C-Reactive Protein-to-Prealbumin and C-Reactive Protein-to-Albumin Ratios as Nutritional and Prognostic Markers in Hospitalized Patients—An Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Nutritional Assessment
2.3. Biochemical Markers
2.4. Variables
2.5. Statistical Analysis
3. Results
3.1. Description of Results
3.2. ROC Curve Analysis
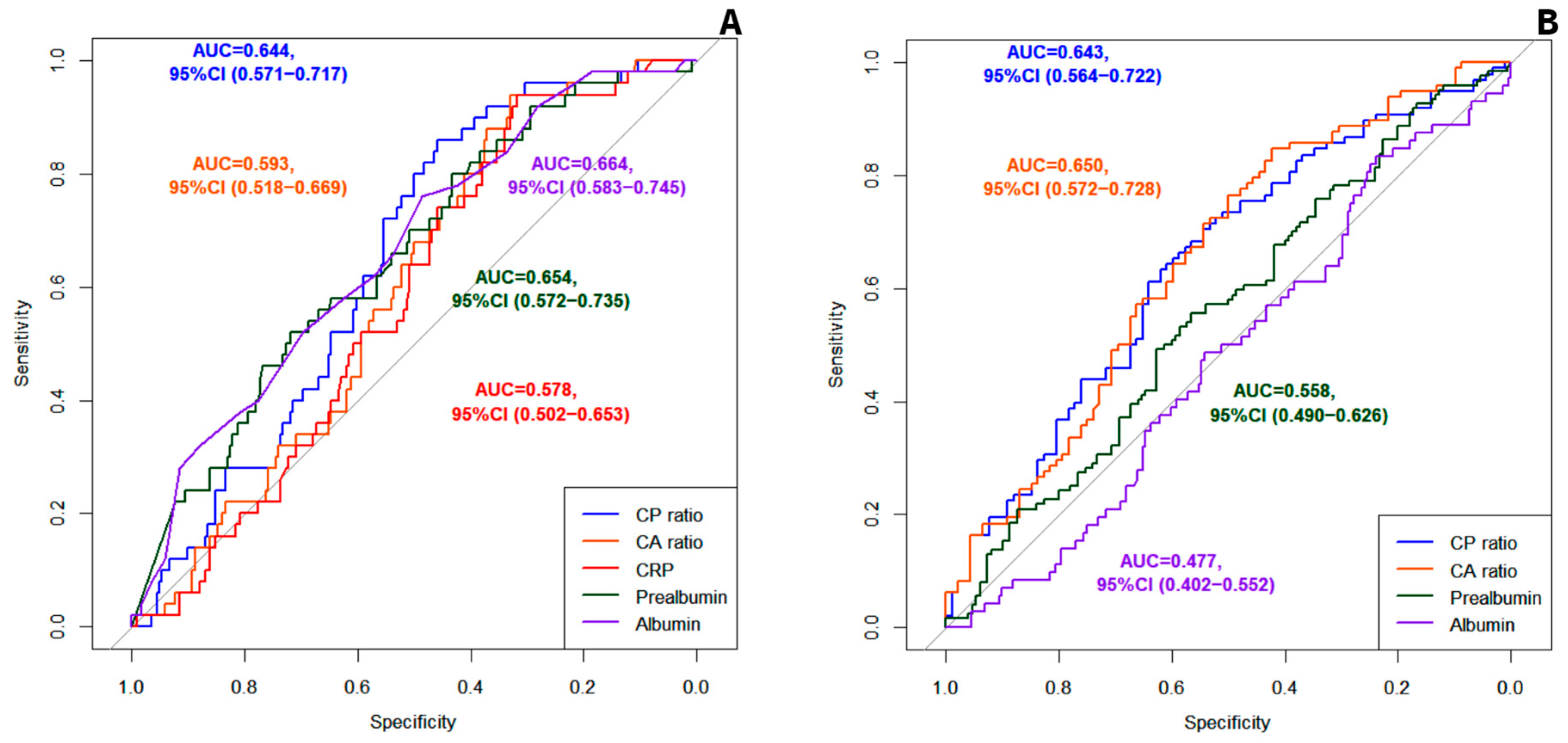
3.2.1. Evaluation of the CP Ratio and the CA Ratio as Predictors of Mortality
3.2.2. Evaluation of the CP Ratio and the CA Ratio as Predictors of Malnutrition
3.2.3. Characteristics of the Subgroups of the Patients Stratified According to the CP Ratio and the CA Ratio Values
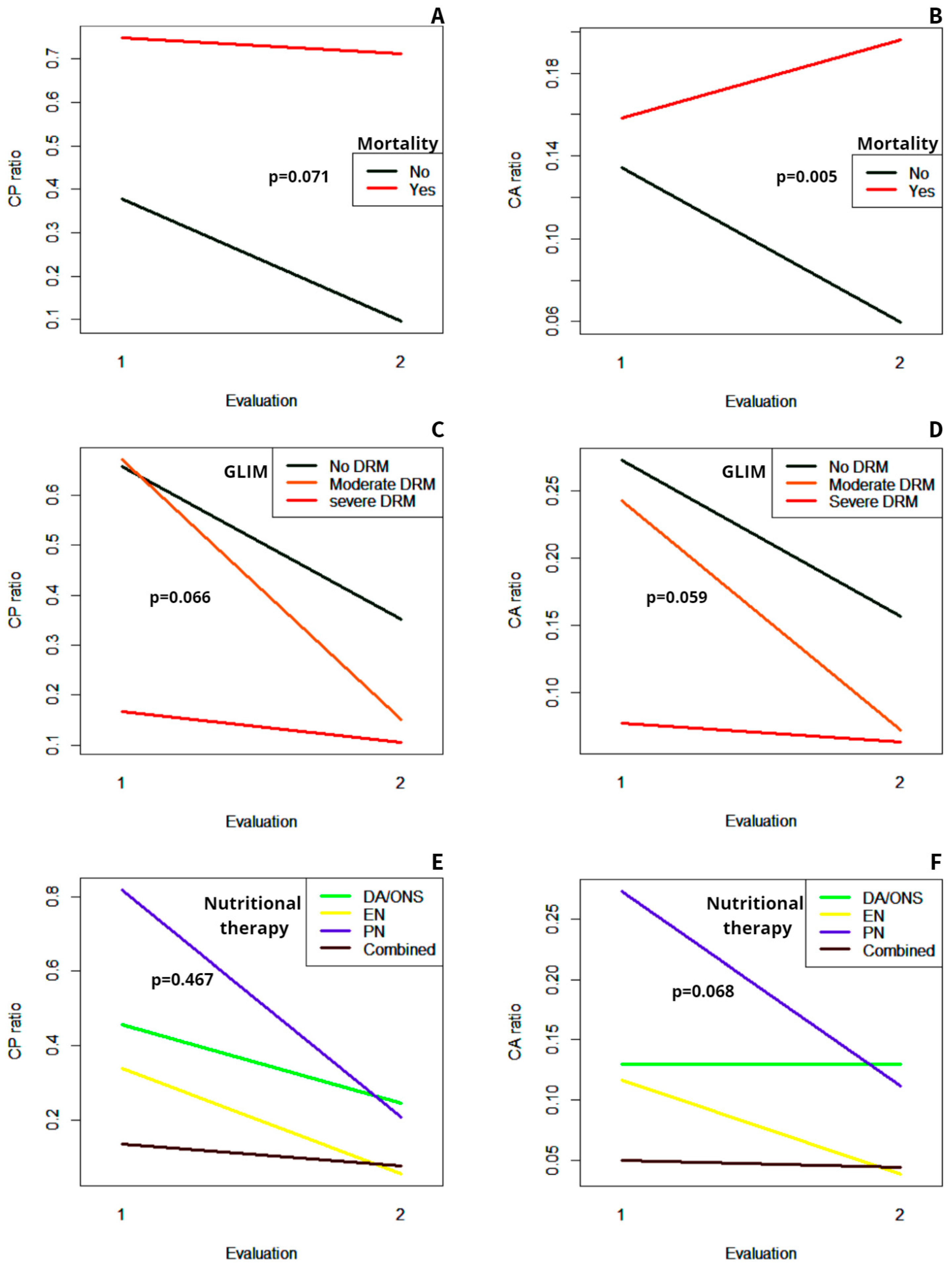
3.3. Evolution of the CP Ratio and the CA Ratio during Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schuetz, P.; Seres, D.; Lobo, D.N.; Gomes, F.; Kaegi-Braun, N.; Stanga, Z. Management of Disease-Related Malnutrition for Patients Being Treated in Hospital. Lancet 2021, 398, 1927–1938. [Google Scholar] [CrossRef] [PubMed]
- Zugasti Murillo, A.; Petrina-Jáuregui, M.E.; Ripa-Ciáurriz, C.; Sánchez Sánchez, R.; Villazón-González, F.; González-Díaz Faes, Á.; Fernández-López, C.; Calles-Romero, L.; Martín Palmero, M.Á.; Riestra-Fernández, M.; et al. SeDREno Study—Prevalence of Hospital Malnutrition According to GLIM Criteria, Ten Years after the PREDyCES Study. Nutr. Hosp. 2021, 38, 1016–1025. [Google Scholar] [CrossRef]
- Barker, L.A.; Gout, B.S.; Crowe, T.C. Hospital Malnutrition: Prevalence, Identification and Impact on Patients and the Healthcare System. Int. J. Environ. Res. Public Health 2011, 8, 514–527. [Google Scholar] [CrossRef]
- Correia, M.I.T.D.; Perman, M.I.; Waitzberg, D.L. Hospital Malnutrition in Latin America: A Systematic Review. Clin. Nutr. 2017, 36, 958–967. [Google Scholar] [CrossRef]
- Alves, L.F.; de Jesus, J.D.S.; Britto, V.N.M.; de Jesus, S.A.; Santos, G.S.; de Oliveira, C.C. GLIM Criteria to Identify Malnutrition in Patients in Hospital Settings: A Systematic Review. JPEN J. Parenter. Enter. Nutr. 2023, 47, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Keller, U. Nutritional Laboratory Markers in Malnutrition. J. Clin. Med. 2019, 8, 775. [Google Scholar] [CrossRef]
- Yamada, T.; Haruki, S.; Minami, Y.; Numata, M.; Hagiwara, N. The C-Reactive Protein to Prealbumin Ratio on Admission and Its Relationship with Outcome in Patients Hospitalized for Acute Heart Failure. J. Cardiol. 2021, 78, 308–313. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, Y.; Wu, H. Regulation of C-Reactive Protein Conformation in Inflammation. Inflamm. Res. 2019, 68, 815–823. [Google Scholar] [CrossRef]
- Dellière, S.; Cynober, L. Is Transthyretin a Good Marker of Nutritional Status? Clin. Nutr. 2017, 36, 364–370. [Google Scholar] [CrossRef]
- Matsunaga, T.; Miyata, H.; Sugimura, K.; Motoori, M.; Asukai, K.; Yanagimoto, Y.; Yamamoto, K.; Akita, H.; Nishimura, J.; Wada, H.; et al. Prognostic Significance of C-Reactive Protein-to-Prealbumin Ratio in Patients with Esophageal Cancer. Yonago Acta Med. 2020, 63, 8–19. [Google Scholar] [CrossRef]
- Llop-Talaveron, J.; Badia-Tahull, M.B.; Leiva-Badosa, E. An Inflammation-Based Prognostic Score, the C-Reactive Protein/Albumin Ratio Predicts the Morbidity and Mortality of Patients on Parenteral Nutrition. Clin. Nutr. 2018, 37, 1575–1583. [Google Scholar] [CrossRef]
- Ignacio de Ulíbarri, J.; González-Madroño, A.; de Villar, N.G.P.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A Tool for Controlling Nutritional Status. First Validation in a Hospital Population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Detsky, A.; McLaughlin, J.R.; Baker, J.; Johnston, N.; Whittaker, S.; Mendelson, R.; Jeejeebhoy, K. What Is Subjective Global Assessment of Nutritional Status? J. Parenter. Enter. Nutr. 1987, 11, 8–13. [Google Scholar] [CrossRef]
- Jensen, G.L.; Cederholm, T.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; de Baptista, G.A.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM Criteria for the Diagnosis of Malnutrition: A Consensus Report from the Global Clinical Nutrition Community. J. Parenter. Enter. Nutr. 2019, 43, 32–40. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Procedures for the Collection of Diagnostic Blood Specimens by Venipuncture, 6th ed.; Approved Standard; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2007; CLSI Document GP41-A6. [Google Scholar]
- Doumas, B.T. Biggs HG Determination of Serum Albumin. In Standard Methods of Clinical Chemistry; Cooper, C.A., Ed.; Academic Press, Inc.: New York, NY, USA, 1972; p. 175. [Google Scholar]
- Hamlin, C.R.; Pankowsky, D.A. Turbidimetric Determination of Transthyretin (Prealbumin) with a Centrifugal Analyzer. Clin. Chem. 1987, 33, 144–146. [Google Scholar] [CrossRef]
- Keevil, B.G.; Nicholls, S.P.; Kilpatrick, E.S. Evaluation of a Latex-Enhanced Immunoturbidimetric Assay for Measuring Low Concentrations of C-Reactive Protein. Ann. Clin. Biochem. 1998, 35 Pt 5, 671–673. [Google Scholar] [CrossRef]
- Li, L.; Dai, L.; Wang, X.; Wang, Y.; Zhou, L.; Chen, M.; Wang, H. Predictive Value of the C-Reactive Protein-to-Prealbumin Ratio in Medical ICU Patients. Biomark. Med. 2017, 11, 329–337. [Google Scholar] [CrossRef]
- Kano, H.; Midorikawa, Y.; Song, P.; Nakayama, H.; Moriguchi, M.; Higaki, T.; Tsuji, S.; Takayama, T. High C-Reactive Protein/Albumin Ratio Associated with Reduced Survival Due to Advanced Stage of Intrahepatic Cholangiocarcinoma. Biosci. Trends 2020, 14, 304–309. [Google Scholar] [CrossRef]
- Liao, C.-K.; Yu, Y.-L.; Lin, Y.-C.; Hsu, Y.-J.; Chern, Y.-J.; Chiang, J.-M.; You, J.-F. Prognostic Value of the C-Reactive Protein to Albumin Ratio in Colorectal Cancer: An Updated Systematic Review and Meta-Analysis. World J. Surg. Oncol. 2021, 19, 139. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Li, J.; Ke, Q.; Wang, L.; Cao, Y.; Liu, J. Clinical Significance of C-Reactive Protein to Albumin Ratio in Patients with Hepatocellular Carcinoma: A Meta-Analysis. Dis. Mark. 2020, 2020, 4867974. [Google Scholar] [CrossRef]
- Özdemir, İ.H.; Özlek, B.; Özen, M.B.; Gündüz, R.; Çetin, N.; Özlek, E.; Yıldız, B.S.; Bilge, A.R. Prognostic Value of C-Reactive Protein/Albumin Ratio in Hypertensive COVID-19 Patients. Clin. Exp. Hypertens. 2021, 43, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.; Ates, I.; Akpinar, M.Y.; Yuksel, M.; Kuzu, U.B.; Kacar, S.; Coskun, O.; Kayacetin, E. Predictive Value of C-Reactive Protein/Albumin Ratio in Acute Pancreatitis. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Sonsöz, M.R.; Karadamar, N.; Yılmaz, H.Ç.; Eroğlu, Z.; Şahin, K.K.; Özateş, Y.; Güler, A.; Tekkeşin, A.İ. C-Reactive Protein to Albumin Ratio Predicts In-Hospital Mortality in Patients with Acute Heart Failure. Turk. Kardiyol. Dern. Ars. 2023, 51, 174–181. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, L.; Sun, F.; Lv, B.; Ge, X.; Shao, L.; Liu, S. C-Reactive Protein/Albumin Ratio on the First Day after Surgery Predicts Short-Term Complications of Gastrectomy for Gastric Cancer. Nutr. Cancer 2022, 74, 3574–3581. [Google Scholar] [CrossRef]
- Paliogiannis, P.; Deidda, S.; Maslyankov, S.; Paycheva, T.; Farag, A.; Mashhour, A.; Misiakos, E.; Papakonstantinou, D.; Mik, M.; Losinska, J.; et al. C Reactive Protein to Albumin Ratio (CAR) as Predictor of Anastomotic Leakage in Colorectal Surgery. Surg. Oncol. 2021, 38, 101621. [Google Scholar] [CrossRef]
- Ren, H.; Zhao, L.; Liu, Y.; Tan, Z.; Luo, G.; Deng, X. The High-Sensitivity C-Reactive Protein to Prealbumin Ratio Predicts Adverse Cardiovascular Events after ST-Elevation Myocardial Infarction. Heart Surg. Forum 2021, 24, E153–E157. [Google Scholar] [CrossRef]
- Reid, M.; Badaloo, A.; Forrester, T.; Morlese, J.F.; Heird, W.C.; Jahoor, F. The Acute-Phase Protein Response to Infection in Edematous and Nonedematous Protein-Energy Malnutrition. Am. J. Clin. Nutr. 2002, 76, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Manary, M.J.; Yarasheski, K.E.; Berger, R.; Abrams, E.T.; Hart, C.A.; Broadhead, R.L. Whole-Body Leucine Kinetics and the Acute Phase Response during Acute Infection in Marasmic Malawian Children. Pediatr. Res. 2004, 55, 940–946. [Google Scholar] [CrossRef]
- Amesty-Valbuena, A.; Pereira, N.; Castillo, J.L.; García, D.; Nuñez, J.R.; Cayana, N.; Morán, A.; Parra, M.A.; Troconiz, C. Inflammation mediators (C reactive protein) in children with proteic-energetic malnutrition and in eutrophic children. Investig. Clin. 2004, 45, 53–62. [Google Scholar]
- Doherty, J.F.; Golden, M.H.; Raynes, J.G.; Griffin, G.E.; McAdam, K.P. Acute-Phase Protein Response Is Impaired in Severely Malnourished Children. Clin. Sci. 1993, 84, 169–175. [Google Scholar] [CrossRef]
| Total Patients (n = 274) | CP Ratio < 0.153 (n = 110) | CP Ratio ≥ 0.153 (n = 164) | p | CA Ratio < 0.040 (n = 76) | CA Ratio ≥ 0.040 (n = 198) | p | ||
|---|---|---|---|---|---|---|---|---|
| Age | 65 (SD 17) | 64 (SD 18) | 66 (SD 16) | 0.303 | 63 (SD 18) | 67 (SD 16) | 0.083 | |
| Sex | Male | 171 (62.4%) | 70 (63.6%) | 101 (61.6%) | 0.731 | 46 (60.5%) | 125 (63.1%) | 0.690 |
| Female | 103 (37.6%) | 40 (36.4%) | 63 (38.4%) | 30 (39.5%) | 73 (36.9%) | |||
| Medical history | Diabetes mellitus | 59 (21.5%) | 22 (20%) | 37 (22.6%) | 0.613 | 15 (19.7%) | 44 (22.2%) | 0.654 |
| Hypertension | 117 (42.7%) | 46 (41.8%) | 71 (43.3% | 0.809 | 31 (40.8%) | 86 (43.4%) | 0.692 | |
| Dyslipidemia | 86 (31.4%) | 33 (30%) | 53 (32.3%) | 0.685 | 23 (30.3%) | 63 (31.8%) | 0.804 | |
| Obesity | 28 (10.2%) | 5 (4.5%) | 23 (14%) | 0.011 | 5 (6.6%) | 23 (11.6%) | 0.218 | |
| IC | 19 (6.9%) | 7 (6.4%) | 12 (7.3%) | 0.886 | 5 (6.6%) | 14 (7.1%) | 0.886 | |
| CHF | 26 (9.5%) | 12 (10.9%) | 14 (8.5%) | 0.551 | 9 (11.8%) | 17 (8.6%) | 0.410 | |
| CKD | 20 (7.3%) | 11 (10%) | 9 (5.5%) | 0.159 | 8 (10.5%) | 12 (6.1%) | 0.203 | |
| Liver disease | 17 (6.2%) | 6 (3.4%) | 11 (6.7%) | 0.673 | 5 (6.6%) | 12 (6.1%) | 0.873 | |
| COPD | 32 (11.7%) | 11 (10%) | 21 (12.8% | 0.479 | 8 (10.5%) | 24 (12.1%) | 0.713 | |
| OSA | 15 (5.5%) | 5 (4.5%) | 10 (6.1%) | 0.580 | 4 (5.3%) | 11 (5.6%) | 0.924 | |
| Cancer | 134 (48.9%) | 49 (44.5%) | 85 (51.8%) | 0.273 | 31 (40.8%) | 103 (52%) | 0.096 | |
| Cause of admission | Surgery | 127 (46.4%) | 42 (38.2%) | 85 (51.8%) | 0.083 | 22 (28.9%) | 105 (53%) | 0.011 |
| Gastrointestinal disease | 33 (12%) | 13 (11.8%) | 20 (12.2%) | 12 (15.8%) | 21 (10.6%) | |||
| Complications of cancer | 29 (10.6%) | 11 (10%) | 18 (11%) | 10 (13.2%) | 19 (9.6%) | |||
| Neurologic disease | 33 (12%) | 19 (17.3%) | 14 (8.5%) | 12 (15.8%) | 21 (10.6%) | |||
| Others | 52 (19%) | 25 (22.7%) | 27 (16.5%) | 20 (26.3%) | 32 (16.2%) | |||
| CONUT screening | No risk | 126 (47.5%) | 69 (64.5%) | 57 (36.1%) | <0.001 | 49 (67.1%) | 77 (40.1%) | 0.001 |
| Moderate risk | 116 (43.8%) | 33 (30.8%) | 83 (52.5%) | 19 (26%) | 97 (50.5%) | |||
| High risk | 23 (8.7%) | 5 (4.7%) | 18 (11.4%) | 5 (6.8%) | 18 (9.4%) | |||
| SGA | A | 71 (25.9%) | 25 (22.7%) | 46 (28%) | 0.013 | 19 (25%) | 52 (26.3%) | 0.202 |
| B | 123 (44.9%) | 42 (38.2%) | 81 (49.4%) | 29 (38.2%) | 94 (47.5%) | |||
| C | 80 (29.2%) | 43 (39.1%) | 37 (22.6%) | 28 (36.8%) | 52 (26.3%) | |||
| DRM according to GLIM | No | 84 (30.7%) | 29 (26.4%) | 55 (33.5%) | 0.023 | 23 (30.3%) | 61 (30.8%) | 0.185 |
| Moderate | 92 (33.6%) | 31 (28.2%) | 61 (37.2%) | 20 (26.3%) | 72 (36.4%) | |||
| Severe | 98 (35.8%) | 50 (45.4%) | 48 (29.3%) | 33 (43.4%) | 65 (32.8%) | |||
| GLIM phenotypic criteria † | No criteria | 83 (30.3%) | 28 (25.5%) | 55 (33.5%) | 0.010 | 22 (28.9%) | 61 (30.8%) | 0.065 |
| 1 | 18 (6.6%) | 5 (4.5%) | 13 (7.9%) | 4 (5.3%) | 14 (7.1%) | |||
| 2 | 4 (1.4%) | 0 | 4 (2.4%) | 0 | 4 (2%) | |||
| 3 | 32 (11.7%) | 8 (7.3%) | 24 (14.6%) | 7 (9.2%) | 25 (12.6%) | |||
| 1 + 2 | 12 (4.4%) | 4 (3.6%) | 8 (4.9%) | 1 (1.3%) | 11 (5.6%) | |||
| 1 + 3 | 60 (21.9%) | 27 (24.5%) | 33 (20.1%) | 16 (21.1%) | 44 (22.2%) | |||
| 2 + 3 | 9 (3.3%) | 6 (5.5%) | 3 (1.8%) | 6 (7.9%) | 3 (1.5%) | |||
| 1 + 2 + 3 | 56 (20.4%) | 32 (29.1%) | 24 (14.6%) | 20 (26.3%) | 36 (18.2%) | |||
| GLIM etiologic criteria †† | No criteria | 9 (3.3%) | 8 (7.3%) | 1 (0.6%) | <0.001 | 8 (10.5%) | 1 (0.5%) | <0.001 |
| 1 | 39 (14.2%) | 33 (30%) | 6 (3.7%) | 32 (42.1%) | 7 (3.5%) | |||
| 2 | 29 (10.6%) | 10 (9.1%) | 19 (11.6%) | 5 (6.6%) | 24 (12.1%) | |||
| 1 + 2 | 197 (71.9%) | 59 (53.6%) | 138 (84.1%) | 31 (40.8%) | 166 (83.8%) | |||
| LOS | 20 (IQR 26) | 21 (IQR 32) | 20(IQR 19) | 0.970 | 20 (IQR 33) | 20.5 (IQR 23) | 0.729 | |
| Complications | Total | 166 (60.6%) | 65 (59.1%) | 101 (61.6%) | 0.679 | 41 (53.9%) | 125 (63.1%) | 0.164 |
| Infectious | 111 (40.5%) | 43 (39.1%) | 68 (41.5%) | 0.695 | 31 (40.8%) | 80 (40.4%) | 0.954 | |
| Non-infectious | 124 (45.3%) | 47 (42.7%) | 77 (47%) | 0.491 | 27 (35.5%) | 97 (49%) | 0.045 | |
| Mortality | 50 (18.2%) | 7 (6.4%) | 43 (26.2%) | <0.001 | 3 (3.9%) | 47 (23.7%) | <0.001 | |
| Readmissions | 72 (26.3%) | 28 (25.5%) | 44 (26.8%) | 0.834 | 15 (19.7%) | 57 (28.8%) | 0.141 |
| CP Ratio AUC | CA Ratio AUC | p | |
|---|---|---|---|
| Mortality | 0.644, 95%CI (0.571 to 0.717) | 0.593, 95%CI (0.518 to 0.669) | 0.029 |
| Total complications | 0.555, 95%CI (0.485 to 0.624) | 0.559, 95%CI (0.489 to 0.629) | 0.731 |
| Infectious complications | 0.539, 95%CI (0.468 to 0.610) | 0.533, 95% CI (0.463 to 0.604) | 0.720 |
| Non-infectious complications | 0.558, 95%CI (0.490 to 0.626) | 0.561, 95%CI (0.493 to 0.629) | 0.851 |
| Readmission | 0.529, 95% CI (0.452 to 0.605) | 0.477, 95%CI (0.402 to 0.552) | 0.499 |
| SGA A (vs. B or C) | 0.546, 95%CI (0.465 to 0.628) | 0.572, 95%CI (0.489 to 0.655) | 0.054 |
| SGA B (vs. C) | 0.614, 95% CI (0.534 to 0.695) | 0.640, 95%CI (0.563 to 0.718) | 0.136 |
| SGA C (vs. A or B) | 0.611, 95%CI (0.537 to 0.685) | 0.643, 95% CI (0.573 to 0.712) | 0.039 |
| DRM according to GLIM (vs. no DRM) | 0.535, 95% CI (0.460 to 0.610) | 0.549, 95%CI (0.473 to 0.625) | 0.375 |
| Severe DRM (vs. moderate) | 0.643, 95%CI (0.564 to 0.722) | 0.650, 95%CI (0.572 to 0.728) | 0.662 |
| Sensitivity | Specificity | PPV | NPV | ||
|---|---|---|---|---|---|
| Prognostic accuracy for predicting mortality | CP ratio ≥ 0.153 | 0.86, 95%CI (0.73 to 0.94) | 0.46, 95%CI (0.39 to 0.53) | 0.26, 95%CI (0.20 to 0.34) | 0.94, 95%CI (0.87 to 0.97) |
| CA ratio ≥ 0.040 | 0.94, 95%CI (0.83 to 0.99) | 0.33, 95%CI (0.26 to 0.39) | 0.24, 95%CI (0.18 to 0.33) | 0.96, 95%CI (0.89 to 0.99) | |
| Diagnostic accuracy for identifying severe DRM | CP ratio < 0.237 | 0.61, 95%CI (0.51 to 0.71) | 0.64, 95%CI (0.53 to 0.74) | 0.65, 95%CI (0.54 to 0.74) | 0.61, 95%CI (0.50 to 0.71) |
| CA ratio < 0.273 | 0.85, 95%CI (0.76 to 0.91) | 0.42, 95%CI (0.32 to 0.53) | 0.61, 95%CI (0.52 to 0.69) | 0.72, 95%CI (0.58 to 0.84). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Moreno, R.M.; Mola Reyes, L.; López-Plaza, B.; Palma Milla, S. C-Reactive Protein-to-Prealbumin and C-Reactive Protein-to-Albumin Ratios as Nutritional and Prognostic Markers in Hospitalized Patients—An Observational Study. Nutrients 2024, 16, 2610. https://doi.org/10.3390/nu16162610
García-Moreno RM, Mola Reyes L, López-Plaza B, Palma Milla S. C-Reactive Protein-to-Prealbumin and C-Reactive Protein-to-Albumin Ratios as Nutritional and Prognostic Markers in Hospitalized Patients—An Observational Study. Nutrients. 2024; 16(16):2610. https://doi.org/10.3390/nu16162610
Chicago/Turabian StyleGarcía-Moreno, Rosa M., Laura Mola Reyes, Bricia López-Plaza, and Samara Palma Milla. 2024. "C-Reactive Protein-to-Prealbumin and C-Reactive Protein-to-Albumin Ratios as Nutritional and Prognostic Markers in Hospitalized Patients—An Observational Study" Nutrients 16, no. 16: 2610. https://doi.org/10.3390/nu16162610
APA StyleGarcía-Moreno, R. M., Mola Reyes, L., López-Plaza, B., & Palma Milla, S. (2024). C-Reactive Protein-to-Prealbumin and C-Reactive Protein-to-Albumin Ratios as Nutritional and Prognostic Markers in Hospitalized Patients—An Observational Study. Nutrients, 16(16), 2610. https://doi.org/10.3390/nu16162610






