Impact of Iron Intake and Reserves on Cognitive Function in Young University Students
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Subjects
2.2. Study Variables
2.2.1. Personal and Socio-Demographic, Lifestyle Data
2.2.2. Physical Activity and Energy Expenditure Data
2.2.3. Anthropometric Data
2.2.4. Dietary Data
2.2.5. Hematological and Biochemical Data
2.2.6. Cognitive Function Study
2.3. Statistical Analysis
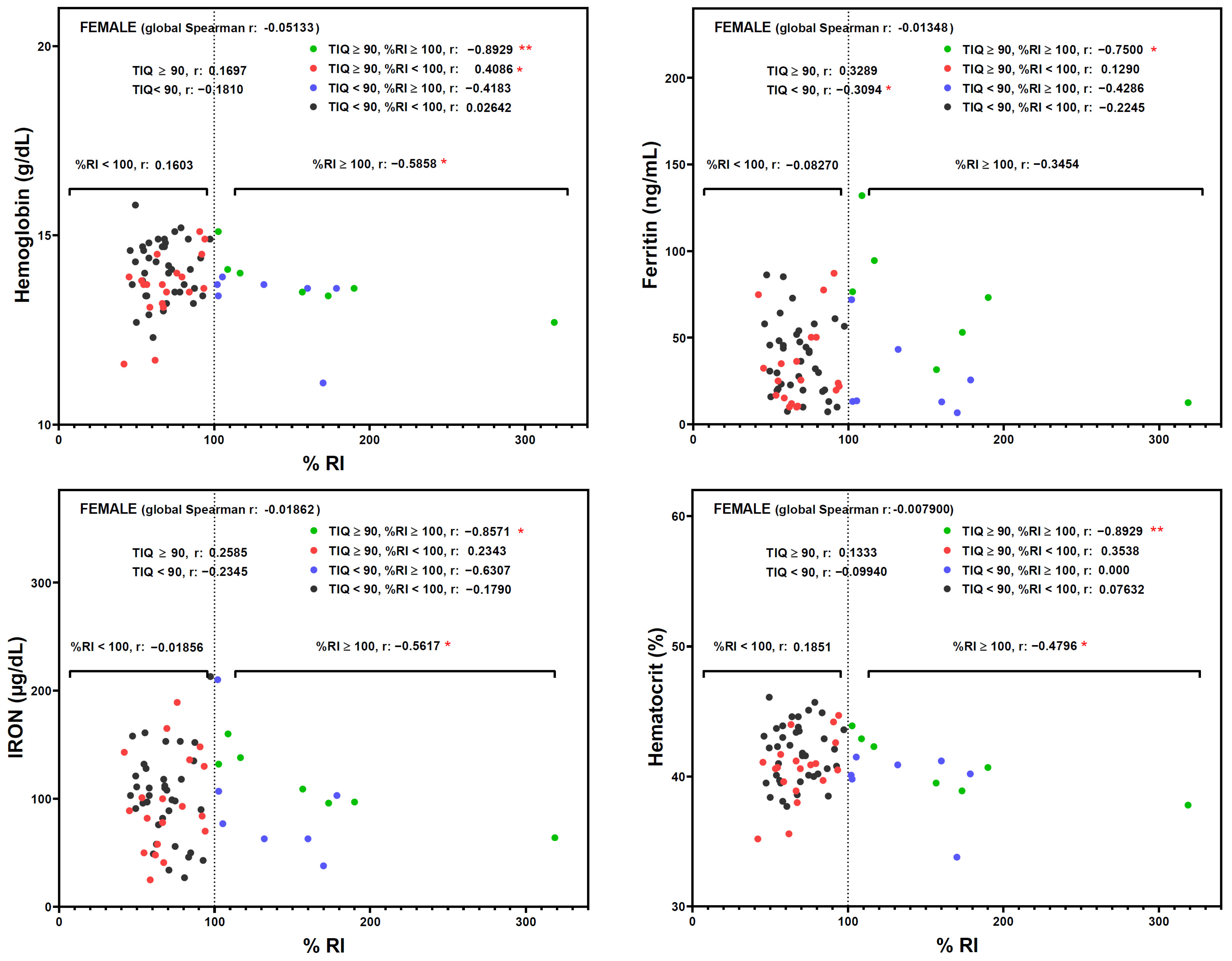
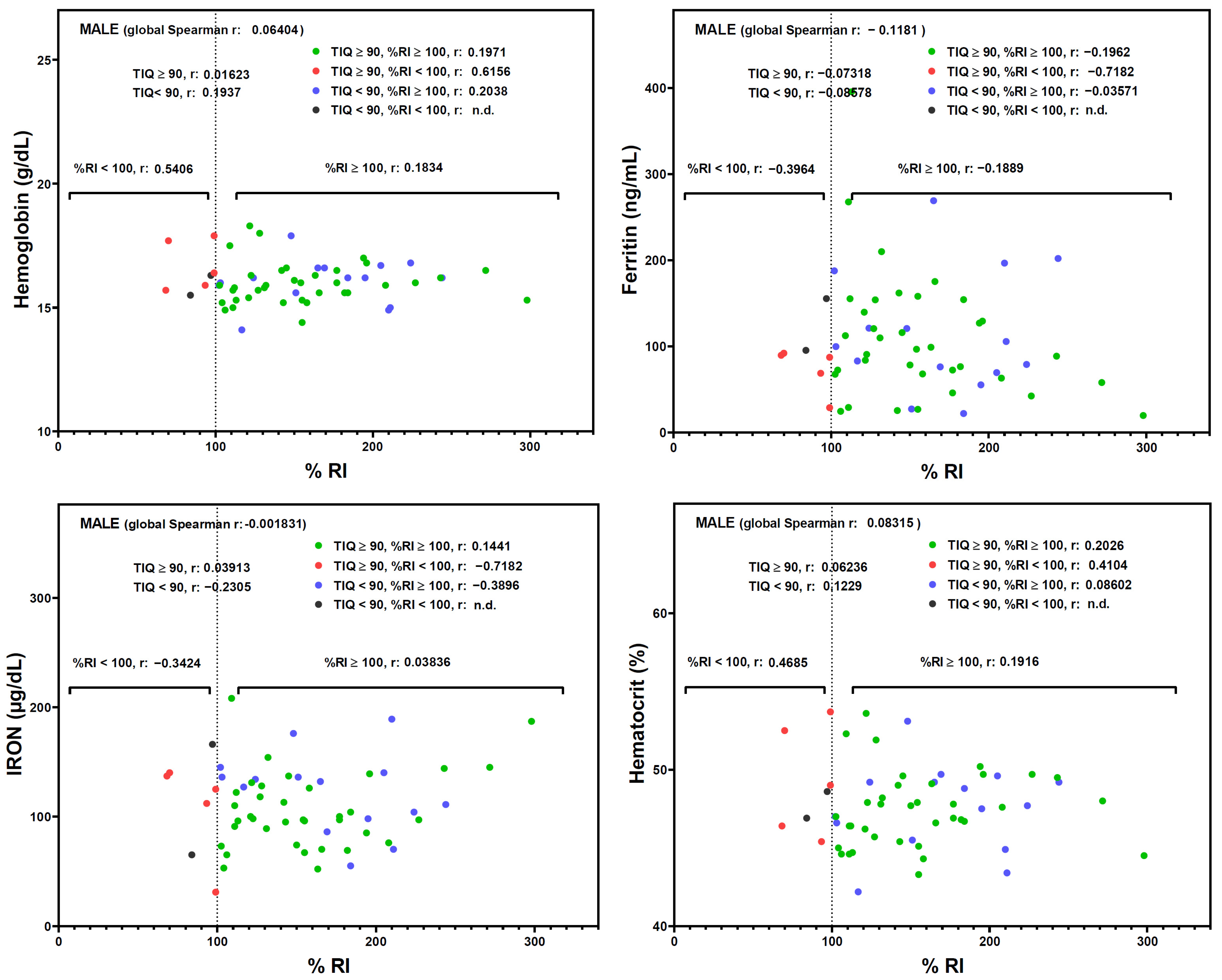
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organisation. Nutrition: Iron Deficiency Anaemia. Available online: http://www.who.int/nutrition/topics/ida/en/ (accessed on 20 September 2023).
- Camaschella, C. Iron deficiency. Blood 2019, 133, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Soppi, E.T. Iron deficiency without anemia—A clinical challenge. Clin. Case Rep. 2018, 6, 1082–1086. [Google Scholar] [CrossRef]
- Stevens, G.A.; Paciorek, C.J.; Flores-Urrutia, M.C.; Borghi, E.; Namaste, S.; Wirth, J.P.; Suchdev, P.S.; Ezzati, M.; Rohner, F.; Flaxman, S.R.; et al. National, regional, and global estimates of anaemia by severity in women and children for 2000-19: A pooled analysis of population-representative data. Lancet Glob. Health 2022, 10, e627–e639. [Google Scholar] [CrossRef]
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Q.; Feng, Y.; Zeng, Y. Iron Deficiency and Iron Deficiency Anemia: Potential risk factors in bone loss. Int. J. Mol. Sci. 2023, 24, 6891. [Google Scholar] [CrossRef] [PubMed]
- Berthou, C.; Iliou, J.P.; Barba, D. Iron, neuro-bioavailability and depression. EJHaem 2022, 3, 263–275. [Google Scholar] [CrossRef]
- Gong, L.; Sun, J.; Cong, S. Levels of iron and iron-related proteins in Alzheimer’s disease: A systematic review and meta-analysis. J. Trace Elem. Med. Biol. 2023, 80, 127304. [Google Scholar] [CrossRef]
- Zhang, N.; Yu, X.; Xie, J.; Xu, H. New insights into the role of ferritin in iron homeostasis and neurodegenerative diseases. Mol. Neurobiol. 2021, 58, 2812–2823. [Google Scholar] [CrossRef]
- Fehsel, K. Why is iron deficiency/anemia linked to Alzheimer’s disease and its comorbidities, and how is it prevented? Biomedicines 2023, 11, 2421. [Google Scholar] [CrossRef]
- Heidelbaugh, J.J. Proton pump inhibitors and risk of vitamin and mineral deficiency: Evidence and clinical implications. Ther. Adv. Drug Saf. 2013, 4, 125–133. [Google Scholar] [CrossRef]
- Munro, M.G.; Mast, A.E.; Powers, J.M.; Kouides, P.A.; O’Brien, S.H.; Richards, T.; Lavin, M.; Levy, B.S. The relationship between heavy menstrual bleeding, iron deficiency, and iron deficiency anemia. Am. J. Obstet. Gynecol. 2023, 229, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pantopoulos, K. Regulation of cellular iron metabolism. Biochem. J. 2011, 434, 365–381. [Google Scholar] [CrossRef]
- Ganz, T. Hepcidin—A regulator of intestinal iron absorption and iron recycling by macrophages. Best Pract. Res. Clin. Haematol. 2005, 18, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, P.; Humeres, A. Iron deficiency on neuronal function. Biometals 2012, 25, 825–835. [Google Scholar] [CrossRef]
- Pivina, L.; Semenova, Y.; Doşa, M.D.; Dauletyarova, M.; Bjørklund, G. Iron deficiency, cognitive functions, and neurobehavioral disorders in children. J. Mol. Neurosci. 2019, 68, 1–10. [Google Scholar] [CrossRef]
- Choe, Y.M.; Suh, G.H.; Lee, B.C.; Choi, I.G.; Lee, J.H.; Kim, H.S.; Kim, J.W. Association between copper and global cognition and the moderating effect of iron. Front. Aging Neurosci. 2022, 14, 811117. [Google Scholar] [CrossRef] [PubMed]
- Fretham, S.J.; Carlson, E.S.; Georgieff, M.K. The role of iron in learning and memory. Adv. Nutr. 2011, 2, 112–121. [Google Scholar] [CrossRef]
- Jáuregui-Lobera, I. Iron deficiency and cognitive functions. Neuropsychiatr. Dis. Treat. 2014, 10, 2087–2095. [Google Scholar] [CrossRef]
- Falkingham, M.; Abdelhamid, A.; Curtis, P.; Fairweather-Tait, S.; Dye, L.; Hooper, L. The effects of oral iron supplementation on cognition in older children and adults: A systematic review and meta-analysis. Nutr. J. 2010, 9, 4. [Google Scholar] [CrossRef]
- Shill, K.B.; Karmakar, P.; Kibria, M.G.; Das, A.; Rahman, M.A.; Hossain, M.S.; Sattar, M.M. Prevalence of iron-deficiency anaemia among university students in Noakhali region, Bangladesh. J. Health Popul. Nutr. 2014, 32, 103–110. [Google Scholar]
- Arija, V.; Fernandez, J.; Salas, J. Iron deficiency and ferropenic anemia in the Spanish population. Med. Clin. 1997, 109, 425–430. [Google Scholar]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.A.; Wagner, P.D. Exercise-induced arterial hypoxemia. J. Appl. Physiol. 1999, 87, 1997–2006. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.W.; Ramseth, D.J.; Chanter, D.O.; Moon, T.E.; Goodman, D.B.; Mendzelevski, B. Electrocardiographic reference ranges derived from 79,743 ambulatory subjects. J. Electrocardiol. 2007, 40, 228–234. [Google Scholar] [CrossRef]
- Ortega, R.M.; Requejo, A.M.; López-Sobaler, A.M. Activity questionnaire. In Nutriguía. Manual of Clinical Nutrition in Primary Care; Requejo, A.M., Ortega, R.M., Eds.; Complutense Madrid: Madrid, Spain, 2006; p. 468. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005; p. 1358. [Google Scholar] [CrossRef]
- World Health Organisation. Energy and protein requirements. Report of a joint FAO/WHO/UNU Expert Consultation. World Health Organ. Tech. Rep. Ser. 1985, 724, 1–206. [Google Scholar]
- Durnin, J.V.; Fidanza, F. Evaluation of nutritional status. Bibl. Nutr. Dieta 1985, 35, 20–30. [Google Scholar]
- Salas-Salvadó, J.; Rubio, M.A.; Barbany, M.; Moreno, B.; de la Seedo, G.C. Consenso SEEDO 2007 para la evaluación del sobrepeso y la obesidad y el establecimiento de criterios de intervención terapéutica. Med. Clín. 2007, 128, 184–196. [Google Scholar] [CrossRef]
- Lean, M.E.; Han, T.S.; Deurenberg, P. Predicting body composition by densitometry from simple anthropometric measurements. Am. J. Clin. Nutr. 1996, 63, 4–14. [Google Scholar] [CrossRef]
- Ortega, R.M.; Requejo, A.M.; López-Sobaler, A.M. Registro de consumo de alimentos y bebidas. In Nutriguía. Manual de Nutrición Clínica en Atención Primaria; Ortega, R.M., Requejo, A.M., Eds.; Editorial Panamericana: Madrid, Spain, 2015. [Google Scholar]
- Ortega, R.M.; Requejo, A.M.; Navia, B.; López Sobaler, A.M.; Aparicio, A. Recommended daily intakes of energy and nutrients for Spanish population. In The Composition of Foods. Basic Tool for Nutritional Assessment; Complutense: Madrid, Spain, 2019. [Google Scholar]
- Department of Nutrition (UCM); Alceingeniería, S.A. DIAL Software for Assessing Diets and Food Calculations; 3.0.0.5 for Windows; Ortega, R.M., López-Sobaler, A.M., Andrés, P., Requejo, A.M., Aparicio, A.M.L., Eds.; Alceingeniería, S.A.: Madrid, Spain, 2013. [Google Scholar]
- Ortega, R.M.; López-Sobaler, A.M.; Andrés, P.; Aparicio, A. Food Nutritional Composition. A Tool for the Design and Evaluation of Food and Diets; Departament of Nutrition and Food Science, Complutense University of Madrid: Madrid, Spain, 2021; Available online: https://www.ucm.es/idinutricion/tablas-de-composicion-nutricional (accessed on 20 January 2020).
- Ortega, R.M.; Quintas, E.; Sánchez, B.; Andrés, P.; Requejo, A.M.; Encinas, A. Underestimation of energy intake in a group of young female university students of Madrid. Rev. Clin. Esp. 1997, 197, 545–549. [Google Scholar]
- Wechsler, D. Escala de Inteligencia de Wechsler para Adultos-IV (WAIS-IV); Pearson Educación: London, UK, 2012. [Google Scholar]
- Ferreira, E.; Calderón, C. Assessment of adults: WAIS-IV. Cognitive Aptitude Assessment. 2022 [Educational Document]. Faculty of Psychology, University of Barcelona. 2022. Available online: http://hdl.handle.net/2445/191582 (accessed on 5 June 2022).
- World Health Organization. Obesity, Preventing and Managing the Global Epidemic-Report of a WHO Consultation on Obesity; World Health Organisation: Geneva, Switzerland, 1997. [Google Scholar]
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation; World Health Organisation: Geneva, Switzerland, 2011. [Google Scholar]
- Hsieh, S.D.; Yoshinaga, H.; Muto, T.; Sakurai, Y.; Kosaka, K. Health risks among Japanese men with moderate body mass index. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 358–362. [Google Scholar] [CrossRef]
- Salas-Salvado, J.; Rubio, M.A.; Barbany, M.; Moreno, B. SEEDO’ 2000 consensus for the evaluation of overweight and obesity and the establishment of therapeutic intervention criteria. Med. Clin. 2000, 115, 587–597. [Google Scholar]
- Andrés, P.; Povea, F. Anexo XII: Valores de referencia para los parámetros hematológicos y bioquímicos indicadores de estado nutricional. In Nutriguía. Manual de Nutrición Clínica en Atención Primaria; Requejo, A., Ortega, R., Eds.; Editorial Complutense: Madrid, Spain, 2000; pp. 509–517. [Google Scholar]
- Painter, P.C.; Smith, J. Appendix. In Tietz Fundamentals of Clinical Chemistry; Burtis, C.A., Ashwood, E.R., Eds.; W.B. Saunders Company: Philadelphia, PA, USA, 1996; pp. 766–830. [Google Scholar]
- World Health Organisation. WHO Guideline on Use of Ferritin Concentrations to Assess Iron Status in Individuals and Populations; World Health Organisation: Geneva, Switzerland, 2020. [Google Scholar]
- Iglesias, M.T.; Mata, G.; Pérez, A.; Hernández, S.; García-Chico, R.; Papadaki, C. Nutritional status of students at university in Madrid. Nutr. Clin. Y Diet. Hosp. 2013, 33, 23–30. [Google Scholar]
- Gallo, L.A.; Gallo, T.F.; Young, S.L.; Fotheringham, A.K.; Barclay, J.L.; Walker, J.L.; Moritz, K.M.; Akison, L.K. Adherence to dietary and physical activity guidelines in Australian undergraduate biomedical students and associations with body composition and metabolic health: A cross-sectional study. Nutrients 2021, 13, 3500. [Google Scholar] [CrossRef]
- Martinez Roldan, C.; Veiga Herreros, P.; Lopez de Andres, A.; Cobo Sanz, J.M.; Carbajal Azcona, A. Nutritional status assessment in a group of university students by means of dietary parameters and body composition. Nutr. Hosp. 2005, 20, 197–203. [Google Scholar]
- García-Meseguer, M.J.; Burriel, F.C.; García, C.V.; Serrano-Urrea, R. Adherence to Mediterranean diet in a Spanish university population. Appetite 2014, 78, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Beaudry, K.M.; Ludwa, I.A.; Thomas, A.M.; Ward, W.E.; Falk, B.; Josse, A.R. First-year university is associated with greater body weight, body composition and adverse dietary changes in males than females. PLoS ONE 2019, 14, e0218554. [Google Scholar] [CrossRef]
- Kremmyda, L.S.; Papadaki, A.; Hondros, G.; Kapsokefalou, M.; Scott, J.A. Differentiating between the effect of rapid dietary acculturation and the effect of living away from home for the first time, on the diets of Greek students studying in Glasgow. Appetite 2008, 50, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Jodhun, B.M.; Pem, D.; Jeewon, R. A systematic review of factors affecting energy intake of adolescent girls. Afr. Health Sci. 2016, 16, 910–922. [Google Scholar] [CrossRef]
- Lacaille, L.J.; Dauner, K.N.; Krambeer, R.J.; Pedersen, J. Psychosocial and environmental determinants of eating behaviors, physical activity, and weight change among college students: A qualitative analysis. J. Am. Coll. Health 2011, 59, 531–538. [Google Scholar] [CrossRef]
- Gallardo-Escudero, A.; Mata-Soto, C.; Fernández-García, M.; Rodríguez-Felices, Y.; Lisbona, F.; Mjm, A.; Aliaga, L.; Planells, E. Assessment of iron status in a group of students at the university of Granada: Lifestyle influence. Ars Pharm. 2010, 51, 375–388. [Google Scholar]
- Samaniego-Vaesken, M.L.; Partearroyo, T.; Olza, J.; Aranceta-Bartrina, J.; Gil, A.; Gonzalez-Gross, M.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G. Iron intake and dietary sources in the Spanish population: Findings from the ANIBES study. Nutrients 2017, 9, 203. [Google Scholar] [CrossRef]
- De Piero, A.; Bassett, N.; Rossi, A.; Sammán, N. Trends in food consumption of university students. Nutr. Hosp. 2015, 31, 1824–1831. [Google Scholar] [CrossRef]
- Requejo, A.M.; Ortega, R.M.; Aparicio, A.; López-Sobaler, A.M. El rombo de la alimentación. Guía útil en la planificación de dietas ajustadas a las pautas recomendadas. Nutr. Clín. Diet. Hosp. 2006, 26, 47–55. [Google Scholar]
- Holzer, I.; Ott, J.; Beitl, K.; Mayrhofer, D.; Heinzl, F.; Ebenbauer, J.; Parry, J.P. Iron status in women with infertility and controls: A case-control study. Front. Endocrinol. 2023, 14, 1173100. [Google Scholar] [CrossRef]
- Fayet-Moore, F.; Petocz, P.; Samman, S. Micronutrient status in female university students: Iron, zinc, copper, selenium, vitamin B12 and folate. Nutrients 2014, 6, 5103–5116. [Google Scholar] [CrossRef] [PubMed]
- Cook, R.L.; O’Dwyer, N.J.; Parker, H.M.; Donges, C.E.; Cheng, H.L.; Steinbeck, K.S.; Cox, E.P.; Franklin, J.L.; Garg, M.L.; Rooney, K.B.; et al. Iron deficiency anemia, not iron deficiency, is associated with reduced attention in healthy young women. Nutrients 2017, 9, 1216. [Google Scholar] [CrossRef]
- Ortega, R.M.; Gonzalez-Fernandez, M.; Paz, L.; Andres, P.; Jimenez, L.M.; Jimenez, M.J.; Gonzalez-Gross, M.; Requejo, A.M.; Gaspar, M.J. Influence of iron status on attention and intellectual performance of a population of Spanish adolescents. Arch. Latinoam. Nutr. 1993, 43, 6–11. [Google Scholar]
- Milman, N.; Clausen, J.; Byg, K.E. Iron status in 268 Danish women aged 18-30 years: Influence of menstruation, contraceptive method, and iron supplementation. Ann. Hematol. 1998, 77, 13–19. [Google Scholar] [CrossRef]
- Juul, S.E.; Derman, R.J.; Auerbach, M. Perinatal iron deficiency: Implications for mothers and infants. Neonatology 2019, 115, 269–274. [Google Scholar] [CrossRef]
- Ibañez-Alcalde, M.M.; Vazquez-Lopez, M.A.; Lopez-Ruzafa, E.; Lendinez-Molinos, F.J.; Bonillo-Perales, A.; Parron-Carreno, T. Prevalence of iron deficiency and related factors in Spanish adolescents. Eur. J. Pediatr. 2020, 179, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Grille, S.; Lorenzo, M.; Acosta, S.; Acosta, N.; Correa, S.; Corral, M.; Quintanilla, C.; Ragone, R.; Guillermo, C. Iron deficiency in reproductive age university women at the School of Medicine, Uruguay. Rev. Fac. Cien Med. Univ. Nac. Cordoba 2020, 77, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Saxena, Y.; Shrivastava, A.; Saxena, V. Effect of gender on correlation of anaemia with body mass index in medical students. Indian J. Physiol. Pharmacol. 2011, 55, 364–369. [Google Scholar]
- Rani, N.A.; Arasegowda, R.; Mukherjee, P.; Dhananjay, S.Y. Prevalence of nutritional deficiency anaemia and its impact on scholastic performance among undergraduate medical students. J. Clin. Diagn. Res. 2017, 11, BC21–BC23. [Google Scholar] [CrossRef]
- Amoaning, R.E.; Amoako, E.S.; Kyiire, G.A.; Owusu, D.D.; Bruce, H.; Simpong, D.L.; Adu, P. Anaemia prevalence more than doubles in an academic year in a cohort of tertiary students: A repeated-measure study in Cape Coast, Ghana. Adv. Hematol. 2022, 2022, 4005208. [Google Scholar] [CrossRef]
- Petranovic, D.; Batinac, T.; Petranovic, D.; Ruzic, A.; Ruzic, T. Iron deficiency anaemia influences cognitive functions. Med. Hypotheses 2008, 70, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Stoltzfus, R.J.; Mullany, L.; Black, R.E. Iron deficiency anaemia. In Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors; Ezzati, M., Lopez, A.D., Rodgers, A., Murray, C.J.L., Eds.; World Health Organization: Geneva, Switzerland, 2004; Volume 1, pp. 163–209. [Google Scholar]
- Khedr, E.; Hamed, S.A.; Elbeih, E.; El-Shereef, H.; Ahmad, Y.; Ahmed, S. Iron states and cognitive abilities in young adults: Neuropsychological and neurophysiological assessment. Eur. Arch. Psychiatry Clin. Neurosci. 2008, 258, 489–496. [Google Scholar] [CrossRef]
- More, S.; Shivkumar, V.B.; Gangane, N.; Shende, S. Effects of iron deficiency on cognitive function in school going adolescent females in rural area of central India. Anemia 2013, 2013, 819136. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Kanani, S.J. Deleterious functional impact of anemia on young adolescent school girls. Indian Pediatr. 2006, 43, 219–226. [Google Scholar]
- Low, M.; Farrell, A.; Biggs, B.A.; Pasricha, S.R. Effects of daily iron supplementation in primary-school-aged children: Systematic review and meta-analysis of randomized controlled trials. Can. Med Assoc. J. 2013, 185, E791–E802. [Google Scholar] [CrossRef]
- Samson, K.L.I.; Fischer, J.A.J.; Roche, M.L. Iron status, anemia, and iron interventions and their associations with cognitive and academic performance in adolescents: A systematic review. Nutrients 2022, 14, 224. [Google Scholar] [CrossRef] [PubMed]


| Female | Male | |
|---|---|---|
| Personal and Lifestyle data | ||
| Age (years) | 19.7 ± 1.6 | 21.2 ± 2.6 *** |
| Place of cohabitation (%) | ||
| Living with parents | 13.4 | 29.5 |
| Student flat | 71.6 | 52.5 |
| Student residence | 14.9 | 18 |
| Smoking habit (%) | ||
| Non-smoker | 67.6 | 78.7 |
| Ex-smoker | 12.5 | 10.4 |
| Smoker | 32.4 | 21.3 |
| Cigarettes/day | 6.0 ± 4.8 | 6.6 ± 4.9 |
| Alcohol consumption (g/day) | 4.1 ± 9.2 | 5.5 ± 13.1 |
| Health data | ||
| Systolic blood pressure (mmHg) (normal values) ref. [24] | 96.5 ± 11.3 (120–129) | 108.4 ± 10.6 *** (120–129) |
| Diastolic blood pressure (mmHg) (normal values) ref. [24] | 60.7 ± 10.8 (80–84) | 68.7 ± 9.7 *** (80–84) |
| Oxygen saturation (%) (normal values) ref. [25] | 97.2 ± 1.5 (>95) | 96.4 ± 4.6 (>95) |
| Heart rate (beats/min) (normal values) ref. [26] | 84.8 ± 13.0 (60–90) | 80.0 ± 12.6 * (60–90) |
| Anthropometric data | ||
| Weight (kg) | 59.2 ± 8.9 | 76 ± 10.9 *** |
| Height (cm) | 161.7 ± 5.7 | 174.6 ± 7.0 *** |
| BMI (kg/m2) | 22.6 ± 3.0 | 25.0 ± 3.9 *** |
| Weight status (%) ref. [31] | ||
| Underweight (BMI < 18.5) | 4.2 | 0.0 |
| Normal weight (BMI: 18.5–24.9) | 77.5 | 54.1 |
| Overweight (BMI: 25.0–29.9) | 16.9 | 34.4 |
| Obese (BMI ≥ 30.0) | 1.4 | 11.5 |
| Waist circumference (cm) | 75.9 ± 8.7 | 88.2 ± 9.4 *** |
| cardiometabolic risk, ref. [31] | ||
| Very low | (<80) | (<94) |
| Incremented | (80 to 87) | (94 to 101) |
| High | (≥88) | (≥102) |
| Hip circumference (cm) | 96 ± 13.87 | 100 ± 8.15 * |
| cardiometabolic risk ref [40] | ||
| Very low | (<80) | (<94) |
| Incremented | (80 to 88) | (94 to 102) |
| High | (>88) | (>102) |
| Waist-to-hip ratio | 0.89 ± 0.91 | 0.88 ± 0.05 |
| cardiometabolic risk ref [41] | ||
| Substantially increased | (≥0.85) | (≥0.90) |
| Waist-to-height ratio | 0.47 ± 0.05 | 0.51 ± 0.06 |
| high risk of lifestyle-related disorders ref [42] | (≥0.5) | (≥0.5) |
| Bicipital fold (mm) | 10.2 ± 4.5 | 8.5± 4.9 * |
| Tricipital crease (mm) | 16.6 ± 10.0 | 12.8 ± 6.8 * |
| Subscapular fold (mm) | 17.0 ± 7.3 | 17.0 ± 6.7 |
| Body fat (%) | 32.3 ± 5.40 | 23.3 ± 7.18 |
| reference values ref [43] | ||
| Limit | (31–33%) | (21–25%) |
| Obesity | (>33%) | (>25%) |
| Fat-free mass (%) | 68 ± 5.40 | 77 ± 7.18 *** |
| Female | Male | |
|---|---|---|
| Energy (Kcal/day) | 1881.8 ± 332.7 | 2550.1 ± 587.7 *** |
| RI (Kcal/day) | ||
| 14–19 years | 2250 | 2800 |
| 20–39 years | 2200 | 2700 |
| RI Contribution (%) | 99.6 ± 18.8 | 97.0 ± 24.9 |
| <100% RI (%) | 64.8 | 59.0 |
| <67% RI (%) | 2.8 | 9.8 |
| Energy Expenditure (Kcal/day) | 1906.4 ± 196.6 | 2654.6 ± 202.1 |
| Underestimation (%) | 0.4 | 3.0 |
| Iron intake (mg/day) | 12.7 ± 6.5 | 17.5 ± 10.9 ** |
| Iron intake (mg/Kcal/day) | 6.8 ± 3.6 | 6.7 ± 2.7 |
| RI (mg/day) | ||
| 14–19 years | 15 | 12 |
| 20–39 years | 15 | 10 |
| RI Contribution (%) | 84.5 ± 43.6 | 167.6 ± 109.1 *** |
| <100% RI (%) | 80.3 | 11.5 |
| <67% RI (%) | 39.4 | 0.0 |
| Hematology | ||
| Red blood cells (mill/µL) (normal values) ref. [44] | 4.7 ± 0.3 (3.5–5.0) | 5.4 ± 0.30 *** (4.3–5.9) |
| Hemoglobin (g/dL) (normal values) ref. [45] IDA (%) (cut-off reference value) | 13.9 ± 0.9 (11.7–15.5) 4.2 (<12 g/dL) | 16.1 ± 0.9 *** (13.2–17.3) 0.0 (<13 g/dL) |
| Hematocrit (%) (normal values) ref. [45] Deficiency (%) | 41.2 ± 2.4 (33–43) 4.2 | 47.7 ±2.6 (39–49) 0.0 |
| MCV (µm3) (normal values) ref. [44] | 88.5 ± 4.4 (86–98) | 88.9 ± 3.0 (86–98) |
| MCH (pg) (normal values) ref. [44] | 29.8 ± 1.7 (27–32) | 30.0 ± 1.1 (27–32) |
| MCHC (%) (normal values) ref. [44] | 33.7 ± 0.5 (33–37) | 33.6 ± 0.7 (33–37) |
| RDW (%) (normal values) ref. [44] | 14.7 ± 1.8 (11–18) | 13.2 ± 1.2 *** (11–18) |
| Biochemistry | ||
| Serum ferritin (ng/mL) | 38.1 ± 26.0 | 109.2 ± 68.5 *** |
| (normal values) ref. [3] | (10–130) | (27–300) |
| Mild ID, ferritin < 30 ng/mL (%) | 47.9 | 13.1 |
| ID, ferritin < 15 ng/mL (%) | 21.1 | 0.0 |
| Serum iron (µg/dL) | 100.9 ± 41.8 | 114.3 ± 41.3 |
| (normal values) ref. [44] | (60–160) | (80–180) |
| Deficiency (%) | 4.2 | 6.6 |
| (cut-off reference value) | (<37 µg/dL) | (<59 µg/dL) |
| Food Group | Female | Male |
|---|---|---|
| Cereals and pulses | 4.0 ± 1.3 | 5.6 ± 2.2 *** |
| Vegetables | 2.1 ± 1.1 | 3.1 ± 1.5 *** |
| Fruits and derivatives | 1.1 ± 1.2 | 1.5 ± 1.1 |
| Meat, fish and eggs | 2.6 ± 1.1 | 3.5 ± 1.4 *** |
| Dairy products | 1.5 ± 0.8 | 2.3 ± 1.7 *** |
| Female | Male | |||
|---|---|---|---|---|
| TIQ < 90 (n = 45) | TIQ ≥ 90 (n = 26) | TIQ < 90 (n = 17) | TIQ ≥ 90 (n = 44) | |
| Energy (Kcal/day) | 1867.0 ± 314.1 | 1907.5 ± 367.7 | 2443.6 ± 582.5 | 2591.2 ± 591.2 |
| Contribution RI (%) | 99.6 ±18.4 | 99.6 ± 19.7 | 91.6 ± 24.5 | 99.0 ± 25.0 |
| Under/Over-estimated | 0.4 ± 18.4 | 0.4 ± 19.7 | 8.4 ± 24.5 | 1.0 ± 25.0 |
| Iron (mg/day) | 11.7 ± 4.6 | 14.3 ± 8.8 | 16.7 ± 5.3 | 17.9 ± 12.4 |
| Contribution RI (%) | 78.1 ± 30.7 | 81.6 ± 31.7 | 160.8 ± 50.0 | 170.2 ± 125.1 |
| Hematology | ||||
| Red cells (mill/µL) | 4.7 ± 0.3 | 4.6 ± 0.3 | 5.3 ± 0.3 | 5.4 ± 0.3 |
| Hemoglobin (g/dL) | 14.0 ± 2.2 | 13.7 ± 0.8 | 16.0 ± 0.9 | 16.1 ± 0.9 |
| Hematocrit (%) | 41.5 ± 6.6 | 40.6 ± 2.4 | 47.6 ± 2.6 | 47.7 ± 2.6 |
| MCV (µm3) | 88.9 ± 3.5 | 87.7 ± 5.6 | 89.5 ± 2.5 | 88.6 ± 3.2 |
| MCH (pg) | 30.0 ± 1.5 | 29.5 ± 2.1 | 30.2 ± 1.1 | 29.9 ± 1.1 |
| MCHC (%) | 33.7 ± 0.5 | 33.6 ± 0.5 | 33.6 ± 0.7 | 33.6 ± 0.7 |
| RDW (%) | 14.6 ± 1.1 | 14.8 ± 2.7 | 13.5 ± 0.9 | 13.1 ± 1.2 |
| Biochemistry | ||||
| Ferritin (ng/mL) | 35.5 ± 22.1 | 42.6 ± 32.2 | 115.6 ± 66.8 | 106.8 ± 69.8 |
| Mild ID, ferritin < 30 ng/mL (%) | 48.9 | 46.2 | 11.8 | 13.6 |
| ID, ferritin < 15 ng/mL (%) | 22.2 | 19.2 | 0.0 | 0.0 |
| Iron (µg/dL) | 100.9 ± 44.2 | 101.0 ± 42.0 | 121.8 ± 38.4 | 111.4 ± 42.5 |
| Deficiency (%) | 4.4 | 3.8 | 5.9 | 6.8 |
| Verbal Comprehension Index (VCI) | Working Memory Index (WMI) | Perceptual Reasoning Index (PRI) | Processing Speed Index (PSI) | |||||
|---|---|---|---|---|---|---|---|---|
| Female | <90 (n = 26) | ≥90 (n = 45) | <90 (n = 35) | ≥90 (n = 36) | <90 (n = 41) | ≥90 (n = 30) | <90 (n = 41) | ≥90 (n = 30) |
| Iron (mg/day) | 11.5 ± 4.1 | 13.3 ± 7.6 | 11.9 ± 4.9 | 13.4 ± 7.8 | 12.3 ±5.2 | 13.1 ± 8.1 | 11.9 ± 7.1 | 13.7 ± 5.7 |
| % RI | 76.8 ± 27.1 | 88.9± 50.6 | 79.3 ± 32.9 | 89.5 ± 52.0 | 82.2 ± 34.6 | 87.6 ± 54.2 | 79.5 ± 47.1 | 91.4 ± 38.1 |
| Iron (μg/dL) | 104.4 ± 42.1 | 98.9 ± 41.9 | 99.9 ± 45.1 | 102.0 ± 38.9 | 96.7 ± 37.8 | 106.7 ± 46.7 | 98.2 ± 42.0 | 104.6 ± 41.9 |
| Hb (g/dL) | 13.9 ± 1.0 | 13.8 ± 0.8 | 13.9 ± 0.9 | 13.8 ± 0.9 | 13.8 ± 0.8 | 13.9 ± 0.9 | 13.9 ± 0.9 | 13.9 ± 0.9 |
| Ferritin (ng/mL) | 37.3 ± 21.1 | 38.6 ± 28.7 | 35.4 ± 22.8 | 40.7 ± 28.9 | 34.1 ± 23.0 | 43.6 ± 29.1 | 39.5 ± 26.1 | 36.2 ± 26.2 |
| Male | <90 (n = 1) | ≥90 (n = 60) | <90 (n = 9) | ≥90 (n = 52) | <90 (n = 26) | ≥90 (n = 35) | <90 (n = 47) | ≥90 (n = 14) |
| Iron (mg/day) | 21.1 ± n.d | 17.5 ± 11.0 * | 14.0 ± 4.7 | 18.1 ± 11.6 * | 16.9 ± 7.8 ** | 18.0 ± 12.8 | 18.7 ± 12.0 ** | 13.5 ± 4.0 |
| % RI | 211.0 ± n.d. | 166.8 ± 109.8 *** | 134.5 ± 48.5 *** | 173.3 ± 115.8 *** | 162.3 ± 77.6 *** | 171.5 ± 128.5 ** | 180.3 ± 120.1 *** | 124.8 ± 36.1 ** |
| Iron (μg/dL) | 70.0 ± n.d. | 115.0 ± 41.3 | 105.9 ± 34.6 | 115.7 ± 42.5 | 115.3 ± 34.8 * | 113.5 ± 46.1 | 122.5 ± 40.1 ** | 86.7 ± 33.4 †† |
| Hb (g/dL) | 15.0 ± n.d. | 16.1 ± 0.9 *** | 16.0 ± 0.5 *** | 16.1 ± 0.9 *** | 16.3 ± 0.9 *** | 16.0 ± 0.9 | 16.1 ± 0.9 *** | 16.0 ± 0.9 *** |
| Ferritin (ng/mL) | 105.6 ± n.d. | 109.3 ± 69.1 *** | 96.4 ± 58.3 *** | 111.5 ± 70.4 *** | 115.1 ± 69.2 | 104.9 ± 68.7 *** | 110.5 ± 75.1 *** | 105.0 ± 40.9 *** |
| Female | Male | |||
|---|---|---|---|---|
| %RI < 100 (n = 57) | %RI ≥ 100 (n = 14) | %RI < 100 (n = 7) | %RI ≥ 100 (n = 54) | |
| Energy (Kcal/day) | 1829.1 ± 361.1 | 2096.7 ± 464.4 * | 1969.3 ± 550.4 | 2625.4 ± 553.6 ** |
| Contribution RI (%) | 96.3 ±19.1 | 113.2 ± 27.3 ** | 75.1 ± 23.9 | 99.8 ± 23.8 * |
| Under/Over-estimated | 3,7 ± 14,3 | −13.2 ± 27.3 ** | 24.9 ± 23.9 | 0.2 ± 23.8 * |
| Iron (mg/day) | 10.2 ± 2.5 | 22.7 ± 8.7 *** | 9.4 ± 1.1 | 18.6 ± 11.2 * |
| Contribution RI (%) | 68.1 ± 16.9 | 151.2 ± 58.1 *** | 87.2 ± 13.4 | 178.0 ± 111.7 * |
| Hematology | ||||
| Red cells (mill/ µL) | 4.7 ± 0.7 | 4.6 ± 0.2 | 5.5 ± 0.3 | 5.4 ± 0.3 |
| Hemoglobin (g/dL) | 13.9 ± 2.0 | 13.5 ± 0.9 | 16.5 ± 1.0 | 16.0 ± 0.9 |
| Hematocrit (%) | 41.4 ± 5.9 | 40.3 ± 2.4 | 48.9 ± 3.1 | 47.5 ± 2.5 |
| MCV (µm3) | 88.5 ± 12.5 | 88.3 ± 3.2 | 89.3 ± 3.2 | 88.8 ± 3.0 |
| MCH (pg) | 29.8 ± 4.3 | 29.7 ± 1.3 | 30.1 ± 1.1 | 20.0 ± 1.1 |
| MCHC (%) | 33.7 ± 4.5 | 33.6 ± 0.5 | 33.7 ± 0.6 | 33.6 ± 0.7 |
| RDW (%) | 14.7 ± 2.7 | 14.6 ± 1.3 | 13.6 ± 1.6 | 13.2 ± 1.1 |
| Biochemistry | ||||
| Ferritin (ng/mL) | 35.9 ± 22.4 | 47.2 ± 37.9 | 88.1 ± 37.6 | 112.0 ± 71.3 |
| Mild ID, ferritin < 30 ng/mL (%) | 49.1 | 42.9 | 14.3 | 13.0 |
| ID, ferritin < 15 ng/mL (%) | 17.5 | 35.7 | 0.0 | 0.0 |
| Iron (µg/dL) | 100.2 ± 43.0 | 104.1 ± 45.2 | 110.9 ± 47.0 | 114.7 ± 41.0 |
| Deficiency (%) | 5.3 | 7.1 | 14.3 | 5.6 |
| TIQ < 90 | TIQ ≥ 90 | ||||
| <100% RI (n = 38) | ≥100% RI (n = 7) | <100% RI (n = 19) | ≥100% RI (n = 7) | ||
| FEMALE | HEMATOLOGY | ||||
| Erythrocyte (106/μL) | 4.7 ± 0.8 | 4.6 ± 0.3 | 4.7 ± 0.3 | 4.6 ± 0.2 | |
| Hemoglobin (g/dL) | 14.1 ± 2.4 | 13.3 ± 1.0 | 13.6 ± 0.9 | 13.8 ± 0.7 | |
| Hematocrit (%) | 41.8 ± 7.1 | 39.6 ± 2.7 | 40.6 ± 2.5 | 40.9 ± 2.3 | |
| MCV (fL) | 89.3 ± 14.7 | 86.8 ± 3.4 | 87.0 ± 6.4 | 89.7 ± 2.3 | |
| MCH (pg) | 30.1 ± 5.0 | 29.1 ± 1.4 | 29.3 ± 2.4 | 30.3 ± 0.9 | |
| MCHC (%) | 33.7 ± 5.4 | 33.5 ± 0.5 | 33.6 ± 0.5 | 33.7 ± 0.6 | |
| RDW (%) | 14.5 ± 2.5 | 15.3 ± 1.4 | 15.1 ± 3.0 | 13.9 ± 0.9 † | |
| BIOCHEMISTRY | |||||
| Ferritin (ng/mL) | 37.2 ± 21.8 | 26.7 ± 23.3 | 33.4 ± 24.0 | 67.6 ± 39.8 *,† | |
| Mild ID, ferritin < 30 ng/mL (%) | 44.7 | 71.4 | 57.9 | 14.3 | |
| ID, ferritin < 15 ng/mL (%) | 15.8 | 57.1 | 21.1 | 14.3 | |
| Iron (μg/dL) | 102.1 ± 42.5 | 94.4 ± 56.3 | 96.3 ± 45.0 | 113.7 ± 32.0 | |
| Iron deficiency (%) | 5.3 | 14.3 | 5.3 | 0.0 | |
| TIQ < 90 | TIQ ≥ 90 | ||||
| <100% RI (n = 2) | ≥100% RI (n = 15) | <100% RI (n = 5) | ≥100% RI (n = 39) | ||
| MALE | HEMATOLOGY | ||||
| Erythrocyte (106/μL) | 5.4 ± 0.2 | 5.3 ± 0.4 | 5.5 ± 0.3 | 5.4 ± 0.3 | |
| Hemoglobin (g/dL) | 15.9 ± 0.6 | 16.1 ± 0.9 | 16.7 ± 1.0 | 16.0 ± 0.9 | |
| Hematocrit (%) | 47.8 ± 1.2 | 47.6 ± 2.8 | 49.4 ± 3.6 | 47.5 ± 2.4 | |
| MCV (fL) | 88.9 ± 1.6 | 89.6 ± 2.6 | 89.4 ± 3.8 | 88.5 ± 3.2 | |
| MCH (pg) | 29.6 ± 0.3 | 30.3 ± 1.2 | 30.3 ± 1.3 | 29.8 ± 1.1 | |
| MCHC (%) | 33.3 ± 0.4 | 33.7 ± 0.8 | 33.9 ± 0.7 | 33.6 ± 0.7 | |
| RDW (%) | 13.5 ± 0.9 | 13.5 ± 1.0 | 13.7 ± 1.8 | 13.0 ± 1.1 | |
| BIOCHEMISTRY | |||||
| Ferritin (ng/mL) | 125.3 ± 42.5 | 114.3 ± 70.4 | 73.2 ± 26.5 | 111.1 ± 72.6 | |
| Mild ID, ferritin < 30 ng/mL (%) | 0.0 | 13.3 | 20.0 | 12.8 | |
| ID, ferritin < 15 ng/mL (%) | 0.0 | 0.0 | 0.0 | 0.0 | |
| Iron (μg/dL) | 115.5 ± 71.4 | 122.6 ± 36.3 | 109.0 ± 45.0 | 111.7 ± 42.7 | |
| Iron deficiency (%) | 0.0 | 6.7 | 20.0 | 5.1 | |
| GROUPS | β0 | β1 | β2 | β3 | β4 | |
|---|---|---|---|---|---|---|
| FEMALE (overall) R2 = 0.1119 n = 71 | Intercept | % RI | Hemoglobin (g/dL) | Iron (µg/dL) | Ferritin (ng/mL) | |
| Unstandardized estimate | 100.1 | 0.04412 | −1.277 | −0.02317 | 0.05324 | |
| 95% CI | 67.94 to 132.2 | 0.001032 to 0.08721 | −3.557 to 1.003 | −0.07512 to 0.02877 | −0.03237 to 0.1388 | |
| Intercept | %RI (z−score) | Hemoglobin (z−score) | Iron (z−score) | Ferritin (z−score) | ||
| Standardized estimate | 85.77 | 1.926 | −1.096 | −0.9678 | 1.385 | |
| 95% CI | 83.96 to 87.59 | 0.04504 to 3.806 | −3.054 to 0.8611 | −3.138 to 1.202 | −0.8421 to 3.612 | |
| p−value | <0.0001 | 0.0449 | 0.2675 | 0.3764 | 0.2187 | |
| TIQ < 90 R2 = 0.04305 n = 45 | Intercept | % RI | Hemoglobin (g/dL) | Iron (µg/dL) | Ferritin (ng/mL) | |
| Unstandardized estimate | 77.47 | −0.006827 | 0.4591 | −0.008289 | −0.04401 | |
| 95% CI | 47.26 to 107.7 | −0.06260 to 0.04894 | −1.628 to 2.546 | −0.05005 to 0.03347 | −0.1321 to 0.04406 | |
| Intercept | % RI (z−score) | Hemoglobin (z−score) | Iron (z−score) | Ferritin (z−score) | ||
| Standardized estimate | 80.75 | −0.298 | 0.3941 | −0.3462 | −1.145 | |
| 95% CI | 79.12 to 82.38 | −2.732 to 2.136 | −1.397 to 2.186 | −2.091 to 1.398 | −3.436 to 1.146 | |
| p−value | <0.0001 | 0.8059 | 0.659 | 0.6904 | 0.3186 | |
| TIQ ≥ 90 R2 = 0.32 n = 26 | Intercept | % RI | Hemoglobin (g/dL) | Iron (µg/dL) | Ferritin (ng/mL) | |
| Unstandardized estimate | 100 | 0.03271 | −0.5735 | −0.02359 | 0.0274 | |
| 95% CI | 75.18 to 124.9 | 0.007172 to 0.05824 | −2.403 to 1.256 | −0.07187 to 0.02469 | −0.03611 to 0.09092 | |
| Intercept | % RI (z−score) | Hemoglobin (z−score) | Iron (z−score) | Ferritin (z−score) | ||
| Standardized estimate | 93.53 | 1.428 | −0.4924 | −0.9852 | 0.713 | |
| 95% CI | 91.98 to 95.07 | 0.3130 to 2.542 | −2.063 to 1.078 | −3.002 to 1.032 | −0.9395 to 2.365 | |
| p−value | <0.0001 | 0.0145 | 0.5215 | 0.3212 | 0.3798 | |
| %RI < 100 R2 = 0.112 n = 57 | Intercept | % RI | Hemoglobin (g/dL) | Iron (µg/dL) | Ferritin (ng/mL) | |
| Unstandardized estimate | 111.6 | 0.1008 | −2.221 | −0.01925 | −0.005461 | |
| 95% CI | 80.21 to 143.0 | −0.03154 to 0.2332 | −4.561 to 0.1195 | −0.07041 to 0.03190 | −0.1049 to 0.09395 | |
| Intercept | % RI (z−score) | Hemoglobin (z−score) | Iron (z−score) | Ferritin (z−score) | ||
| Standardized estimate | 87.18 | 4.401 | −1.907 | −0.8043 | −0.1421 | |
| 95% CI | 84.26 to 90.10 | −1.377 to 10.18 | −3.916 to 0.1026 | −2.941 to 1.333 | −2.728 to 2.444 | |
| p−value | <0.0001 | 0.1324 | 0.0624 | 0.4535 | 0.9126 | |
| %RI ≥ 100 R2 = 0.6387 n = 14 | Intercept | % RI | Hemoglobin (g/dL) | Iron (µg/dL) | Ferritin (ng/mL) | |
| Unstandardized estimate | 31.55 | 0.1423 | 2.129 | −0.03486 | 0.1948 | |
| 95% CI | −70.11 to 133.2 | 0.04026 to 0.2443 | −5.227 to 9.485 | −0.2035 to 0.1338 | 0.001661 to 0.3880 | |
| Intercept | % RI (z−score) | Hemoglobin (z−score) | Iron (z−score) | Ferritin (z−score) | ||
| Standardized estimate | 76.98 | 6.21 | 1.827 | −1.456 | 5.069 | |
| 95% CI | 68.55 to 85.41 | 1.757 to 10.66 | −4.488 to 8.142 | −8.501 to 5.589 | 0.04321 to 10.10 | |
| p−value | 0.5005 | 0.0116 | 0.5291 | 0.6513 | 0.0484 | |
| TIQ < 90 %RI < 100 R2 = 0.05356 n = 38 | Intercept | % RI | Hemoglobin (g/dL) | Iron (µg/dL) | Ferritin (ng/mL) | |
| Unstandardized estimate | 78.8 | 0.03312 | 0.1907 | −0.009043 | −0.03565 | |
| 95% CI | 47.06 to 110.5 | −0.08951 to 0.1558 | −2.081 to 2.462 | −0.05323 to 0.03514 | −0.1270 to 0.05565 | |
| Intercept | % RI (z−score) | Hemoglobin (z−score) | Iron (z−score) | Ferritin (z−score) | ||
| Standardized estimate | 81.97 | 1.446 | 0.1637 | −0.3777 | −0.9276 | |
| 95% CI | 79.20 to 84.73 | −3.907 to 6.799 | −1.786 to 2.114 | −2.223 to 1.468 | −3.303 to 1.448 | |
| p−value | <0.0001 | 0.5863 | 0.8654 | 0.6798 | 0.4326 | |
| TIQ < 90 %RI ≥ 100 R2 = 0.9707 n = 7 | Intercept | % RI | Hemoglobin (g/dL) | Iron (µg/dL) | Ferritin (ng/mL) | |
| Unstandardized estimate | 40.78 | 0.1883 | 0.5949 | 0.09269 | −0.1822 | |
| 95% CI | −14.88 to 96.44 | 0.07538 to 0.3013 | −3.078 to 4.268 | −0.005881 to 0.1913 | −0.4032 to 0.03889 | |
| Intercept | % RI (z−score) | Hemoglobin (z−score) | Iron (z−score) | Ferritin (z−score) | ||
| Standardized estimate | 67.35 | 8.221 | 0.5107 | 3.872 | −4.74 | |
| 95% CI | 60.91 to 73.79 | 3.290 to 13.15 | −2.642 to 3.664 | −0.2457 to 7.989 | −10.49 to 1.012 | |
| p−value | 0.0876 | 0.0189 | 0.558 | 0.056 | 0.0712 | |
| TIQ ≥ 90 %RI < 100 R2 = 0.2247 n = 19 | Intercept | % RI | Hemoglobin (g/dL) | Iron (µg/dL) | Ferritin (ng/mL) | |
| Unstandardized estimate | 107.5 | 0.1166 | −1.505 | −0.02436 | 0.01431 | |
| 95% CI | 78.71 to 136.2 | −0.01943 to 0.2526 | −3.905 to 0.8948 | −0.07744 to 0.02872 | −0.08320 to 0.1118 | |
| Intercept | % RI (z−score) | Hemoglobin (z−score) | Iron (z−score) | Ferritin (z−score) | ||
| Standardized estimate | 94.55 | 5.088 | −1.292 | −1.018 | 0.3724 | |
| 95% CI | 92.05 to 97.04 | −0.8479 to 11.02 | −3.353 to 0.7682 | −3.235 to 1.200 | −2.165 to 2.909 | |
| p−value | <0.0001 | 0.0873 | 0.2 | 0.3417 | 0.7576 | |
| TIQ ≥ 90 %RI ≥ 100 R2 = 0.4673 n = 7 | Intercept | % RI | Hemoglobin (g/dL) | Iron (µg/dL) | Ferritin (ng/mL) | |
| Unstandardized estimate | 128.7 | −0.01839 | −0.8551 | −0.211 | 0.1014 | |
| 95% CI | −263.6 to 521.0 | −0.3953 to 0.3586 | −24.84 to 23.13 | −1.310 to 0.8877 | −0.4680 to 0.6707 | |
| Intercept | % RI (z−score) | Hemoglobin (z−score) | Iron (z−score) | Ferritin (z−score) | ||
| Standardized estimate | 97.86 | −0.8026 | −0.7341 | −8.812 | 2.637 | |
| 95% CI | 60.17 to 135.5 | −17.26 to 15.65 | −21.32 to 19.86 | −54.70 to 37.08 | −12.18 to 17.45 | |
| p−value | 0.2936 | 0.8532 | 0.8922 | 0.4956 | 0.5238 |
| GROUPS | β0 | β1 | β2 | β3 | β4 | |
|---|---|---|---|---|---|---|
| MALE (overall) R2 = 0.02492 n = 58 | Intercept | % RI | Hemoglobin (g/dL) | Iron (µg/dL) | Ferritin (ng/mL) | |
| Unstandardized estimate | 98.73 | −0.018 | 0.2229 | −0.008961 | −0.02229 | |
| 95% CI | 38.25 to 159.2 | −0.08071 to 0.04470 | −3.499 to 3.945 | −0.09718 to 0.07926 | −0.06842 to 0.02384 | |
| Intercept | % RI (z−score) | Hemoglobin (z−score) | Iron (z−score) | Ferritin (z−score) | ||
| Standardized estimate | 96.19 | −0.9055 | 0.192 | −0.3298 | −1.547 | |
| 95% CI | 93.13 to 99.25 | −4.059 to 2.248 | −3.014 to 3.398 | −3.577 to 2.917 | −4.748 to 1.654 | |
| p−value | 0.0019 | 0.5671 | 0.9049 | 0.8393 | 0.3368 | |
| TIQ < 90 R2 = 0.2877 n = 17 | Intercept | % RI | Hemoglobin (g/dL) | Iron (µg/dL) | Ferritin (ng/mL) | |
| Unstandardized estimate | 83.12 | −0.02122 | 0.5637 | −0.04979 | 0.003212 | |
| 95% CI | 48.03 to 118.2 | −0.06015 to 0.01771 | −1.666 to 2.794 | −0.1066 to 0.007063 | −0.02860 to 0.03502 | |
| Intercept | % RI (z−score) | Hemoglobin (z−score) | Iron (z−score) | Ferritin (z−score) | ||
| Standardized estimate | 83.79 | −1.067 | 0.4856 | −1.833 | 0.2229 | |
| 95% CI | 81.82 to 85.75 | −3.025 to 0.8905 | −1.435 to 2.407 | −3.925 to 0.2599 | −1.984 to 2.430 | |
| p−value | 0.0002 | 0.2579 | 0.5919 | 0.0806 | 0.8295 | |
| TIQ ≥ 90 R2 = 0.08355 n = 41 | Intercept | % RI | Hemoglobin (g/dL) | Iron (µg/dL) | Ferritin (ng/mL) | |
| Unstandardized estimate | 107.6 | −0.001585 | −0.706 | 0.06816 | −0.01536 | |
| 95% CI | 50.93 to 164.2 | −0.06043 to 0.05726 | −4.155 to 2.743 | −0.01602 to 0.1523 | −0.05664 to 0.02592 | |
| Intercept | % RI (z−score) | Hemoglobin (z−score) | Iron (z−score) | Ferritin (z−score) | ||
| Standardized estimate | 101.9 | −0.07972 | −0.6082 | 2.509 | −1.066 | |
| 95% CI | 99.09 to 104.7 | −3.039 to 2.880 | −3.579 to 2.363 | −0.5895 to 5.607 | −3.930 to 1.799 | |
| p−value | 0.0005 | 0.9567 | 0.6805 | 0.1093 | 0.4554 | |
| %RI < 100 R2 = 0.2705 n = 7 | Intercept | % RI | Hemoglobin (g/dL) | Iron (µg/dL) | Ferritin (ng/mL) | |
| Unstandardized estimate | 88.18 | −0.2856 | 2.96 | 0.03285 | −0.1766 | |
| 95% CI | −669.8 to 846.1 | −3.262 to 2.691 | −38.39 to 44.31 | −0.8644 to 0.9301 | −1.228 to 0.8749 | |
| Intercept | % RI (z−score) | Hemoglobin (z−score) | Iron (z−score) | Ferritin (z−score) | ||
| Standardized estimate | 77 | −14.36 | 2.55 | 1.209 | −12.26 | |
| 95% CI | −121.3 to 275.3 | −164.1 to 135.3 | −33.07 to 38.17 | −31.82 to 34.23 | −85.22 to 60.71 | |
| p−value | 0.6663 | 0.7198 | 0.7872 | 0.8893 | 0.545 | |
| %RI ≥ 100 R2 = 0.01318 n = 51 | Intercept | % RI | Hemoglobin (g/dL) | Iron (µg/dL) | Ferritin (ng/mL) | |
| Unstandardized estimate | 102.3 | −0.001139 | −0.2044 | −0.01506 | −0.01424 | |
| 95% CI | 38.25 to 166.4 | −0.07247 to 0.07019 | −4.253 to 3.844 | −0.1124 to 0.08225 | −0.06094 to 0.03246 | |
| Intercept | % RI (z−score) | Hemoglobin (z−score) | Iron (z−score) | Ferritin (z−score) | ||
| Standardized estimate | 95.68 | −0.05727 | −0.1761 | −0.5544 | −0.9881 | |
| 95% CI | 92.42 to 98.94 | −3.645 to 3.530 | −3.663 to 3.311 | −4.136 to 3.027 | −4.229 to 2.252 | |
| p−value | 0.0024 | 0.9745 | 0.9195 | 0.7568 | 0.5424 | |
| TIQ < 90 %RI < 100 R2 = 0 n = 2 | Intercept | % RI | Hemoglobin (g/dL) | Iron (µg/dL) | Ferritin (ng/mL) | |
| Unstandardized estimate | 0 | 0 | 0 | 0 | 0 | |
| 95% CI | 0 | 0 | 0 | 0 | 0 | |
| Intercept | % RI (z−score) | Hemoglobin (z−score) | Iron (z−score) | Ferritin (z−score) | ||
| Standardized estimate | 0 | 0 | 0 | 0 | 0 | |
| 95% CI | 0 | 0 | 0 | 0 | 0 | |
| p−value | 0 | 0 | 0 | 0 | 0 | |
| TIQ < 90 %RI ≥ 100 R2 = 0.1921 n = 15 | Intercept | % RI | Hemoglobin (g/dL) | Iron (µg/dL) | Ferritin (ng/mL) | |
| Unstandardized estimate | 81.88 | −0.01621 | 0.5568 | −0.04543 | 0.002051 | |
| 95% CI | 41.49 to 122.3 | −0.06965 to 0.03723 | −1.938 to 3.052 | −0.1194 to 0.02856 | −0.03412 to 0.03822 | |
| Intercept | % RI (z−score) | Hemoglobin (z−score) | Iron (z−score) | Ferritin (z−score) | ||
| Standardized estimate | 83.56 | −0.8151 | 0.4797 | −1.672 | 0.1423 | |
| 95% CI | 80.95 to 86.17 | −3.503 to 1.872 | −1.669 to 2.629 | −4.395 to 1.051 | −2.367 to 2.652 | |
| p−value | 0.0011 | 0.5145 | 0.6297 | 0.2012 | 0.902 | |
| TIQ ≥ 90 %RI < 100 R2 = 1 n = 5 | Intercept | % RI | Hemoglobin (g/dL) | Iron (µg/dL) | Ferritin (ng/mL) | |
| Unstandardized estimate | −391.8 | 3.285 | −0.07885 | 0.9341 | 1.571 | |
| 95% CI | 0 | 0 | 0 | 0 | 0 | |
| Intercept | % RI (z−score) | Hemoglobin (z−score) | Iron (z−score) | Ferritin (z−score) | ||
| Standardized estimate | 378.1 | 165.2 | −0.06792 | 34.38 | 109 | |
| 95% CI | 0 | 0 | 0 | 0 | 0 | |
| p−value | 0 | 0 | 0 | 0 | 0 | |
| TIQ ≥ 90 %RI ≥ 100 R2 = 0.1151 n = 36 | Intercept | % RI | Hemoglobin (g/dL) | Iron (µg/dL) | Ferritin (ng/mL) | |
| Unstandardized estimate | 113.1 | 0.01916 | −1.319 | 0.06772 | −0.01067 | |
| 95% CI | 53.65 to 172.6 | −0.04525 to 0.08358 | −5.067 to 2.429 | −0.02419 to 0.1596 | −0.05083 to 0.02950 | |
| Intercept | % RI (z−score) | Hemoglobin (z−score) | Iron (z−score) | Ferritin (z−score) | ||
| Standardized estimate | 101.2 | 0.9638 | −1.136 | 2.492 | −0.7402 | |
| 95% CI | 98.40 to 103.9 | −2.276 to 4.203 | −4.365 to 2.092 | −0.8903 to 5.875 | −3.527 to 2.047 | |
| p−value | 0.0005 | 0.5484 | 0.4783 | 0.143 | 0.5919 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimas-Benedicto, C.; Albasanz, J.L.; Bermejo, L.M.; Castro-Vázquez, L.; Sánchez-Melgar, A.; Martín, M.; Martínez-García, R.M. Impact of Iron Intake and Reserves on Cognitive Function in Young University Students. Nutrients 2024, 16, 2808. https://doi.org/10.3390/nu16162808
Dimas-Benedicto C, Albasanz JL, Bermejo LM, Castro-Vázquez L, Sánchez-Melgar A, Martín M, Martínez-García RM. Impact of Iron Intake and Reserves on Cognitive Function in Young University Students. Nutrients. 2024; 16(16):2808. https://doi.org/10.3390/nu16162808
Chicago/Turabian StyleDimas-Benedicto, Carmen, José Luis Albasanz, Laura M. Bermejo, Lucía Castro-Vázquez, Alejandro Sánchez-Melgar, Mairena Martín, and Rosa M. Martínez-García. 2024. "Impact of Iron Intake and Reserves on Cognitive Function in Young University Students" Nutrients 16, no. 16: 2808. https://doi.org/10.3390/nu16162808
APA StyleDimas-Benedicto, C., Albasanz, J. L., Bermejo, L. M., Castro-Vázquez, L., Sánchez-Melgar, A., Martín, M., & Martínez-García, R. M. (2024). Impact of Iron Intake and Reserves on Cognitive Function in Young University Students. Nutrients, 16(16), 2808. https://doi.org/10.3390/nu16162808








