Association between Dietary Inflammatory Index and Hyperemesis Gravidarum
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Assessment of Dietary Intakes
2.3. Assessment of DII
2.4. Assessment of HG
- (1)
- PUQE questionnaire score ≥13;
- (2)
- Inability to eat normally due to severe nausea or vomiting, seriously affecting daily living activities;
- (3)
- Dehydration and weight loss after pregnancy exceeding 5% of pre-pregnancy weight;
- (4)
- Seeking medical attention due to NVP and receiving medical interventions;
- (5)
- Beginning of symptoms in the first trimester;
- (6)
- No other causes identified of nausea and vomiting, such as gastrointestinal or urinary tract, infections, etc.
2.5. Assessment of Other Variables
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Characteristic | Participants | Non-HG (n = 1866) | HG (n = 167) | p |
|---|---|---|---|---|
| Age (years) | 31.3 ± 3.4 | 31.3 ± 3.4 | 31.2 ± 3.5 | 0.856 |
| Week of gestation (weeks), median (IQR) | 12.0 (9.7, 12.7) | 12.0 (9.3, 12.7) | 12.3 (11.5, 12.7) | <0.001 |
| Parity (primigravida), n (%) | 1449 (71.3) | 1330 (71.3) | 119 (71.3) | 0.996 |
| Total energy intake (kcal/d), median (IQR) | 1413.0 (1049.3, 1872.2) | 1407.9 (1050.1, 1875.2) | 1421.9 (1024.8, 1816.5) | 0.911 |
| Physical activity (<median MET-min/week), n (%) | 777 (38.2) | 713 (38.2) | 64 (38.3) | 0.977 |
| Pre-pregnancy BMI (<24 kg/m2), n (%) | 1634 (80.4) | 1500 (80.4) | 134 (80.2) | 0.964 |
| Annual household income (<CNY 100,000), n (%) | 690 (33.9) | 627 (33.6) | 63 (37.7) | 0.281 |
| Educational level (under college), n (%) | 755 (37.1) | 683 (36.6) | 72 (43.1) | 0.095 |
| Employment status (no), n (%) | 417 (20.5) | 381 (20.4) | 36 (21.6) | 0.727 |
| Smoking (yes), n (%) | 61 (3.0) | 58 (3.1) | 3 (1.8) | 0.341 |
| Alcohol drinking (yes), n (%) | 70 (3.4) | 66 (3.5) | 4 (2.4) | 0.438 |
| Use of nutritional supplements (yes), n (%) | 804 (39.5) | 729 (39.1) | 75 (44.9) | 0.139 |
| DII, median (IQR) | +0.01 (−1.55, +1.56) | −0.02 (−1.56, +1.56) | +0.10 (−1.46, +1.41) | 0.890 |
| Variables | Tertiles of Dietary Inflammatory Index Scores | p | ||
|---|---|---|---|---|
| T1 (n = 678) | T2 (n = 677) | T3 (n = 678) | ||
| Carbohydrate (g/d) | 277.80 (225.12, 350.47) | 195.99 (167.05, 236.79) | 136.19 (110.8, 167.06) | <0.001 |
| Total fat (g/d) | 62.96 (49.93, 81.34) | 41.79 (33.47, 51.71) | 26.22 (20.34, 33.60) | <0.001 |
| Protein (g/d) | 102.38 (83.84, 127.36) | 64.02 (56.31, 74.74) | 41.08 (32.57, 48.81) | <0.001 |
| Cholesterol (mg/d) | 389.33 (264.91, 489.69) | 262.89 (180.24, 375.13) | 167.37 (86.39, 241.47) | <0.001 |
| Vitamin B12 (μg/d) | 0.71 (0.48, 1.05) | 0.53 (0.41, 0.80) | 0.44 (0.32, 0.64) | <0.001 |
| Fe (mg/d) | 24.50 (20.26, 30.67) | 14.91 (13.13, 17.12) | 9.45 (7.79, 10.96) | <0.001 |
| Alcohol (g/d) | 0.68 (0.68, 0.68) | 0.68 (0.68, 0.68) | 0.68 (0.68, 0.68) | 0.038 |
| MUFA (g/d) | 15.88 (11.87, 22.22) | 10.36 (7.31, 13.44) | 5.61 (4.08, 8.65) | <0.001 |
| PUFA (g/d) | 9.22 (6.79, 11.98) | 4.94 (3.93, 6.44) | 2.87 (2.22, 3.68) | <0.001 |
| Fiber (g/d) | 16.53 (13.51, 21.33) | 9.50 (8.10, 11.35) | 5.34 (4.25, 6.55) | <0.001 |
| Vitamin B6 (mg/d) | 1.84 (1.51, 2.46) | 1.17 (0.97, 1.36) | 0.69 (0.57, 0.88) | <0.001 |
| Folic acid (mg/d) | 551.21 (467.28, 701.22) | 331.34 (288.62, 377.46) | 194.74 (155.40, 233.71) | <0.001 |
| Niacin (mg/d) | 19.55 (15.67, 26.07) | 12.72 (10.56, 16.07) | 8.340 (6.89, 10.29) | <0.001 |
| Riboflavin (mg/d) | 1.30 (1.07, 1.65) | 0.93 (0.72, 0.97) | 0.51 (0.40, 0.63) | <0.001 |
| Thiamin (mg/d) | 1.05 (0.88, 1.32) | 0.67 (0.57, 0.75) | 0.40 (0.31, 0.48) | <0.001 |
| Vitamin A (RE) | 902.59 (651.66, 1182.75) | 485.94 (364.11, 643.09) | 270.08 (213.20, 355.35) | <0.001 |
| Vitamin C (mg/d) | 224.00 (174.29, 296.98) | 133.50 (103.76, 166.63) | 73.33 (53.81, 98.46) | <0.001 |
| Vitamin D (μg/d) | 2.07 (1.06, 2.70) | 1.22 (0.48, 2.21) | 0.52 (0.289, 1.24) | <0.001 |
| Vitamin E (mg/d) | 33.17 (23.11, 42.39) | 16.56 (13.76, 19.46) | 9.65 (7.94, 12.29) | <0.001 |
| Zn (mg/d) | 12.66 (10.55, 16.01) | 8.00 (6.92, 9.46) | 5.14 (4.14, 6.10) | <0.001 |
| Se (mg/d) | 45.73 (36.64, 62.33) | 30.94 (25.81, 38.23) | 20.27 (16.25, 25.19) | <0.001 |
| Mg (mg/d) | 504.54 (433.42, 633.17) | 318.90 (285.40, 350.73) | 200.38 (165.93, 230.61) | <0.001 |
| Model | Tertiles of Dietary Inflammatory Index Scores (OR, 95%CI) | Ptrend | Per SD Increase | ||
|---|---|---|---|---|---|
| T1 | T2 | T3 | |||
| DII-23 | 1.00 (Ref.) | 1.48 (0.98, 2.22) | 1.65 (1.04, 2.62) | 0.032 | 1.24 (1.03, 1.50) |
| DII-except Energy | 1.00 (Ref.) | 1.43 (0.95, 2.14) | 1.64 (1.03, 2.62) | 0.035 | 1.23 (1.02, 1.48) |
| DII-except Carbohydrate | 1.00 (Ref.) | 1.42 (0.94, 2.12) | 1.62 (1.02, 2.59) | 0.038 | 1.22 (1.01, 1.47) |
| DII-except Total fat | 1.00 (Ref.) | 1.41 (0.94, 2.12) | 1.62 (1.02, 2.58) | 0.040 | 1.22 (1.01, 1.47) |
| DII-except Protein | 1.00 (Ref.) | 1.42 (0.94, 2.12) | 1.61 (1.01, 2.56) | 0.041 | 1.22 (1.01, 1.47) |
| DII-except Cholesterol | 1.00 (Ref.) | 1.41 (0.94, 2.12) | 1.61 (1.01, 2.55) | 0.042 | 1.21 (1.01, 1.46) |
| DII-except Vitamin B12 | 1.00 (Ref.) | 1.40 (0.93, 2.10) | 1.67 (1.05, 2.66) | 0.029 | 1.23 (1.02, 1.49) |
| DII-except Fe | 1.00 (Ref.) | 1.47 (0.98, 2.21) | 1.65 (1.03, 2.62) | 0.032 | 1.23 (1.02, 1.48) |
| DII-except Alcohol | 1.00 (Ref.) | 1.37 (0.91, 2.05) | 1.69 (1.07, 2.66) | 0.025 | 1.24 (1.03, 1.49) |
| DII-except MUFA | 1.00 (Ref.) | 1.48 (0.98, 2.22) | 1.64 (1.03, 2.61) | 0.033 | 1.23 (1.02, 1.48) |
| DII-except PUFA | 1.00 (Ref.) | 1.37 (0.91, 2.05) | 1.54 (0.98, 2.44) | 0.060 | 1.20 (0.99, 1.44) |
| DII-except Fiber | 1.00 (Ref.) | 1.49 (0.99, 2.24) | 1.64 (1.03, 2.61) | 0.033 | 1.23 (1.02, 1.48) |
| DII-except Vitamin B6 | 1.00 (Ref.) | 1.50 (1.00, 2.24) | 1.55 (0.98, 2.46) | 0.054 | 1.20 (1.00, 1.44) |
| DII-except Folic acid | 1.00 (Ref.) | 1.39 (0.92, 2.08) | 1.65 (1.04, 2.62) | 0.031 | 1.23 (1.02, 1.48) |
| DII-except Niacin | 1.00 (Ref.) | 1.50 (1.00, 2.24) | 1.54 (0.97, 2.43) | 0.059 | 1.19 (0.99, 1.43) |
| DII-except Riboflavin | 1.00 (Ref.) | 1.44 (0.96, 2.16) | 1.67 (1.06, 2.65) | 0.027 | 1.24 (1.03, 1.49) |
| DII-except Thiamin | 1.00 (Ref.) | 1.44 (0.96, 2.17) | 1.67 (1.06, 2.65) | 0.027 | 1.23 (1.02, 1.49) |
| DII-except Vitamin A | 1.00 (Ref.) | 1.35 (0.89, 2.02) | 1.72 (1.09, 2.72) | 0.020 | 1.25 (1.04, 1.50) |
| DII-except Vitamin C | 1.00 (Ref.) | 1.33 (0.89, 1.99) | 1.59 (1.00, 2.52) | 0.047 | 1.21 (1.00, 1.46) |
| DII-except Vitamin D | 1.00 (Ref.) | 1.54 (1.03, 2.29) | 1.46 (0.92, 2.32) | 0.097 | 1.17 (0.97, 1.41) |
| DII-except Vitamin E | 1.00 (Ref.) | 1.37 (0.91, 2.06) | 1.56 (0.98, 2.47) | 0.056 | 1.20 (1.00, 1.44) |
| DII-except Zn | 1.00 (Ref.) | 1.44 (0.96, 2.15) | 1.52 (0.96, 2.41) | 0.068 | 1.19 (0.99, 1.43) |
| DII-except Se | 1.00 (Ref.) | 1.42 (0.95, 2.13) | 1.56 (0.99, 2.46) | 0.054 | 1.20 (1.00, 1.44) |
| DII-except Mg | 1.00 (Ref.) | 1.32 (0.88, 1.99) | 1.61 (1.03, 2.54) | 0.038 | 1.22 (1.01, 1.46) |
References
- ACOG Practice Bulletin No. 189: Nausea and Vomiting of Pregnancy. Obstet. Gynecol. 2018, 131, e15–e30. [CrossRef]
- Fejzo, M.S.; Trovik, J.; Grooten, I.J.; Sridharan, K.; Roseboom, T.J.; Vikanes, Å.; Painter, R.C.; Mullin, P.M. Nausea and vomiting of pregnancy and hyperemesis gravidarum. Nat. Rev. Dis. Primers 2019, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.I.; Nori, W.; Abdulrahman Hadi, B.A. Hyperemesis gravidarum and risks of placental dysfunction disorders. J. Pak. Med. Assoc. 2021, 71 (Suppl. S9), S24–S28. [Google Scholar]
- Fiaschi, L.; Nelson-Piercy, C.; Gibson, J.; Szatkowski, L.; Tata, L.J. Adverse Maternal and Birth Outcomes in Women Admitted to Hospital for Hyperemesis Gravidarum: A Population-Based Cohort Study. Paediatr. Perinat. Epidemiol. 2018, 32, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Nijsten, K.; Jansen, L.A.W.; Limpens, J.; Finken, M.J.J.; Koot, M.H.; Grooten, I.J.; Roseboom, T.J.; Painter, R.C. Long-term health outcomes of children born to mothers with hyperemesis gravidarum: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2022, 227, 414–429.e7. [Google Scholar] [CrossRef] [PubMed]
- Soysal, C.; Işıkalan, M.M.; Bıyık, İ.; Erten, Ö.; İnce, O. The relationship between inflammation markers and ketonuria in hyperemesis gravidarum. J. Obstet. Gynaecol. Res. 2021, 47, 3078–3083. [Google Scholar] [CrossRef] [PubMed]
- Dal, Y.; Akkuş, F.; Karagün, Ş.; Çolak, H.; Coşkun, A. Are serum delta neutrophil index and other inflammatory marker levels different in hyperemesis gravidarum? J. Obstet. Gynaecol. Res. 2023, 49, 828–834. [Google Scholar] [CrossRef] [PubMed]
- McParlin, C.; O’Donnell, A.; Robson, S.C.; Beyer, F.; Moloney, E.; Bryant, A.; Bradley, J.; Muirhead, C.R.; Nelson-Piercy, C.; Newbury-Birch, D.; et al. Treatments for Hyperemesis Gravidarum and Nausea and Vomiting in Pregnancy: A Systematic Review. JAMA 2016, 316, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Li, L.; Long, Z.; Ma, X.; Chen, F.; Ma, L.; Zhang, S.; Lin, J. Association between Dietary Patterns and the Risk of Hyperemesis Gravidarum. Nutrients 2023, 15, 3300. [Google Scholar] [CrossRef]
- Shivappa, N. Diet and Chronic Diseases: Is There a Mediating Effect of Inflammation? Nutrients 2019, 11, 1639. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hebert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public. Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Kyozuka, H.; Murata, T.; Fukuda, T.; Yamaguchi, A.; Yasuda, S.; Suzuki, D.; Kanno, A.; Sato, A.; Ogata, Y.; Hosoya, M.; et al. Preconception dietary inflammatory index and hypertension disorders of pregnancy: The Japan environment and children’s study. Pregnancy Hypertens. 2022, 28, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Hébert, J.R.; Akhoundan, M.; Mirmiran, P.; Rashidkhani, B. Association between inflammatory potential of diet and odds of gestational diabetes mellitus among Iranian women. J. Matern. Fetal Neonatal Med. 2019, 32, 3552–3558. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chang, Q.; Du, Q.; Dang, S.; Zeng, L.; Yan, H. Dietary Inflammatory Index during Pregnancy and Congenital Heart Defects. Nutrients 2023, 15, 2262. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, N.P.A.; Carvalho, T.R.; Gonçalves, C.; da Silva, P.H.A.; de Melo Romão, L.G.; Kwak-Kim, J.; Cavalcante, M.B. The Dietary Inflammatory Index as a predictor of pregnancy outcomes: Systematic review and meta-analysis. J. Reprod. Immunol. 2022, 152, 103651. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Zhang, E.; Liu, R.; Zhang, Y.; Wang, C.; Gao, S.; Su, S.; Gao, X.; Wu, Q.; Yang, X.; et al. The China birth cohort study (CBCS). Eur. J. Epidemiol. 2022, 37, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, Z.; Shi, J.; Liu, S.; Li, L.; Ding, L.; Zhao, J.; Pan, Y.; Lei, H.; He, T.; et al. The association between prepregnancy dietary fatty acids and risk of gestational diabetes mellitus: A prospective cohort study. Clin. Nutr. 2024, 43, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Cross, C.L.; Daniel, W.W. Biostatistics: A Foundation for Analysis in the Health Sciences; John Wiley & Sons, Inc: New York, NY, USA, 2018. [Google Scholar]
- Cheng, Y.; Yan, H.; Dibley, M.J.; Shen, Y.; Li, Q.; Zeng, L. Validity and reproducibility of a semi-quantitative food frequency questionnaire for use among pregnant women in rural China. Asia Pac. J. Clin. Nutr. 2008, 17, 166–177. [Google Scholar] [PubMed]
- Cheng, Y.; Dibley, M.J.; Zhang, X.; Zeng, L.; Yan, H. Assessment of dietary intake among pregnant women in a rural area of western China. BMC Public Health 2009, 9, 222. [Google Scholar] [CrossRef] [PubMed]
- Salari-Moghaddam, A.; Keshteli, A.H.; Afshar, H.; Esmaillzadeh, A.; Adibi, P. Association between dietary inflammatory index and psychological profile in adults. Clin. Nutr. 2019, 38, 2360–2368. [Google Scholar] [CrossRef]
- Koren, G.; Boskovic, R.; Hard, M.; Maltepe, C.; Navioz, Y.; Einarson, A. Motherisk-PUQE (pregnancy-unique quantification of emesis and nausea) scoring system for nausea and vomiting of pregnancy. Am. J. Obstet. Gynecol. 2002, 186, S228–S231. [Google Scholar] [CrossRef] [PubMed]
- Birkeland, E.; Stokke, G.; Tangvik, R.J.; Torkildsen, E.A.; Boateng, J.; Wollen, A.L.; Albrechtsen, S.; Flaatten, H.; Trovik, J. Norwegian PUQE (Pregnancy-Unique Quantification of Emesis and nausea) identifies patients with hyperemesis gravidarum and poor nutritional intake: A prospective cohort validation study. PLoS ONE 2015, 10, e0119962. [Google Scholar] [CrossRef] [PubMed]
- Koot, M.H.; Grooten, I.J.; Post, J.; Bais, J.M.J.; Ris-Stalpers, C.; Naaktgeboren, C.A.; Niemeijer, M.N.; Bremer, H.A.; van der Ham, D.P.; Heidema, W.M.; et al. Ketonuria is not associated with hyperemesis gravidarum disease severity. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 254, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Jansen, L.A.W.; Koot, M.H.; Van’t Hooft, J.; Dean, C.R.; Bossuyt, P.M.M.; Ganzevoort, W.; Gauw, N.; Van der Goes, B.Y.; Rodenburg, J.; Roseboom, T.J.; et al. The windsor definition for hyperemesis gravidarum: A multistakeholder international consensus definition. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 266, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Jansen, L.A.W.; Shaw, V.; Grooten, I.J.; Koot, M.H.; Dean, C.R.; Painter, R.C. Diagnosis and treatment of hyperemesis gravidarum. CMAJ 2024, 196, E477–E485. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B. Predictive values of body mass index and waist circumference to risk factors of related diseases in Chinese adult population. Zhonghua Liu Xing Bing. Xue Za Zhi 2002, 23, 5–10. [Google Scholar]
- Wang, C.; Wei, Y.; Zhang, X.; Zhang, Y.; Xu, Q.; Sun, Y.; Su, S.; Zhang, L.; Liu, C.; Feng, Y.; et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am. J. Obstet. Gynecol. 2017, 216, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.L.; Yang, S.T.; Wang, P.H. Body mass index and gestational weight gain of the pregnant women in Taiwan. Taiwan. J. Obstet. Gynecol. 2024, 63, 288–290. [Google Scholar] [CrossRef]
- Vahid, F.; Shivappa, N.; Hekmatdoost, A.; Hebert, J.R.; Davoodi, S.H.; Sadeghi, M. Association between Maternal Dietary Inflammatory Index (DII) and abortion in Iranian women and validation of DII with serum concentration of inflammatory factors: Case-control study. Appl. Physiol. Nutr. Metab. 2017, 42, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Casas, R.; Castro-Barquero, S.; Crovetto, F.; Larroya, M.; Ruiz-León, A.M.; Segalés, L.; Nakaki, A.; Youssef, L.; Benitez, L.; Casanovas-Garriga, F.; et al. Maternal Dietary Inflammatory Index during Pregnancy Is Associated with Perinatal Outcomes: Results from the IMPACT BCN Trial. Nutrients 2022, 14, 2284. [Google Scholar] [CrossRef]
- Ishibashi, M.; Kyozuka, H.; Yamaguchi, A.; Fujimori, K.; Hosoya, M.; Yasumura, S.; Masahito, K.; Sato, A.; Ogata, Y.; Hashimoto, K. Effect of proinflammatory diet before pregnancy on gestational age and birthweight: The Japan Environment and Children’s Study. Matern. Child. Nutr. 2020, 16, e12899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, K.; Mi, B.; Liu, H.; Wang, Y.; Huo, Y.; Ma, L.; Liu, D.; Jing, H.; Liu, J.; et al. Maternal Dietary Inflammatory Potential and Offspring Birth Outcomes in a Chinese Population. J. Nutr. 2023, 153, 1512–1523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, Y.; Zhong, C.; Zhou, X.; Liu, C.; Li, Q.; Chen, R.; Gao, Q.; Li, X.; Zhang, H.; et al. Association between dietary inflammatory index and gestational diabetes mellitus risk in a prospective birth cohort study. Nutrition 2021, 87–88, 111193. [Google Scholar] [CrossRef] [PubMed]
- Verberg, M.F.; Gillott, D.J.; Al-Fardan, N.; Grudzinskas, J.G. Hyperemesis gravidarum, a literature review. Hum. Reprod. Update 2005, 11, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Panesar, N.S. Human chorionic gonadotropin: A secretory hormone. Med. Hypotheses 1999, 53, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Carson, D.A.; Bhat, S.; Hayes, T.C.L.; Gharibans, A.A.; Andrews, C.N.; O’Grady, G.; Varghese, C. Abnormalities on Electrogastrography in Nausea and Vomiting Syndromes: A Systematic Review, Meta-Analysis, and Comparison to Other Gastric Disorders. Dig. Dis. Sci. 2022, 67, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Lagiou, P.; Tamimi, R.; Mucci, L.A.; Trichopoulos, D.; Adami, H.O.; Hsieh, C.C. Nausea and vomiting in pregnancy in relation to prolactin, estrogens, and progesterone: A prospective study. Obstet. Gynecol. 2003, 101, 639–644. [Google Scholar] [CrossRef]
- Chortatos, A.; Haugen, M.; Iversen, P.O.; Vikanes, Å.; Magnus, P.; Veierød, M.B. Nausea and vomiting in pregnancy: Associations with maternal gestational diet and lifestyle factors in the Norwegian Mother and Child Cohort Study. BJOG Int. J. Obstet. Gynaecol. 2013, 120, 1642–1653. [Google Scholar] [CrossRef] [PubMed]
- Oruç, A.S.; Mert, I.; Akturk, M.; Aslan, E.; Polat, B.; Buyukkagnıcı, U.; Danışman, N. Ghrelin and motilin levels in hyperemesis gravidarum. Arch. Gynecol. Obstet. 2013, 287, 1087–1092. [Google Scholar] [CrossRef]
- Kokhan, E.P.; Kvashin, V.A. Temporary cannulation of the blood vessels (review of the literature). Voen. Med. Zh 1983, 8, 33–36. [Google Scholar]
- Signorello, L.B.; Harlow, B.L.; Wang, S.; Erick, M.A. Saturated fat intake and the risk of severe hyperemesis gravidarum. Epidemiology 1998, 9, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Haugen, M.; Vikanes, A.; Brantsaeter, A.L.; Meltzer, H.M.; Grjibovski, A.M.; Magnus, P. Diet before pregnancy and the risk of hyperemesis gravidarum. Br. J. Nutr. 2011, 106, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Crozier, S.R.; Inskip, H.M.; Godfrey, K.M.; Cooper, C.; Robinson, S.M. Nausea and vomiting in early pregnancy: Effects on food intake and diet quality. Matern. Child. Nutr. 2017, 13, e12389. [Google Scholar] [CrossRef]
- London, V.; Grube, S.; Sherer, D.M.; Abulafia, O. Hyperemesis Gravidarum: A Review of Recent Literature. Pharmacology 2017, 100, 161–171. [Google Scholar] [CrossRef]
- Ruiz-Canela, M.; Zazpe, I.; Shivappa, N.; Hébert, J.R.; Sánchez-Tainta, A.; Corella, D.; Salas-Salvadó, J.; Fitó, M.; Lamuela-Raventós, R.M.; Rekondo, J.; et al. Dietary inflammatory index and anthropometric measures of obesity in a population sample at high cardiovascular risk from the PREDIMED (PREvención con DIeta MEDiterránea) trial. Br. J. Nutr. 2015, 113, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Nurmi, M.; Rautava, P.; Gissler, M.; Vahlberg, T.; Polo-Kantola, P. Incidence and risk factors of hyperemesis gravidarum: A national register-based study in Finland, 2005–2017. Acta Obstet. Gynecol. Scand. 2020, 99, 1003–1013. [Google Scholar] [CrossRef]
- Wei, Y.; Meng, Y.; Li, N.; Wang, Q.; Chen, L. The effects of low-ratio n-6/n-3 PUFA on biomarkers of inflammation: A systematic review and meta-analysis. Food Funct. 2021, 12, 30–40. [Google Scholar] [CrossRef]
- Heshmati, J.; Morvaridzadeh, M.; Maroufizadeh, S.; Akbari, A.; Yavari, M.; Amirinejad, A.; Maleki-Hajiagha, A.; Sepidarkish, M. Omega-3 fatty acids supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 149, 104462. [Google Scholar] [CrossRef]
- Biberoglu, E.H.; Kirbas, A.; Dirican, A.; Genc, M.; Avci, A.; Doganay, B.; Uygur, D.; Biberoglu, K. Alterations in lipid peroxidation and T-cell function in women with hyperemesis gravidarum. J. Obstet. Gynaecol. 2016, 36, 93–96. [Google Scholar] [CrossRef]
- Qiu, W.; Lu, H.; Qi, Y.; Wang, X. Dietary fat intake and ovarian cancer risk: A meta-analysis of epidemiological studies. Oncotarget 2016, 7, 37390–37406. [Google Scholar] [CrossRef]
- Michel, M.E.; Alanio, E.; Bois, E.; Gavillon, N.; Graesslin, O. Wernicke encephalopathy complicating hyperemesis gravidarum: A case report. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 149, 118–119. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, S.; Akdağ Cırık, D.; Demirtaş, C.; Timur, H.; Şahin, A.; Danışman, N.; Uygur, D. Do vitamin D and high-sensitivity-C reactive protein levels differ in patients with hyperemesis gravidarum? A preliminary study. Turk. J. Obstet. Gynecol. 2016, 13, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Hu, F. Vitamin D and hyperemesis gravidarum: A mendelian randomization study. J. Gynecol. Obstet. Hum. Reprod. 2023, 52, 102678. [Google Scholar] [CrossRef] [PubMed]
- Celik, F.; Guzel, A.I.; Kuyumcuoglu, U.; Celik, Y. Dietary antioxidant levels in hyperemesis gravidarum: A case control study. Ginekol. Pol. 2011, 82, 840–844. [Google Scholar] [PubMed]
- Mizumoto, Y.; Okuyama, T.; Endo, R.; Nakajima, H.; Hiramatsu, H.; Horie, M.; Masuda, H.; Kobayashi, S.; Saeki, H.; Abe, M. Studies on hypogeusia in hyperemesis gravidarum. Nihon Sanka Fujinka Gakkai Zasshi 1994, 46, 35–41. [Google Scholar] [PubMed]
- Hovdenak, N.; Haram, K. Influence of mineral and vitamin supplements on pregnancy outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 164, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Masuhiro, K.; Matsuzaki, N.; Nishino, E.; Taniguchi, T.; Kameda, T.; Li, Y.; Saji, F.; Tanizawa, O. Trophoblast-derived interleukin-1 (IL-1) stimulates the release of human chorionic gonadotropin by activating IL-6 and IL-6-receptor system in first trimester human trophoblasts. J. Clin. Endocrinol. Metab. 1991, 72, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Matsuzaki, N.; Masuhiro, K.; Kameda, T.; Taniguchi, T.; Saji, F.; Yone, K.; Tanizawa, O. Trophoblast-derived tumor necrosis factor-alpha induces release of human chorionic gonadotropin using interleukin-6 (IL-6) and IL-6-receptor-dependent system in the normal human trophoblasts. J. Clin. Endocrinol. Metab. 1992, 74, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, P.B.; Gücer, F.; Sayin, N.C.; Yüksel, M.; Yüce, M.A.; Yardim, T. Maternal serum cytokine levels in women with hyperemesis gravidarum in the first trimester of pregnancy. Fertil. Steril. 2003, 79, 498–502. [Google Scholar] [CrossRef]
- Yanushpolsky, E.H.; Ozturk, M.; Polgar, K.; Berkowitz, R.S.; Hill, J.A. The effects of cytokines on human chorionic gonadotropin (hCG) production by a trophoblast cell line. J. Reprod. Immunol. 1993, 25, 235–247. [Google Scholar] [CrossRef]
- Nishino, E.; Matsuzaki, N.; Masuhiro, K.; Kameda, T.; Taniguchi, T.; Takagi, T.; Saji, F.; Tanizawa, O. Trophoblast-derived interleukin-6 (IL-6) regulates human chorionic gonadotropin release through IL-6 receptor on human trophoblasts. J. Clin. Endocrinol. Metab. 1990, 71, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; van Zon, S.; Adams, A.; Schmidt-Arras, D.; Laurence, A.D.J.; Uhlig, H.H. The Human GP130 Cytokine Receptor and Its Expression-an Atlas and Functional Taxonomy of Genetic Variants. J. Clin. Immunol. 2023, 44, 30. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Arras, D.; Galun, E.; Rose-John, S. The two facets of gp130 signalling in liver tumorigenesis. Semin. Immunopathol. 2021, 43, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Saito, S. Cytokine network at the feto-maternal interface. J. Reprod. Immunol. 2000, 47, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, X.; Hao, S.; Zhao, H.; Pang, L.; Wang, L.; Ren, H.; Wang, C.; Mao, H. Human chorionic gonadotropin and IL-35 contribute to the maintenance of peripheral immune tolerance during pregnancy through mediating the generation of IL-10(+) or IL-35(+) Breg cells. Exp. Cell Res. 2019, 383, 111513. [Google Scholar] [CrossRef]
- Gadsby, R.; Barnie-Adshead, A.; Grammatoppoulos, D.; Gadsby, P. Nausea and vomiting in pregnancy: An association between symptoms and maternal prostaglandin E2. Gynecol. Obstet. Investig. 2000, 50, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.L.; Hooper, W.C.; Jones, D.P.; Ashfaq, S.; Rhodes, S.D.; Weintraub, W.S.; Harrison, D.G.; Quyyumi, A.A.; Vaccarino, V. Association between novel oxidative stress markers and C-reactive protein among adults without clinical coronary heart disease. Atherosclerosis 2005, 178, 115–121. [Google Scholar] [CrossRef]
- Schwedler, S.B.; Kuhlencordt, P.J.; Ponnuswamy, P.P.; Hatiboglu, G.; Quaschning, T.; Widder, J.; Wanner, C.; Potempa, L.A.; Galle, J. Native C-reactive protein induces endothelial dysfunction in ApoE−/− mice: Implications for iNOS and reactive oxygen species. Atherosclerosis 2007, 195, e76–e84. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, S.B.; Ayaydın Yılmaz, K.I.; Altuntaş, N.B.; Tekin, Y.B. Relationship between combined systemic inflammatory indices with presence and severity of hyperemesis gravidarum. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 8868–8876. [Google Scholar] [CrossRef]
- Peres, L.C.; Bandera, E.V.; Qin, B.; Guertin, K.A.; Shivappa, N.; Hebert, J.R.; Abbott, S.E.; Alberg, A.J.; Barnholtz-Sloan, J.; Bondy, M.; et al. Dietary inflammatory index and risk of epithelial ovarian cancer in African American women. Int. J. Cancer 2017, 140, 535–543. [Google Scholar] [CrossRef]
- Cucó, G.; Fernández-Ballart, J.; Sala, J.; Viladrich, C.; Iranzo, R.; Vila, J.; Arija, V. Dietary patterns and associated lifestyles in preconception, pregnancy and postpartum. Eur. J. Clin. Nutr. 2006, 60, 364–371. [Google Scholar] [CrossRef] [PubMed]

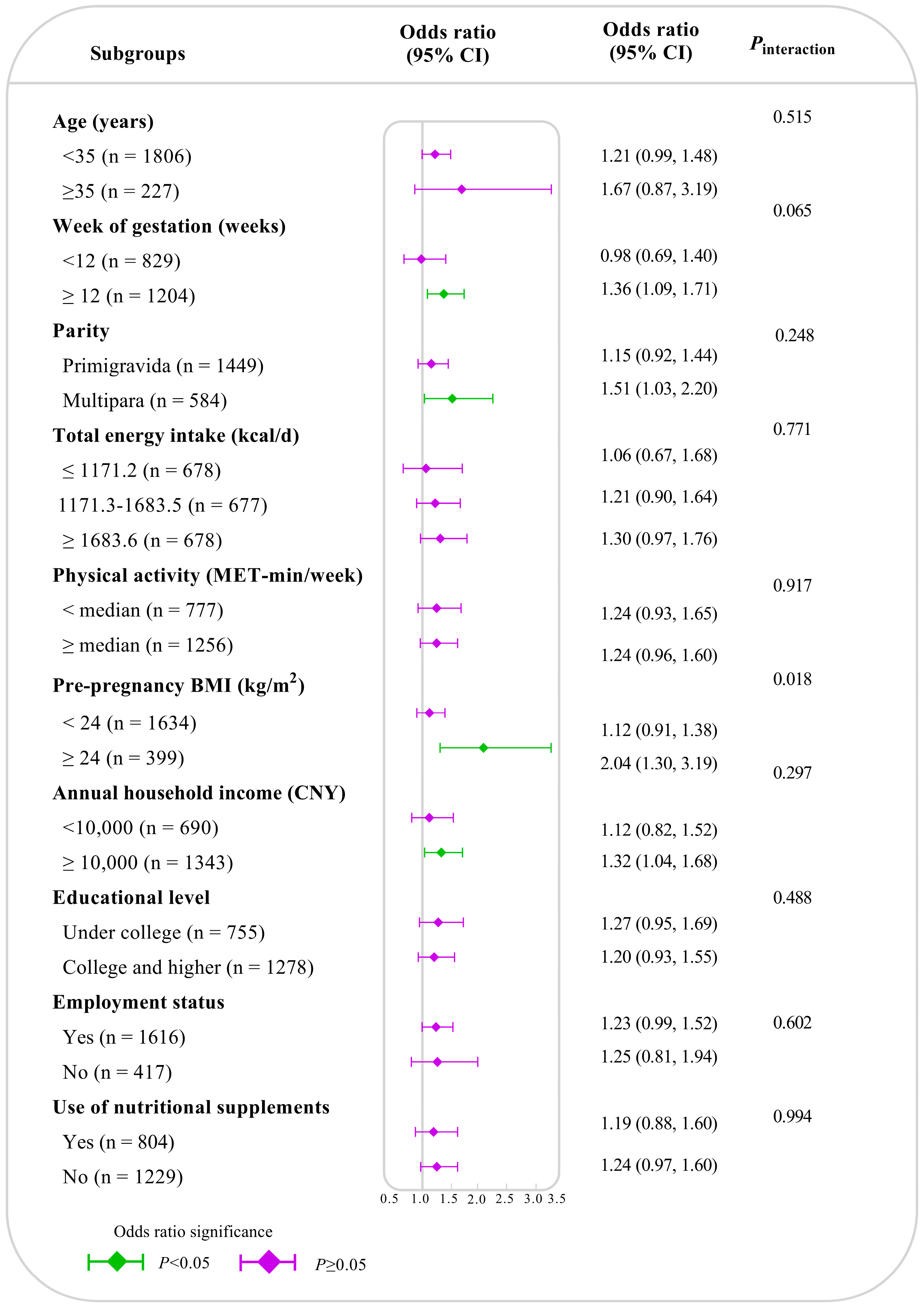
| Characteristic | Tertiles of Dietary Inflammatory Index Scores | p | ||
|---|---|---|---|---|
| T1 (n = 678) | T2 (n = 677) | T3 (n = 678) | ||
| Age (years) | 31.4 ± 3.5 | 31.3 ± 3.4 | 31.1 ± 3.4 | 0.160 |
| Week of gestation (weeks), median (IQR) | 12.2 (10.0, 12.7) | 12.2 (9.7, 12.7) | 12.0 (9.5, 12.7) | 0.533 |
| Parity (primigravida), n (%) | 457 (67.4) | 485 (71.6) | 507 (74.8) | 0.011 |
| Total energy intake (kcal/d), median (IQR) | 2036.6 (1713.3, 2510.2) | 1403.7 (1214.3, 1633.1) | 944.9 (772.3, 1114.0) | <0.001 |
| Physical activity (<median), n (%) | 274 (40.4) | 231 (34.1) | 272 (40.1) | 0.027 |
| Pre-pregnancy BMI (<24 kg/m2), n (%) | 549 (81.0) | 533 (78.7) | 552 (81.4) | 0.410 |
| Annual household income (<CNY 100,000), n (%) | 216 (31.9) | 229 (33.8) | 245 (36.1) | 0.250 |
| Educational level (under college), n (%) | 255 (37.6) | 236 (34.9) | 264 (38.9) | 0.285 |
| Employment status (no), n (%) | 143 (21.1) | 137 (20.2) | 137 (20.2) | 0.900 |
| Smoking (yes), n (%) | 20 (2.9) | 20 (3.0) | 21 (3.1) | 0.984 |
| Alcohol drinking (yes), n (%) | 21 (3.1) | 22 (3.2) | 27 (4.0) | 0.634 |
| Use of nutritional supplements (yes), n (%) | 298 (44.0) | 255 (37.7) | 251 (37.0) | 0.016 |
| DII, median (IQR) | −2.06 (−2.74, −1.55) | +0.01 (−0.52, +0.50) | +2.11 (+1.55, +2.76) | |
| Variables | Number | HG | p |
|---|---|---|---|
| Age (continuous) | 2033 | 1.02 (0.96, 1.07) | 0.592 |
| Week of gestation (continuous) | 2033 | 1.19 (1.10, 2.10) | <0.001 |
| Parity a (primigravida/multipara) | 1449/584 | 0.97 (0.65, 1.44) | 0.864 |
| Total energy intake | 2033 | ||
| ≤1171.2 kcal/d | 678 | 1.00 | |
| 1171.3–1683.5 kcal/d | 677 | 1.74 (1.12, 2.71) | 0.014 |
| ≥1683.6 kcal/d | 678 | 2.35 (1.46, 3.77) | <0.001 |
| Physical activity b (<median/≥median) | 777/1256 | 0.99 (0.71, 1.38) | 0.951 |
| Pre-pregnancy BMI c (<24 kg/m2/≥24 kg/m2) | 1634/399 | 0.98 (0.65, 1.47) | 0.913 |
| Annual household income d (<CNY 100,000/≥CNY 100,000) | 690/1343 | 0.88 (0.62, 1.25) | 0.467 |
| Educational level e (under college/college and higher) | 755/1278 | 0.74 (0.52, 1.06) | 0.103 |
| Employment status f (no/yes) | 417/1616 | 1.04 (0.68, 1.58) | 0.872 |
| Smoking f (no/yes) | 1972/61 | 0.53 (0.16, 1.76) | 0.299 |
| Alcohol drinking f (no/yes) | 1963/70 | 0.66 (0.23, 1.87) | 0.431 |
| Use of nutritional supplements f (no/yes) | 1229/804 | 1.30 (0.94, 1.80) | 0.120 |
| Model | Tertiles of Dietary Inflammatory Index Scores (OR, 95%CI) | Ptrend | Per SD Increase | ||
|---|---|---|---|---|---|
| T1 (≤−1.00) | T2 (−0.99~0.97) | T3 (≥0.98) | |||
| case/control | 53/625 | 60/617 | 54/624 | ||
| Model 1 | 1.00 (Ref.) | 1.15 (0.78, 1.69) | 1.02 (0.69, 1.52) | 0.921 | 1.01 (0.86, 1.18) |
| Model 2 | 1.00 (Ref.) | 1.42 (0.95, 2.12) | 1.59 (1.01, 2.51) | 0.046 | 1.22 (1.01, 1.48) |
| Model 3 | 1.00 (Ref.) | 1.48 (0.98, 2.22) | 1.65 (1.04, 2.62) | 0.032 | 1.24 (1.03, 1.50) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhi, S.; Zhang, L.; Cheng, W.; Jin, Y.; Long, Z.; Gu, W.; Ma, L.; Zhang, S.; Lin, J. Association between Dietary Inflammatory Index and Hyperemesis Gravidarum. Nutrients 2024, 16, 2618. https://doi.org/10.3390/nu16162618
Zhi S, Zhang L, Cheng W, Jin Y, Long Z, Gu W, Ma L, Zhang S, Lin J. Association between Dietary Inflammatory Index and Hyperemesis Gravidarum. Nutrients. 2024; 16(16):2618. https://doi.org/10.3390/nu16162618
Chicago/Turabian StyleZhi, Shihan, Lan Zhang, Wenjie Cheng, Yuan Jin, Zhaoqing Long, Wei Gu, Le Ma, Shunming Zhang, and Jing Lin. 2024. "Association between Dietary Inflammatory Index and Hyperemesis Gravidarum" Nutrients 16, no. 16: 2618. https://doi.org/10.3390/nu16162618
APA StyleZhi, S., Zhang, L., Cheng, W., Jin, Y., Long, Z., Gu, W., Ma, L., Zhang, S., & Lin, J. (2024). Association between Dietary Inflammatory Index and Hyperemesis Gravidarum. Nutrients, 16(16), 2618. https://doi.org/10.3390/nu16162618








