop “Un Mare di Agrumi: Dalla Coltivazione al Prodotto Finito; L’Erborista: Pisa, Italy, 2018. [Google Scholar]
- Deiana, M.; Montoro, P.; Jerkovic, I.; Atzeri, A.; Marijanovic, Z.; Serreli, G.; Piacente, S.; Tuberoso, C.I.G. First characterization of Pompia intrea candied fruit: The headspace chemical profile, polar extract composition and its biological activities. Food Res. Int. 2019, 120, 620–630. [Google Scholar] [CrossRef] [PubMed]
- D’Aquino, S.; Fronteddu, F.; Usai, M.; Palma, A. Qualitative and physiological properties of ‘Pompia’, a citron-like fruit. Plant Genet. Resour. Newsl. 2005, 143, 40–45. [Google Scholar]
- D’Aquino, S.; Piga, A.; Agabbio, M.C.S.; Molinu, M.G. Decay Control of “Femminello Santa Teresa” Lemon Fruits by Prestorage High Temperature Conditioning; Office for Official Publications of the European Communities: Luxembourg, 1998. [Google Scholar]
- D’Aquino, S.; Agabbio, M.; Angioni, M.; Delogu, M.; Tedde, M. Evoluzione dei parametri di qualità interna e visivi di limoni (CV “Di Massa”) conservati in condizioni di mercato. Atti Del Convegno Internazionale Produzioni Aliment. E Qual. Della Vita 2000, 4–8. [Google Scholar]
- Agabbio, M.; Molinu, M.; Mura, D.; D’Aquino, S.; Delogu, M. L’arancio “Tardivo di San Vito”, cultivar bionda a maturazione tardiva. In Biodiversità: Germoplasma Locale E Sua Valorizzazione. Atti Del 4 Congresso Nazionale, Alghero; 1998; Volume II, pp. 8–11. [Google Scholar]
- Eaks, I.L. Respiratory response, ethylene production, and response to ethylene of citrus fruit during ontogeny. Plant Physiol. 1970, 45, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Orru, R.; Zucca, P.; Falzoi, M.; Atzori, E.; Rescigno, A.; Padiglia, A. First step towards the biomolecular characterization of Pompia, an endemic Citrus-like fruit from Sardinia (Italy). Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2017, 151, 464–473. [Google Scholar]
- Luro, F.; Viglietti, G.; Marchi, E.; Costantino, G.; Scarpa, G.M.; Tomi, F.; Paoli, M.; Curk, F.; Ollitrault, P. Genetic, morphological and chemical investigations reveal the genetic origin of Pompia (C. medica tuberosa Risso & Poiteau)—An old endemic Sardinian citrus fruit. Phytochemistry 2019, 168, 112083. [Google Scholar] [PubMed]
- El-Otmani, M.; Coggins, C.W. Fruit age and growth regulator effects on the quantity and structure of the epicuticular wax of ‘Washington’navel orange fruit. J. Am. Soc. Hortic. Sci. 1985, 110, 371–378. [Google Scholar] [CrossRef]
- Viglietti, G.; Galla, G.; Porceddu, A.; Barcaccia, G.; Curk, F.; Luro, F.; Scarpa, G.M. Karyological analysis and DNA barcoding of Pompia citron: A first step toward the identification of its relatives. Plants 2019, 8, 83. [Google Scholar] [CrossRef]
- Posadino, A.M.; Giordo, R.; Pintus, G.; Mohammed, S.A.; Orhan, I.E.; Fokou, P.V.T.; Sharopov, F.; Adetunji, C.O.; Gulsunoglu-Konuskan, Z.; Ydyrys, A. Medicinal and mechanistic overview of artemisinin in the treatment of human diseases. Biomed. Pharmacother. 2023, 163, 114866. [Google Scholar] [CrossRef] [PubMed]
- Posadino, A.M.; Giordo, R.; Ramli, I.; Zayed, H.; Nasrallah, G.K.; Wehbe, Z.; Eid, A.H.; Gürer, E.S.; Kennedy, J.F.; Aldahish, A.A. An updated overview of cyanidins for chemoprevention and cancer therapy. Biomed. Pharmacother. 2023, 163, 114783. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Al-Mansoob, M.; Ahmad, S.M.; Haider, M.Z.; Eid, A.H.; Posadino, A.M.; Pintus, G.; Giordo, R. Resveratrol-mediated regulation of mitochondria biogenesis-associated pathways in neurodegenerative diseases: Molecular insights and potential therapeutic applications. Curr. Neuropharmacol. 2023, 21, 1184. [Google Scholar] [CrossRef] [PubMed]
- Ramli, I.; Posadino, A.M.; Giordo, R.; Fenu, G.; Fardoun, M.; Iratni, R.; Eid, A.H.; Zayed, H.; Pintus, G. Effect of resveratrol on pregnancy, prenatal complications and pregnancy-associated structure alterations. Antioxidants 2023, 12, 341. [Google Scholar] [CrossRef]
- Ramli, I.; Cheriet, T.; Posadino, A.M.; Giordo, R.; Zayed, H.; Eid, A.H.; Pintus, G. Potential Therapeutic Targets of Resveratrol in the Prevention and Treatment of Pulmonary Fibrosis. Front. Biosci. (Landmark Ed.) 2023, 28, 198. [Google Scholar] [CrossRef]
- Anmol, R.J.; Marium, S.; Hiew, F.T.; Han, W.C.; Kwan, L.K.; Wong, A.K.Y.; Khan, F.; Sarker, M.M.R.; Chan, S.Y.; Kifli, N. Phytochemical and therapeutic potential of Citrus grandis (L.) Osbeck: A review. J. Evid.-Based Integr. Med. 2021, 26, 2515690X211043741. [Google Scholar] [CrossRef]
- Fenu, G.; Carai, A.; Foddai, M.; Azara, E.; Careddu, S.; Usai, M. Composition and seasonal variation of Citrus monstruosa essential oil from Sardinia. Int. J. Essent. Oil Ther. 2010, 4, 23–25. [Google Scholar]
- Frizzo, C.D.; Lorenzo, D.; Dellacassa, E. Composition and seasonal variation of the essential oils from two mandarin cultivars of southern Brazil. J. Agric. Food Chem. 2004, 52, 3036–3041. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach; Department of Pharmaceutical Sciences, University of Nottingham: Nottingham, UK, 1998. [Google Scholar]
- Flamini, G.; Pistelli, L.; Nardoni, S.; Ebani, V.V.; Zinnai, A.; Mancianti, F.; Ascrizzi, R.; Pistelli, L. Essential oil composition and biological activity of “Pompia”, a Sardinian Citrus ecotype. Molecules 2019, 24, 908. [Google Scholar] [CrossRef]
- Badalamenti, N.; Bruno, M.; Schicchi, R.; Geraci, A.; Leporini, M.; Gervasi, L.; Tundis, R.; Loizzo, M.R. Chemical compositions and antioxidant activities of essential oils, and their combinations, obtained from flavedo by-product of seven cultivars of Sicilian Citrus aurantium L. Molecules 2022, 27, 1580. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.L.; Simas, D.L.R.; Pinheiro, M.M.G.; Moreno, D.S.A.; Alviano, C.S.; da Silva, A.J.R.; Dias Fernandes, P. Anti-inflammatory properties and chemical characterization of the essential oils of four citrus species. PLoS ONE 2016, 11, e0153643. [Google Scholar] [CrossRef] [PubMed]
- Lota, M.L.; de Rocca Serra, D.; Tomi, F.; Bessiere, J.M.; Casanova, J. Chemical composition of peel and leaf essential oils of Citrus medica L. and C. limonimedica Lush. Flavour Fragr. J. 1999, 14, 161–166. [Google Scholar] [CrossRef]
- Khan, M.K.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Dangles, O.; Chemat, F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010, 119, 851–858. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, X.; He, W.H.; Xu, H.G.; Yuan, F.; Gao, Y.X. Investigation into the antioxidant activity and chemical composition of alcoholic extracts from defatted marigold (Tagetes erecta L.) residue. Fitoterapia 2012, 83, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Marongiu, F.; Castangia, I.; Catalan-Latorre, A.; Caddeo, C.; Bacchetta, G.; Ennas, G.; Zaru, M.; Fadda, A.M.; Manconi, M. Protective effect of grape extract phospholipid vesicles against oxidative stress skin damages. Ind. Crops Prod. 2016, 83, 561–567. [Google Scholar] [CrossRef]
- Chen, H.; Zuo, Y.G.; Deng, Y.W. Separation and determination of flavonoids and other phenolic compounds in cranberry juice by high-performance liquid chromatography. J. Chromatogr. A 2001, 913, 387–395. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Manconi, M.; Manca, M.L.; Marongiu, F.; Caddeo, C.; Castangia, I.; Petretto, G.L.; Pintore, G.; Sarais, G.; D’hallewin, G.; Zaru, M.; et al. Chemical characterization of Citrus limon var. pompia and incorporation in phospholipid vesicles for skin delivery. Int. J. Pharm. 2016, 506, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Iwashina, T. The structure and distribution of the flavonoids in plants. J. Plant Res. 2000, 113, 287–299. [Google Scholar] [CrossRef]
- Peterson, J.J.; Dwyer, J.T.; Beecher, G.R.; Bhagwat, S.A.; Gebhardt, S.E.; Haytowitz, D.B.; Holden, J.M. Flavanones in oranges, tangerines (mandarins), tangors, and tangelos: A compilation and review of the data from the analytical literature. J. Food Compos. Anal. 2006, 19, S66–S73. [Google Scholar] [CrossRef]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid composition of citrus juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef]
- Manconi, M.; Manca, M.L.; Caddeo, C.; Sarais, G.; Palmieri, A.; D’Hallewin, G.; Fadda, A.M. Citrus limon extract loaded in vesicular systems for the protection of oral cavity. Medicines 2018, 5, 108. [Google Scholar] [CrossRef] [PubMed]
- Kohlert, C.; van Rensen, I.; Marz, R.; Schindler, G.; Graefe, E.U.; Veit, M. Bioavailability and pharmacokinetics of natural volatile terpenes in animals and humans. Planta Med. 2000, 66, 495–505. [Google Scholar] [CrossRef]
- Fancello, F.; Petretto, G.L.; Zara, S.; Sanna, M.L.; Addis, R.; Maldini, M.; Foddai, M.; Rourke, J.P.; Chessa, M.; Pintore, G. Chemical characterization, antioxidant capacity and antimicrobial activity against food related microorganisms of Citrus limon var. pompia leaf essential oil. LWT-Food Sci. Technol. 2016, 69, 579–585. [Google Scholar] [CrossRef]
- Abderrezak, M.; Abaza, I.; Aburjai, T.; Kabouche, A.; Kabouche, Z. Comparative compositions of essential oils of Citrus aurantium growing in different soils. J. Mater. Environ. Sci 2014, 5, 1913–1918. [Google Scholar]
- Ellouze, I.; Abderrabba, M.; Sabaou, N.; Mathieu, F.; Lebrihi, A.; Bouajila, J. Season’s variation impact on Citrus aurantium leaves essential oil: Chemical composition and biological activities. J. Food Sci. 2012, 77, T173–T180. [Google Scholar] [CrossRef] [PubMed]
- Fancello, F.; Petretto, G.L.; Marceddu, S.; Venditti, T.; Pintore, G.; Zara, G.; Mannazzu, I.; Budroni, M.; Zara, S. Antimicrobial activity of gaseous Citrus limon var pompia leaf essential oil against Listeria monocytogenes on ricotta salata cheese. Food Microbiol. 2020, 87, 103386. [Google Scholar] [CrossRef]
- Fancello, F.; Zara, S.; Petretto, G.L.; Chessa, M.; Addis, R.; Rourke, J.P.; Pintore, G. Essential oils from three species of Mentha harvested in Sardinia: Chemical characterization and evaluation of their biological activity. Int. J. Food Prop. 2017, 20, 1751–1761. [Google Scholar] [CrossRef]
- Usach, I.; Margarucci, E.; Manca, M.L.; Caddeo, C.; Aroffu, M.; Petretto, G.L.; Manconi, M.; Peris, J.E. Comparison between Citral and Pompia Essential Oil Loaded in Phospholipid Vesicles for the Treatment of Skin and Mucosal Infections. Nanomaterials 2020, 10, 286. [Google Scholar] [CrossRef]
- Petretto, G.; Foddai, M.; Maldini, M.; Chessa, M.; Venditti, T.; D’hallewin, G.; Pintore, G. A novel device for the study of antimicrobial activity by vapor-contact of volatile substances on food products. Commun. Agric. Appl. Biol. Sci. 2013, 78, 65–72. [Google Scholar] [PubMed]
- Liu, S.; Lou, Y.; Li, Y.; Zhang, J.; Li, P.; Yang, B.; Gu, Q. Review of phytochemical and nutritional characteristics and food applications of Citrus L. fruits. Front. Nutr. 2022, 9, 968604. [Google Scholar] [CrossRef] [PubMed]
- Tripoli, E.; La Guardia, M.; Giammanco, S.; Di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Zhao, P.; Duan, L.; Guo, L.; Dou, L.-L.; Dong, X.; Zhou, P.; Li, P.; Liu, E.-H. Chemical and biological comparison of the fruit extracts of Citrus wilsonii Tanaka and Citrus medica L. Food Chem. 2015, 173, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Delle Monache, S.; Sanità, P.; Trapasso, E.; Ursino, M.R.; Dugo, P.; Russo, M.; Ferlazzo, N.; Calapai, G.; Angelucci, A.; Navarra, M. Mechanisms underlying the anti-tumoral effects of Citrus bergamia juice. PLoS ONE 2013, 8, e61484. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Bai, J.; Plotto, A.; Ritenour, M.A. Citrus fruit quality assessment; producer and consumer perspectives. Stewart Postharvest Rev. 2014, 10, 1–7. [Google Scholar]
- Liu, Y.; Heying, E.; Tanumihardjo, S.A. History, global distribution, and nutritional importance of citrus fruits. Compr. Rev. Food Sci. Food Saf. 2012, 11, 530–545. [Google Scholar] [CrossRef]
- Girones-Vilaplana, A.; Moreno, D.A.; Garcia-Viguera, C. Phytochemistry and biological activity of Spanish Citrus fruits. Food Funct. 2014, 5, 764–772. [Google Scholar] [CrossRef]
- Toh, J.Y.; Tan, V.M.H.; Lim, P.C.Y.; Lim, S.T.; Chong, M.F.F. Flavonoids from Fruit and Vegetables: A Focus on Cardiovascular Risk Factors. Curr. Atheroscler Rep. 2013, 15, 368. [Google Scholar] [CrossRef]
- Barberis, A.; Deiana, M.; Spissu, Y.; Azara, E.; Fadda, A.; Serra, P.A.; D’hallewin, G.; Pisano, M.; Serreli, G.; Orrù, G. Antioxidant, antimicrobial, and other biological properties of Pompia juice. Molecules 2020, 25, 3186. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Riquelme, M.T.; Porras, N.; Ferreres, F. Effect of the rootstock and interstock grafted in lemon tree (Citrus limon (L.) Burm.) on the flavonoid content of lemon juice. J. Agric. Food Chem. 2004, 52, 324–331. [Google Scholar] [CrossRef]
- Khan, M.K.; Dangles, O. A comprehensive review on flavanones, the major citrus polyphenols. J. Food Compos. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- Navarra, M.; Mannucci, C.; Delbo, M.; Calapai, G. Citrus bergamia essential oil: From basic research to clinical application. Front. Pharmacol. 2015, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Dugo, P.; Navarra, M.; Raymo, V.; Dugo, G.; Mondello, L. Study on the chemical composition variability of some processed bergamot (Citrus bergamia) essential oils. Flavour Fragr. J. 2010, 25, 4–12. [Google Scholar] [CrossRef]
- Hossain, R.; Quispe, C.; Khan, R.A.; Saikat, A.S.M.; Ray, P.; Ongalbek, D.; Yeskaliyeva, B.; Jain, D.; Smeriglio, A.; Trombetta, D. Propolis: An update on its chemistry and pharmacological applications. Chin. Med. 2022, 17, 100. [Google Scholar] [CrossRef]
- Giordo, R.; Cossu, A.; Porcu, M.C.; Cappuccinelli, R.; Biosa, G.; Sharifi-Rad, J.; Pretti, L.; Nasrallah, G.K.; Pintus, G.; Posadino, A.M. Cytoprotective, antioxidant, and anti-migratory activity of Pistacia lentiscus L. supercritical carbon dioxide extract on primary human endothelial cells. Nat. Prod. Res. 2023, 37, 2681–2687. [Google Scholar] [PubMed]
- Cossu, A.; Posadino, A.M.; Giordo, R.; Emanueli, C.; Sanguinetti, A.M.; Piscopo, A.; Poiana, M.; Capobianco, G.; Piga, A.; Pintus, G. Apricot melanoidins prevent oxidative endothelial cell death by counteracting mitochondrial oxidation and membrane depolarization. PLoS ONE 2012, 7, e48817. [Google Scholar] [CrossRef]
- Incani, A.; Serra, G.; Atzeri, A.; Melis, M.P.; Serreli, G.; Bandino, G.; Sedda, P.; Campus, M.; Tuberoso, C.I.; Deiana, M. Extra virgin olive oil phenolic extracts counteract the pro-oxidant effect of dietary oxidized lipids in human intestinal cells. Food Chem. Toxicol. 2016, 90, 171–180. [Google Scholar] [CrossRef]
- Singh, P.; Shukla, R.; Prakash, B.; Kumar, A.; Singh, S.; Mishra, P.K.; Dubey, N.K. Chemical profile, antifungal, antiaflatoxigenic and antioxidant activity of Citrus maxima Burm. and Citrus sinensis (L.) Osbeck essential oils and their cyclic monoterpene, DL-limonene. Food Chem. Toxicol. 2010, 48, 1734–1740. [Google Scholar] [CrossRef]
- Aggarwal, K.K.; Khanuja, S.P.S.; Ahmad, A.; Kumar, T.R.S.; Gupta, V.K.; Kumar, S. Antimicrobial activity profiles of the two enantiomers of limonene and carvone isolated from the oils of Mentha spicata and Anethum sowa. Flavour Fragr. J. 2002, 17, 59–63. [Google Scholar] [CrossRef]
- Elson, C.E.; Maltzman, T.H.; Boston, J.L.; Tanner, M.A.; Gould, M.N. Anti-carcinogenic activity of d-limonene during the initiation and promotion/progression stages of DMBA-induced rat mammary carcinogenesis. Carcinogenesis 1988, 9, 331–332. [Google Scholar] [CrossRef]
- Jokić, S.; Jerković, I.; Pavić, V.; Aladić, K.; Molnar, M.; Kovač, M.J.; Vladimir-Knežević, S. Terpenes and cannabinoids in supercritical CO2 extracts of industrial hemp inflorescences: Optimization of extraction, antiradical and antibacterial activity. Pharmaceuticals 2022, 15, 1117. [Google Scholar] [CrossRef] [PubMed]
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.-D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.-M.; Stefanescu, R.; Bild, V.; Melnic, S. Medicinal plants of the family Lamiaceae in pain therapy: A review. Pain Res. Manag. 2018, 2018, 7801543. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.A.; Russo, E.B.; Smith, K.M. Pharmacological foundations of cannabis chemovars. Planta Med. 2018, 84, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Jerkovic, I. Volatile Benzene Derivatives as Honey Biomarkers. Synlett 2013, 24, 2331–2334. [Google Scholar] [CrossRef]
- Di Donna, L.; Iacopetta, D.; Cappello, A.R.; Gallucci, G.; Martello, E.; Fiorillo, M.; Dolce, V.; Sindona, G. Hypocholesterolaemic activity of 3-hydroxy-3-methyl-glutaryl flavanones enriched fraction from bergamot fruit (Citrus bergamia): “In Vivo” studies. J. Funct. Foods 2014, 7, 558–568. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Van der Fels-Klerx, H.J.; Peters, R.J.B.; Van Boekel, M.A.J.S. Acrylamide and 5-hydroxymethylfurfural formation during baking of biscuits: Part I: Effects of sugar type. Food Chem. 2016, 192, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Pasias, I.N.; Kiriakou, I.K.; Proestos, C. HMF and diastase activity in honeys: A fully validated approach and a chemometric analysis for identification of honey freshness and adulteration. Food Chem. 2017, 229, 425–431. [Google Scholar] [CrossRef]
- Pereira, V.; Albuquerque, F.M.; Ferreira, A.C.; Cacho, J.; Marques, J.C. Evolution of 5-hydroxymethylfurfural (HMF) and furfural (F) in fortified wines submitted to overheating conditions. Food Res. Int. 2011, 44, 71–76. [Google Scholar] [CrossRef]
- Koleva, I.I.; Van Beek, T.A.; Linssen, J.P.; Groot, A.d.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2002, 13, 8–17. [Google Scholar] [CrossRef]
- Giordo, R.; Nasrallah, G.K.; Posadino, A.M.; Galimi, F.; Capobianco, G.; Eid, A.H.; Pintus, G. Resveratrol-elicited pkc inhibition counteracts nox-mediated endothelial to mesenchymal transition in human retinal endothelial cells exposed to high glucose. Antioxidants 2021, 10, 224. [Google Scholar] [CrossRef]
- Bhagani, H.; Nasser, S.A.; Dakroub, A.; El-Yazbi, A.F.; Eid, A.A.; Kobeissy, F.; Pintus, G.; Eid, A.H. The mitochondria: A target of polyphenols in the treatment of diabetic cardiomyopathy. Int. J. Mol. Sci. 2020, 21, 4962. [Google Scholar] [CrossRef] [PubMed]
- Giordo, R.; Wehbe, Z.; Posadino, A.M.; Erre, G.L.; Eid, A.H.; Mangoni, A.A.; Pintus, G. Disease-associated regulation of non-coding RNAs by resveratrol: Molecular insights and therapeutic applications. Front. Cell Dev. Biol. 2022, 10, 894305. [Google Scholar] [CrossRef] [PubMed]
- Ramli, I.; Posadino, A.M.; Zerizer, S.; Spissu, Y.; Barberis, A.; Djeghim, H.; Azara, E.; Bensouici, C.; Kabouche, Z.; Rebbas, K. Low concentrations of Ambrosia maritima L. phenolic extract protect endothelial cells from oxidative cell death induced by H2O2 and sera from Crohn’s disease patients. J. Ethnopharmacol. 2023, 300, 115722. [Google Scholar] [CrossRef] [PubMed]
- Popović-Djordjević, J.; Quispe, C.; Giordo, R.; Kostić, A.; Stanković, J.S.K.; Fokou, P.V.T.; Carbone, K.; Martorell, M.; Kumar, M.; Pintus, G. Natural products and synthetic analogues against HIV: A perspective to develop new potential anti-HIV drugs. Eur. J. Med. Chem. 2022, 233, 114217. [Google Scholar] [CrossRef]
- Bacanlı, M.; Başaran, A.A.; Başaran, N. The antioxidant and antigenotoxic properties of citrus phenolics limonene and naringin. Food Chem. Toxicol. 2015, 81, 160–170. [Google Scholar] [CrossRef]
- Choi, H.-S.; Song, H.S.; Ukeda, H.; Sawamura, M. Radical-scavenging activities of citrus essential oils and their components: Detection using 1,1-diphenyl-2-picrylhydrazyl. J. Agric. Food Chem. 2000, 48, 4156–4161. [Google Scholar] [CrossRef]
- Mir, S.A.; Wani, S.; Ahmad, M.; Wani, T.A.; Gani, A.; Mir, S.; Masoodi, F. Effect of packaging and storage on the physicochemical and antioxidant properties of quince candy. J. Food Sci. Technol. 2015, 52, 7313–7320. [Google Scholar] [CrossRef]
- Miletić, N.; Popović, B.; Mitrović, O.; Kandić, M.; Leposavić, A. Phenolic compounds and antioxidant capacity of dried and candied fruits commonly consumed in Serbia. Czech J. Food Sci. 2014, 32, 360–368. [Google Scholar] [CrossRef]
- Suarez, J.; Herrera, M.; Marhuenda, E. In Vitro scavenger and antioxidant properties of hesperidin and neohesperidin dihydrochalcone. Phytomedicine 1998, 5, 469–473. [Google Scholar] [CrossRef]
- Yu, J.; Wang, L.; Walzem, R.L.; Miller, E.G.; Pike, L.M.; Patil, B.S. Antioxidant activity of citrus limonoids, flavonoids, and coumarins. J. Agric. Food Chem. 2005, 53, 2009–2014. [Google Scholar] [CrossRef] [PubMed]
- Barroso, M.F.; De-Los-Santos-Álvarez, N.; Delerue-Matos, C.; Oliveira, M.B.P.P. Towards a reliable technology for antioxidant capacity and oxidative damage evaluation: Electrochemical (bio) sensors. Biosens. Bioelectron. 2011, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Barberis, A.; Spissu, Y.; Bazzu, G.; Fadda, A.; Azara, E.; Sanna, D.; Schirra, M.; Serra, P.A. Development and characterization of an ascorbate oxidase-based sensor–biosensor system for telemetric detection of AA and antioxidant capacity in fresh orange juice. Anal. Chem. 2014, 86, 8727–8734. [Google Scholar] [CrossRef] [PubMed]
- Barberis, A.; Spissu, Y.; Fadda, A.; Azara, E.; Bazzu, G.; Marceddu, S.; Angioni, A.; Sanna, D.; Schirra, M.; Serra, P.A. Simultaneous amperometric detection of ascorbic acid and antioxidant capacity in orange, blueberry and kiwi juice, by a telemetric system coupled with a fullerene-or nanotubes-modified ascorbate subtractive biosensor. Biosens. Bioelectron. 2015, 67, 214–223. [Google Scholar] [CrossRef]
- Barberis, A.; Bazzu, G.; Calia, G.; Puggioni, G.M.; Rocchitta, G.G.; Migheli, R.; Schirra, M.; Desole, M.S.; Serra, P.A. New ultralow-cost telemetric system for a rapid electrochemical detection of vitamin C in fresh orange juice. Anal. Chem. 2010, 82, 5134–5140. [Google Scholar] [CrossRef] [PubMed]
- Chamulitrat, W. Nitric oxide inhibited peroxyl and alkoxyl radical formation with concomitant protection against oxidant injury in intestinal epithelial cells. Arch. Biochem. Biophys. 1998, 355, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Incani, A.; Atzeri, A.; Angioni, A.; Campus, M.; Cauli, E.; Zurru, R.; Deiana, M. Antioxidant effect of natural table olives phenolic extract against oxidative stress and membrane damage in enterocyte-Like cells. J. Food Sci. 2017, 82, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Cimino, C.; Maurel, O.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.; Pignatello, R.; Carbone, C. Essential oils: Pharmaceutical applications and encapsulation strategies into lipid-based delivery systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef]
- Palmas, L.; Aroffu, M.; Petretto, G.L.; Escribano-Ferrer, E.; Díez-Sales, O.; Usach, I.; Peris, J.-E.; Marongiu, F.; Ghavam, M.; Fais, S. Entrapment of Citrus limon var. pompia essential oil or pure citral in liposomes tailored as mouthwash for the treatment of oral cavity diseases. Pharmaceuticals 2020, 13, 216. [Google Scholar] [CrossRef]




| Compound | Fancello et al., 2020—mg/mL [43] | Fancello et al., 2017—mg/mL [44] |
|---|---|---|
| Linalyl-acetate/geraniol | 298.65 | 256.30 |
| Limonene | 256.87 | - |
| Geranial | 98.39 | 213.8 |
| Neral | 86.81 | 172.9 |
| Myrcene | 10.47 | 15.3 |
| Nerol | 5.47 | 37.5 |
| Linalool | 84.13 | 8.5 |
| Geranyl acetate | - | 18.9 |
| Epten-2-one(6-methyl) | - | 13 |
| α-Phellandrene | 7.07 | - |
| γ-Terpinene | 7.08 | - |
| Z beta O-cymene | - | 8.0 |
| E beta O-cymene | 52.77 | 71.7 |
| Terpinolene | 14.47 | 3.3 |
| Citronellal | 13.85 | 5.4 |
| Compound | Flamini et al., 2019 RA% [25] | Usach et al., 2020 RA% [45] |
|---|---|---|
| Limonene | 28.64 | 29.7 |
| Trans-β-ocimene | 10.50 | 4.4 |
| Linalool | 0.56 | 11.0 |
| Citronellal | 1.27 | 0.2 |
| Nerol | 1.49 | 2.9 |
| Neral | 18.84 | 6.8 |
| Geranial | 24.44 | 11.1 |
| Geranyl acetate | 3.94 | - |
| Linalyl acetate | - | 20.9 |
| α-Terpineol | 41.18 | 8.4 |
| Neryl acetate | 13.56 | 5.5 |
| Cariophyllene | - | 1.3 |
| Carene | - | 21.7 |
| Chrysanthenol cis | - | 3.0 |
| Verbanol iso | - | 10.8 |
| Compound | Chemical Class | Chemical Structure | Plant Part |
|---|---|---|---|
| Rhoifolin 4-glucoside or apigenin 7-O-neohesperidoside 4-glucoside | Flavonoids | 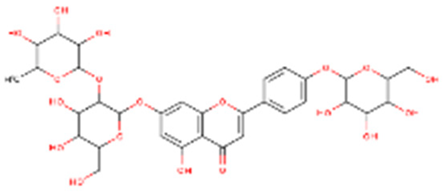 | juice |
| Delta-2- carene | Terpenoids | 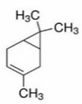 | E.O. leaf |
| Stellarin-2 or chrysoeriol 6,8-C-glucoside | Flavonoids | 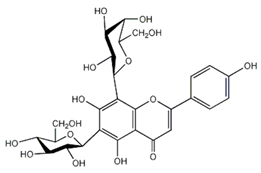 | juice |
| Diosmetin 6,8-diglucoside | Flavonoids |  | juice |
| Diosmin | Flavonoids | 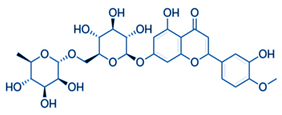 | juice |
| Eriocitrin | Flavonoids | 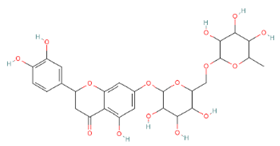 | rind extract |
| Ferulic acid | Terpenoids | 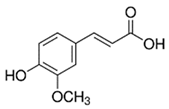 | rind extract |
| Gallic acid | Terpenoids | 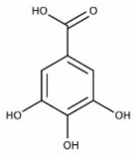 | rind extract |
| Geranial | Terpenoids | 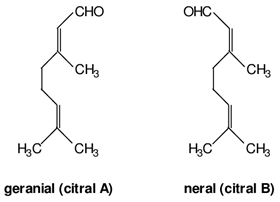 | E.O. leaf and E.O. rind |
| Neral | Terpenoids | E.O. leaf and E.O. rind | |
| Geranyl acetate | Terpenoids | 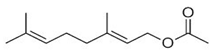 | E.O. leaf |
| Isorhamnetin 3-o-rutinoside | Flavonoids | 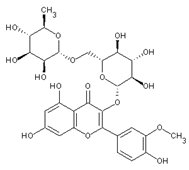 | juice |
| Limonene | Terpenoids |  | E.O. leaf and E.O. rind |
| Linalil-acetato/geraniolo | Terpenoids | 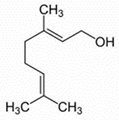 | E.O. leaf |
| Linalool | Terpenoids | 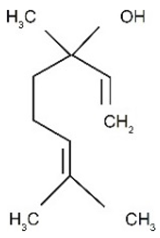 | E.O. leaf and E.O. rind |
| Myrcene | Terpenoids | 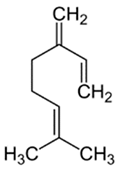 | E.O. leaf and E.O. rind |
| Naringin | Flavonoids | 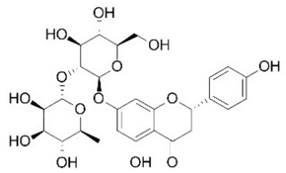 | rind extract |
| Neoeriocitrin | Flavonoids | 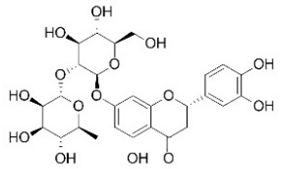 | rind extract |
| Nerol | Terpenoids |  | E.O. leaf |
| (e)-βeta-ocimene | Terpenoids | 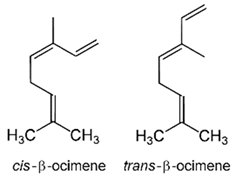 | E.O. leaf and E.O. rind |
| (z)-βeta-ocimene | Terpenoids | E.O. leaf and E.O. rind | |
| Hesperidin | Flavonoids |  | rind extract and juice |
| Neohesperidin | Flavonoids | 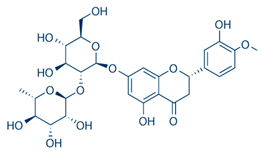 | rind extract |
| Myricetin-3-galactosyde | Flavonoids | 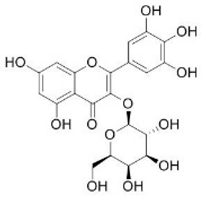 | rind extract |
| Quinic acid | Terpenoids | 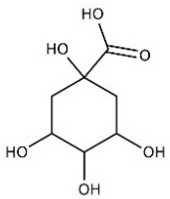 | rind extract |
| Robinin | Flavonoids | 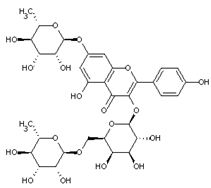 | rind extract |
| Rutin | Flavonoids | 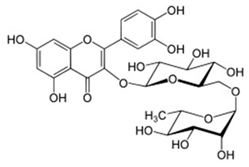 | rind extract |
| Sinapic acid | Terpenoids | 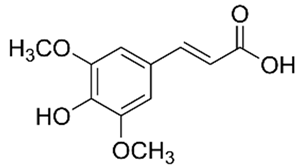 | rind extract |
| α-Terpineolo | Terpenoids |  | E.O. leaf |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Posadino, A.M.; Maccioccu, P.; Eid, A.H.; Giordo, R.; Pintus, G.; Fenu, G. Citrus limon var. pompia Camarda var. nova: A Comprehensive Review of Its Botanical Characteristics, Traditional Uses, Phytochemical Profile, and Potential Health Benefits. Nutrients 2024, 16, 2619. https://doi.org/10.3390/nu16162619
Posadino AM, Maccioccu P, Eid AH, Giordo R, Pintus G, Fenu G. Citrus limon var. pompia Camarda var. nova: A Comprehensive Review of Its Botanical Characteristics, Traditional Uses, Phytochemical Profile, and Potential Health Benefits. Nutrients. 2024; 16(16):2619. https://doi.org/10.3390/nu16162619
Chicago/Turabian StylePosadino, Anna Maria, Paola Maccioccu, Ali H. Eid, Roberta Giordo, Gianfranco Pintus, and Grazia Fenu. 2024. "Citrus limon var. pompia Camarda var. nova: A Comprehensive Review of Its Botanical Characteristics, Traditional Uses, Phytochemical Profile, and Potential Health Benefits" Nutrients 16, no. 16: 2619. https://doi.org/10.3390/nu16162619
APA StylePosadino, A. M., Maccioccu, P., Eid, A. H., Giordo, R., Pintus, G., & Fenu, G. (2024). Citrus limon var. pompia Camarda var. nova: A Comprehensive Review of Its Botanical Characteristics, Traditional Uses, Phytochemical Profile, and Potential Health Benefits. Nutrients, 16(16), 2619. https://doi.org/10.3390/nu16162619








