Lactoferrin Supplementation during Pregnancy and Lactation Protects Adult Male Rat Offspring from Hypertension Induced by Maternal Adenine Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Study Design
2.2. NO-Related Parameters
2.3. SCFAs and Receptors
2.4. 16S rRNA Gene Sequencing and Analysis
2.5. Statistics
3. Results
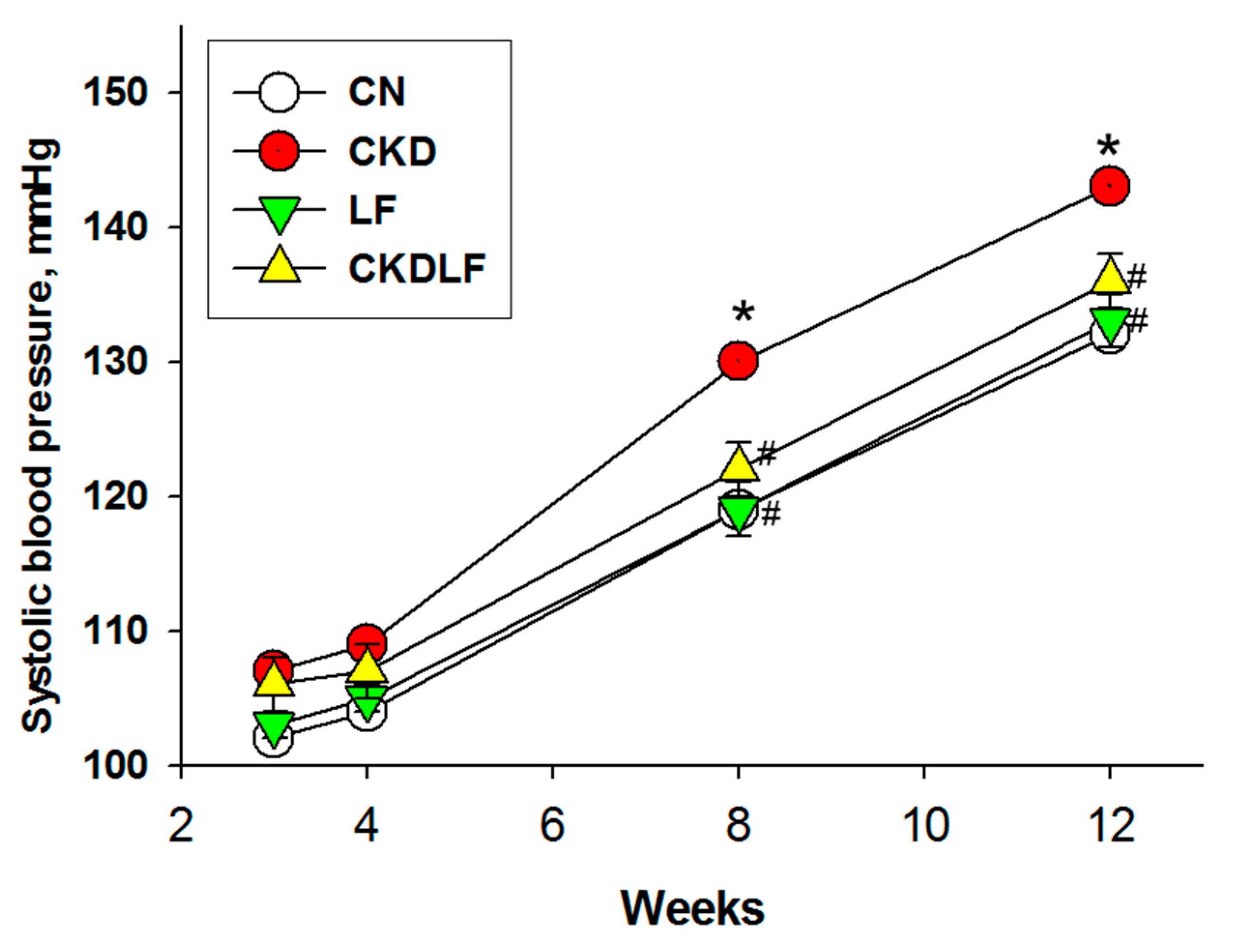
3.1. Body Weight and Blood Pressure
3.2. NO Pathway
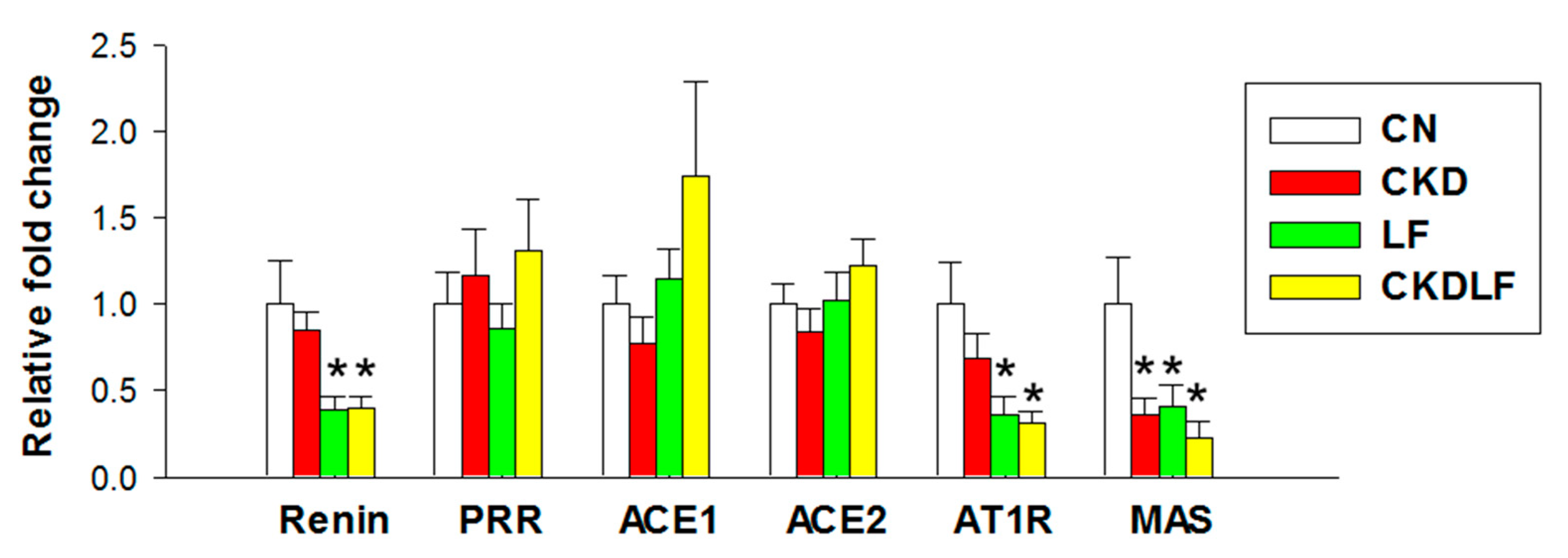
3.3. RAS
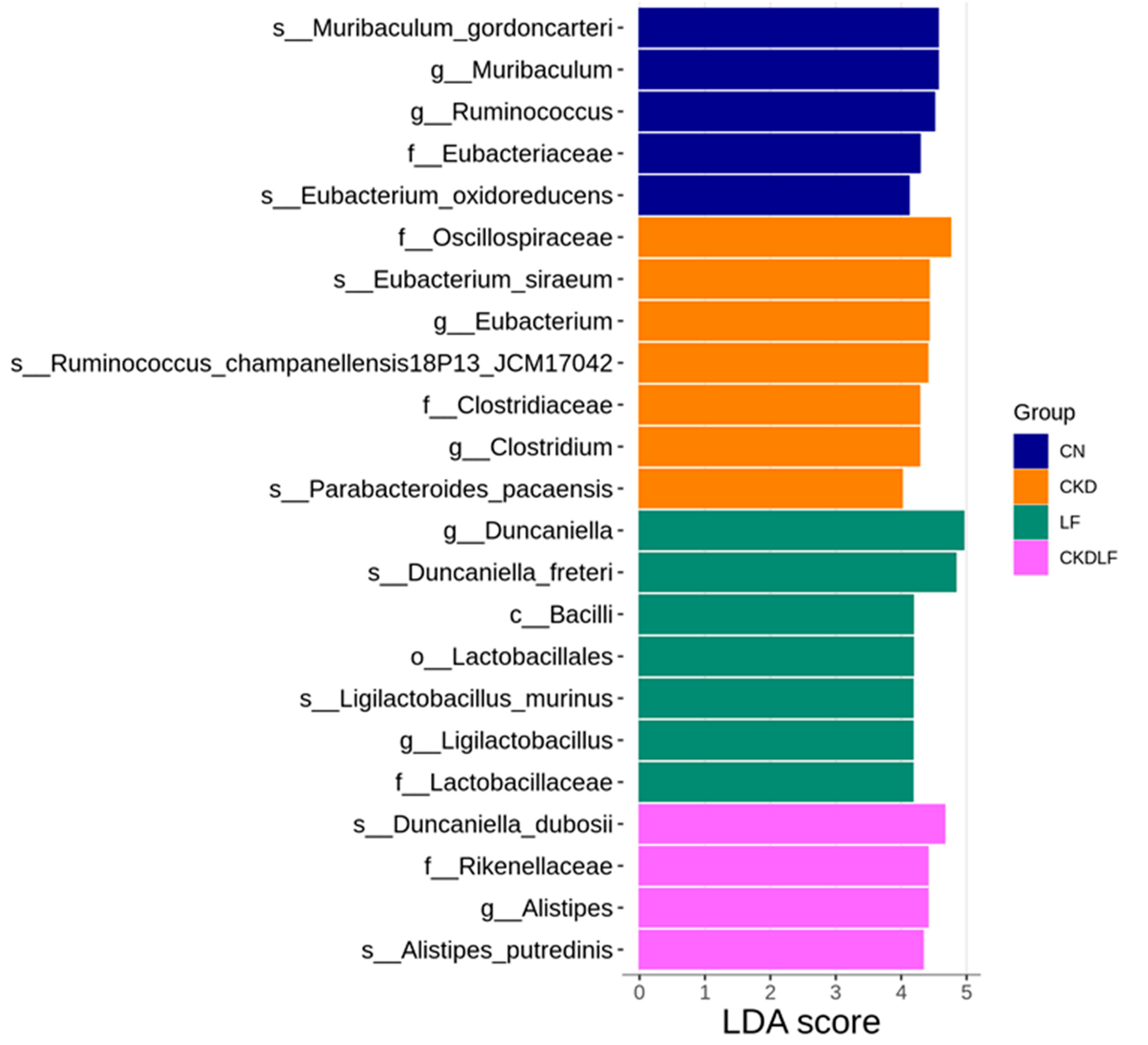
3.4. Differences in Gut Microbiota Composition
3.5. SCFAs and Receptors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shini, V.S.; Udayarajan, C.T.; Nisha, P. A comprehensive review on lactoferrin: A natural multifunctional glycoprotein. Food Funct. 2022, 13, 11954–11972. [Google Scholar] [CrossRef] [PubMed]
- Abrink, M.; Larsson, E.; Gobl, A.; Hellman, L. Expression of lactoferrin in the kidney: Implications for innate immunity and iron metabolism. Kidney Int. 2000, 57, 2004–2010. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P. Clinical Benefits of Lactoferrin for Infants and Children. J. Pediatr. 2016, 173, S43–S52. [Google Scholar] [CrossRef] [PubMed]
- Conesa, C.; Bellés, A.; Grasa, L.; Sánchez, L. The Role of Lactoferrin in Intestinal Health. Pharmaceutics 2023, 15, 1569. [Google Scholar] [CrossRef] [PubMed]
- Verduci, E.; Giannì, M.L.; Vizzari, G.; Vizzuso, S.; Cerasani, J.; Mosca, F.; Zuccotti, G.V. The Triad Mother-Breast Milk-Infant as Predictor of Future Health: A Narrative Review. Nutrients 2021, 13, 486. [Google Scholar] [CrossRef] [PubMed]
- Haschke, F.; Haiden, N.; Thakkar, S.K. Nutritive and Bioactive Proteins in Breastmilk. Ann. Nutr. Metab. 2016, 69, 17–26. [Google Scholar] [CrossRef]
- Carr, L.E.; Virmani, M.D.; Rosa, F.; Munblit, D.; Matazel, K.S.; Elolimy, A.A.; Yeruva, L. Role of Human Milk Bioactives on Infants’ Gut and Immune Health. Front. Immunol. 2021, 12, 604080. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.J.; Reynolds, R.M.; Hardy, D.B. Developmental origins of health and disease: Current knowledge and potential mechanisms. Nutr. Rev. 2017, 75, 951–970. [Google Scholar] [CrossRef]
- Fleming, T.P.; Velazquez, M.A.; Eckert, J.J. Embryos, DOHaD and David Barker. J. Dev. Orig. Health Dis. 2015, 6, 377–383. [Google Scholar] [CrossRef]
- Piccoli, G.B.; Alrukhaimi, M.; Liu, Z.H.; Zakharova, E.; Levin, A.; World Kidney Day Steering Committee. What we do and do not know about women and kidney diseases; Questions unanswered and answers unquestioned: Reflection on World Kidney Day and International Woman’s Day. Physiol. Int. 2018, 105, 1–18. [Google Scholar] [CrossRef]
- Diwan, V.; Brown, L.; Gobe, G.C. Adenine-induced chronic kidney disease in rats. Nephrology 2018, 23, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Yang, H.W.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Maternal adenine-induced chronic kidney disease programs hypertension in adult male rat offspring: Implications of nitric oxide and gut microbiome derived metabolites. Int. J. Mol. Sci. 2020, 21, 7237. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Kono, I.; Kuriki, T.; Funahashi, S.; Fushimi, S.; Iqbal, M.; Okada, S.; Toyokuni, S. Bovine lactoferrin ameliorates ferric nitrilotriacetate-induced renal oxidative damage in rats. J. Clin. Biochem. Nutr. 2012, 51, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Safaeian, L.; Zabolian, H. Antioxidant effects of bovine lactoferrin on dexamethasone-induced hypertension in rat. ISRN Pharmacol. 2014, 2014, 943523. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Takeuchi, T.; Ozaki, T.; Shimizu, H.; Ando, K.; Miyamoto, A.; Harada, E. Bovine lactoferrin has a nitric oxide-dependent hypotensive effect in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R359–R365. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fernández-Musoles, R.; Salom, J.B.; Martínez-Maqueda, D.; López-Díez, J.J.; Recio, I.; Manzanares, P. Antihypertensive effects of lactoferrin hydrolyzates: Inhibition of angiotensin- and endothelin-converting enzymes. Food Chem. 2013, 139, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, B.; Zhang, F.; Liu, X.; Zhang, Y.; Peng, W.; Teng, D.; Mao, R.; Yang, N.; Hao, Y.; et al. Interaction between Dietary Lactoferrin and Gut Microbiota in Host Health. J. Agric. Food Chem. 2024, 72, 7596–7606. [Google Scholar] [CrossRef] [PubMed]
- O'Donnell, J.A.; Zheng, T.; Meric, G.; Marques, F.Z. The gut microbiome and hypertension. Nat. Rev. Nephrol. 2023, 19, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 442–456. [Google Scholar] [CrossRef]
- Paauw, N.D.; van Rijn, B.B.; Lely, A.T.; Joles, J.A. Pregnancy as a critical window for blood pressure regulation in mother and child: Programming and reprogramming. Acta Physiol. 2017, 219, 241–259. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar]
- Blais, A.; Lan, A.; Boluktas, A.; Grauso-Culetto, M.; Chaumontet, C.; Blachier, F.; Davila, A.M. Lactoferrin Supplementation during Gestation and Lactation Is Efficient for Boosting Rat Pup Development. Nutrients 2022, 14, 2814. [Google Scholar] [CrossRef] [PubMed]
- Reckelhoff, J.F. Gender differences in the regulation of blood pressure. Hypertension 2001, 37, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Bode-Böger, S.M.; Scalera, F.; Ignarro, L.J. The L-arginine paradox: Importance of the L-arginine/asymmetrical dimethylarginine ratio. Pharmacol. Ther. 2007, 114, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.F.; Hsu, C.N. Perinatal Propionate Supplementation Protects Adult Male Offspring from Maternal Chronic Kidney Disease-Induced Hypertension. Nutrients 2022, 14, 3435. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Tain, Y.L.; Huang, L.T. Restoration of asymmetric dimethylarginine-nitric oxide balance to prevent the development of hypertension. Int. J. Mol. Sci. 2014, 15, 11773–11782. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Targeting on Asymmetric Dimethylarginine-Related Nitric Oxide-Reactive Oxygen Species Imbalance to Reprogram the Development of Hypertension. Int. J. Mol. Sci. 2016, 17, 2020. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. Targeting the Renin–Angiotensin–Aldosterone System to Prevent Hypertension and Kidney Disease of Developmental Origins. Int. J. Mol. Sci. 2021, 22, 2298. [Google Scholar] [CrossRef]
- Chappell, M.C.; Marshall, A.C.; Alzayadneh, E.M.; Shaltout, H.A.; Diz, D.I. Update on the Angiotensin converting enzyme 2-Angiotensin (1-7)-MAS receptor axis: Fetal programing, sex differences, and intracellular pathways. Front. Endocrinol. 2014, 4, 201. [Google Scholar] [CrossRef]
- Navar, L.G.; Kobori, H.; Prieto, M.C.; Gonzalez-Villalobos, R.A. Intrarenal renin-angiotensin system in hypertension. Hypertension 2011, 57, 355–362. [Google Scholar] [CrossRef]
- García-Tejedor, A.; Manzanares, P.; Castelló-Ruiz, M.; Moscardó, A.; Marcos, J.F.; Salom, J.B. Vasoactive properties of antihypertensive lactoferrin-derived peptides in resistance vessels: Effects in small mesenteric arteries from SHR rats. Life Sci. 2017, 186, 118–124. [Google Scholar] [CrossRef]
- Chen, P.W.; Liu, Z.S.; Kuo, T.C.; Hsieh, M.C.; Li, Z.W. Prebiotic effects of bovine lactoferrin on specific probiotic bacteria. Biometals 2017, 30, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.H.; Wang, G.; Luo, L.; Ding, W.; Xu, J.Y.; Yu, Z.; Qin, L.Q.; Wan, Z. Dietary lactoferrin has differential effects on gut microbiota in young versus middle-aged APPswe/PS1dE9 transgenic mice but no effects on cognitive function. Food Nutr. Res. 2021, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- Palmu, J.; Lahti, L.; Niiranen, T. Targeting Gut Microbiota to Treat Hypertension: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 1248. [Google Scholar] [CrossRef]
- Sun, S.; Lulla, A.; Sioda, M.; Winglee, K.; Wu, M.C.; Jacobs, D.R.; Shikany, J.M.; Lloyd-Jones, D.M.; Launer, L.J.; Fodor, A.A.; et al. Gut Microbiota Composition and Blood Pressure: The CARDIA Study. Hypertension 2019, 73, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Guerrero Sanchez, M.; Passot, S.; Campoy, S.; Olivares, M.; Fonseca, F. Ligilactobacillus salivarius functionalities, applications, and manufacturing challenges. Appl. Microbiol. Biotechnol. 2022, 106, 57–80. [Google Scholar] [CrossRef]
- Mukohda, M.; Yano, T.; Matsui, T.; Nakamura, S.; Miyamae, J.; Toyama, K.; Mitsui, R.; Mizuno, R.; Ozaki, H. Treatment with Ligilactobacillus murinus lowers blood pressure and intestinal permeability in spontaneously hypertensive rats. Sci. Rep. 2023, 13, 15197. [Google Scholar] [CrossRef]
- Xu, J.; Moore, B.N.; Pluznick, J.L. Short-Chain Fatty Acid Receptors and Blood Pressure Regulation: Council on Hypertension Mid-Career Award for Research Excellence 2021. Hypertension 2022, 79, 2127–2137. [Google Scholar] [CrossRef] [PubMed]




| Standard Chow Diet | 10% LF Diet | |
|---|---|---|
| Nutrients | ||
| Protein (g %) | 14 | 14 |
| Carbohydrate (g %) | 73 | 73 |
| Fat gm (g %) | 4 | 4 |
| Ingredients | ||
| Casein | 140 | 140 |
| Cysteine | 1.8 | 1.8 |
| Corn starch | 495.7 | 495.7 |
| Maltodextrin | 125 | 125 |
| Sucrose | 100 | 100 |
| Cellulose | 50 | 50 |
| Soybean oil | 40 | 40 |
| t-butylhydroquinone | 0.008 | 0.008 |
| Mineral mix | 35 | 35 |
| Vitamin mix | 10 | 10 |
| Choline bitartrate | 25 | 25 |
| Lactoferrin | 0 | 10 |
| Total | 1000 | 1010 |
| Energy (kcal/g) | 3.8 | 3.8 |
| Gene | Accession No. | 5′ Primer | 3′ Primer |
|---|---|---|---|
| Olfr78 | NM_173293.2 | 5 gaggaagctcacttttggtttgg 3 | 5 cagcttcaatgtccttgtcacag 3 |
| Ffar3 | NM_001108912.1 | 5 tcttcaccaccgtctatctcac 3 | 5 cacaagtcctgccaccctc 3 |
| Ffar2 | NM_001005877.3 | 5 ctgcctgggatcgtctgtg 3 | 5 cataccctcggccttctgg 3 |
| Hcar2 | NM_181476.2 | 5 cggtggtctactatttctcc 3 | 5 cccctggaatacttctgatt 3 |
| Ren | J02941.1 | 5 aacattaccagggcaactttcact 3 | 5 acccccttcatggtgatctg 3 |
| Atp6ap2 | AB188298.1 | 5 gaggcagtgaccctcaacat 3 | 5 ccctcctcacacaacaaggt 3 |
| Ace1 | U03734.1 | 5 caccggcaaggtctgctt 3 | 5 cttggcatagtttcgtgaggaa 3 |
| Agtr1a | NM_030985.4 | 5 gctgggcaacgagtttgtct 3 | 5 cagtccttcagctggatcttca 3 |
| Ace2 | NM_001012006.2 | 5 acccttcttacatcagccctactg 3 | 5 tgtccaaaacctaccccacatat 3 |
| Mas1 | J03823.1 | 5 catctctcctctcggctttgtg 3 | 5 cctcatccggaagcaaagg 3 |
| R18s | X01117 | 5 gccgcggtaattccagctcca 3 | 5 cccgcccgctcccaagatc 3 |
| Groups | CN | CKD | LF | CKDLF |
|---|---|---|---|---|
| Body weight (BW) (g) | 290 ± 12 | 289 ± 9 | 355 ± 9 *# | 419 ± 9 *# |
| Left kidney weight (KW) (g) | 1.40 ± 0.06 | 1.40 ± 0.04 | 1.85 ± 0.09 *# | 1.91 ± 0.07 *# |
| Ratio of KW to BW (g/kg) | 0.48 ± 0.01 | 0.48 ± 0.01 | 0.52 ± 0.02 * | 0.46 ± 0.02 $ |
| Systolic BP (mmHg) | 132 ± 1 | 143 ± 1 * | 133 ±1 # | 136 ± 1 # |
| Diastolic BP (mmHg) | 88 ± 3 | 91 ± 2 | 82 ± 1 # | 80 ± 2 # |
| Mean arterial pressure (mmHg) | 103 ± 2 | 109 ± 2 * | 99 ± 1 # | 100 ± 1 # |
| Group | CN | CKD | LF | CKDLF |
|---|---|---|---|---|
| L-citrulline (μM) | 52.8 ± 3.3 | 53.5 ± 4.2 | 52.5 ± 4 | 55.9 ± 3.6 |
| L-arginine (μM) | 352.3 ± 16.5 | 353.9 ± 24.1 | 377 ± 42.1 | 396.6 ± 22.8 |
| ADMA (μM) | 1.81 ± 0.1 | 2.6 ± 0.05 * | 1.76 ± 0.17 # | 2.02 ± 0.19 # |
| SDMA (μM) | 1.82 ± 0.12 | 1.69 ± 0.15 | 1.51 ± 0.08 | 1.65 ± 0.24 |
| L-arginine-to-ADMA ratio (μM/μM) | 200.2 ± 15.9 | 135.3 ± 7.2 * | 225.8 ± 32.8 # | 207.5 ± 19.9 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tain, Y.-L.; Hou, C.-Y.; Chen, W.-L.; Liao, W.-T.; Hsu, C.-N. Lactoferrin Supplementation during Pregnancy and Lactation Protects Adult Male Rat Offspring from Hypertension Induced by Maternal Adenine Diet. Nutrients 2024, 16, 2607. https://doi.org/10.3390/nu16162607
Tain Y-L, Hou C-Y, Chen W-L, Liao W-T, Hsu C-N. Lactoferrin Supplementation during Pregnancy and Lactation Protects Adult Male Rat Offspring from Hypertension Induced by Maternal Adenine Diet. Nutrients. 2024; 16(16):2607. https://doi.org/10.3390/nu16162607
Chicago/Turabian StyleTain, You-Lin, Chih-Yao Hou, Wei-Ling Chen, Wei-Ting Liao, and Chien-Ning Hsu. 2024. "Lactoferrin Supplementation during Pregnancy and Lactation Protects Adult Male Rat Offspring from Hypertension Induced by Maternal Adenine Diet" Nutrients 16, no. 16: 2607. https://doi.org/10.3390/nu16162607
APA StyleTain, Y.-L., Hou, C.-Y., Chen, W.-L., Liao, W.-T., & Hsu, C.-N. (2024). Lactoferrin Supplementation during Pregnancy and Lactation Protects Adult Male Rat Offspring from Hypertension Induced by Maternal Adenine Diet. Nutrients, 16(16), 2607. https://doi.org/10.3390/nu16162607










