Healthy Dietary Patterns with and without Meat Improved Cardiometabolic Disease Risk Factors in Adults: A Randomized Crossover Controlled Feeding Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Participant Inclusion Criteria
2.3. Ethics
2.4. Baseline Dietary Assessment
2.5. Dietary Interventions
2.6. Clinical Assessments
2.7. Dietary Satisfaction
2.8. Statistics
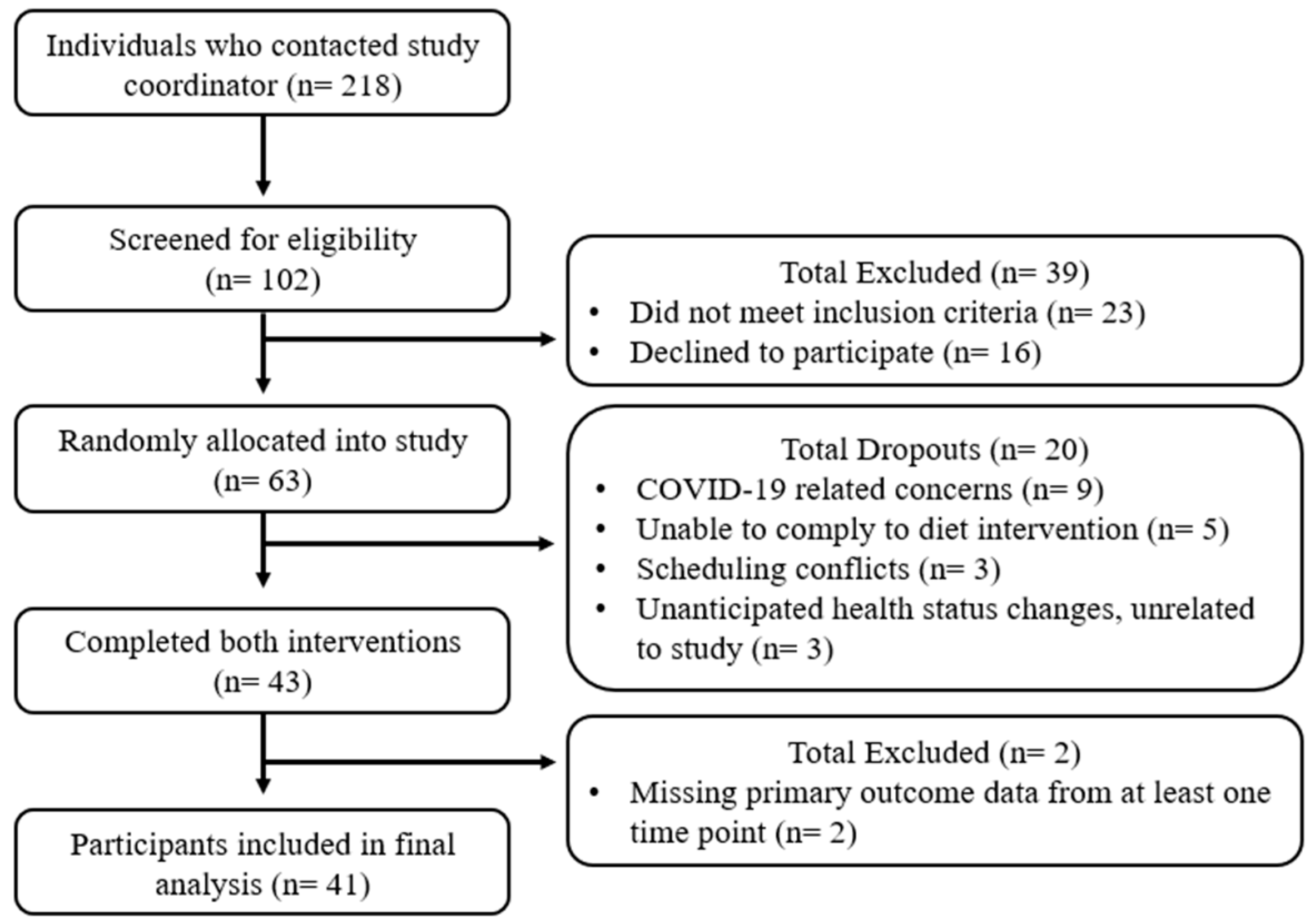
3. Results
3.1. Subject Characteristics
3.2. Dietary Adherence and Satisfaction
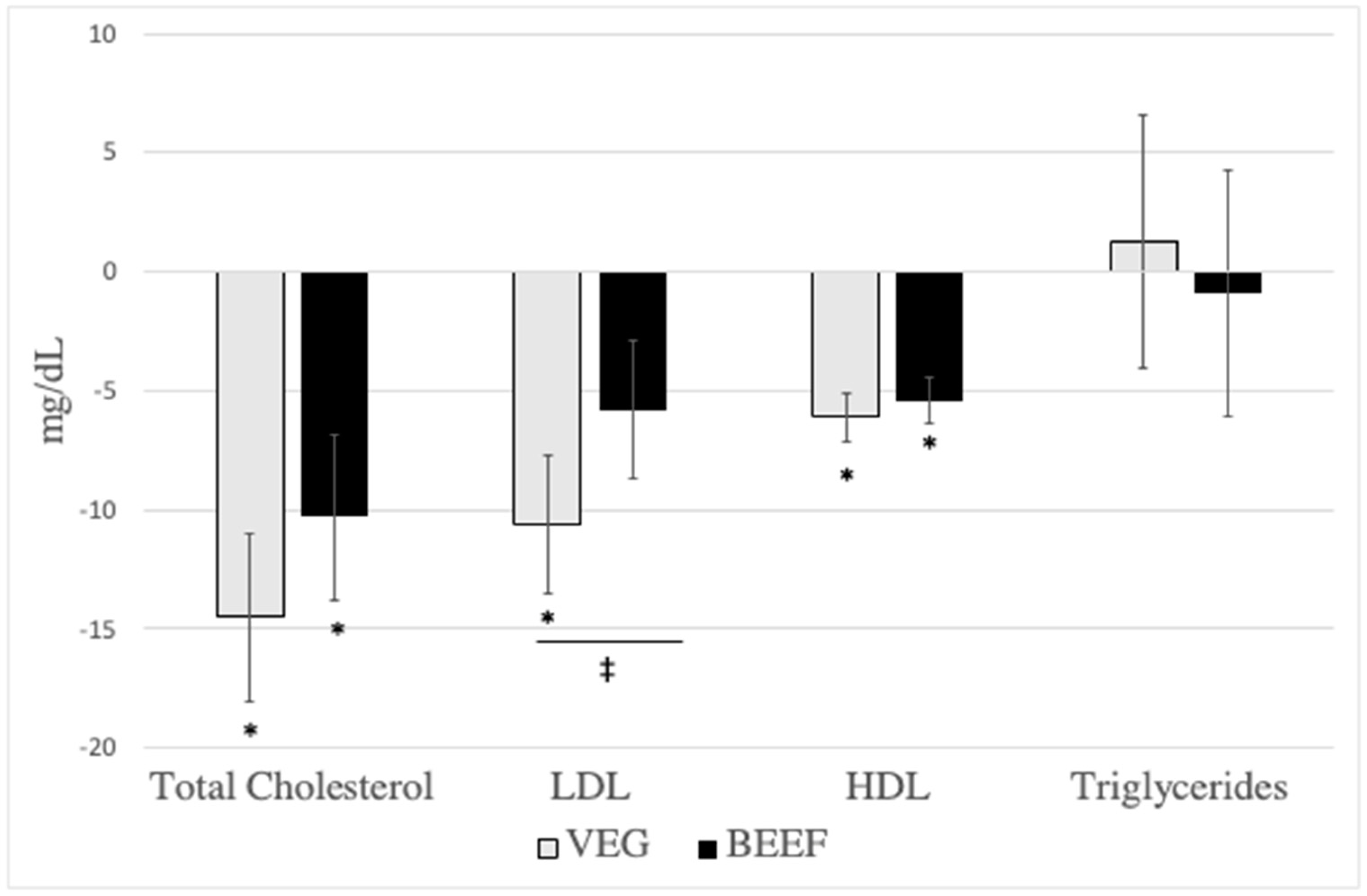
3.3. Cardiometabolic Disease Risk Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shan, Z.; Rehm, C.D.; Rogers, G.; Ruan, M.; Wang, D.D.; Hu, F.B.; Mozaffarian, D.; Zhang, F.F.; Bhupathiraju, S.N. Trends in Dietary Carbohydrate, Protein, and Fat Intake and Diet Quality Among US Adults, 1999–2016. JAMA 2019, 322, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaar, L.; Satija, A.; Wang, D.D.; Rimm, E.B.; Smith-Warner, S.A.; Stampfer, M.J.; Hu, F.B.; Willett, W.C. Red meat intake and risk of coronary heart disease among US men: Prospective cohort study. BMJ 2020, 371, m4141. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Sun, Q.; Bernstein, A.M.; Manson, J.E.; Willett, W.C.; Hu, F.B. Changes in red meat consumption and subsequent risk of type 2 diabetes mellitus: Three cohorts of US men and women. JAMA Intern. Med. 2013, 173, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; USDA: Washington, DC, USA, 2020. Available online: https://www.dietaryguidelines.gov/ (accessed on 15 March 2021).
- Lichtenstein, A.H.; Appel, L.J.; Vadiveloo, M.; Hu, F.B.; Kris-Etherton, P.M.; Rebholz, C.M.; Sacks, F.M.; Thorndike, A.N.; Van Horn, L.; Wylie-Rosett, J. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e472–e487. [Google Scholar] [CrossRef] [PubMed]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition Therapy for Adults With Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef] [PubMed]
- Sayer, R.D.; Wright, A.J.; Chen, N.; Campbell, W.W. Dietary Approaches to Stop Hypertension diet retains effectiveness to reduce blood pressure when lean pork is substituted for chicken and fish as the predominant source of protein. Am. J. Clin. Nutr. 2015, 102, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Roussell, M.A.; Hill, A.M.; Gaugler, T.L.; West, S.G.; Heuvel, J.P.; Alaupovic, P.; Gillies, P.J.; Kris-Etherton, P.M. Beef in an Optimal Lean Diet study: Effects on lipids, lipoproteins, and apolipoproteins. Am. J. Clin. Nutr. 2012, 95, 9–16. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, L.E.; Paddon-Jones, D.; Wright, A.J.; Campbell, W.W. A Mediterranean-style eating pattern with lean, unprocessed red meat has cardiometabolic benefits for adults who are overweight or obese in a randomized, crossover, controlled feeding trial. Am. J. Clin. Nutr. 2018, 108, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Roussell, M.A.; Hill, A.M.; Gaugler, T.L.; West, S.G.; Ulbrecht, J.S.; Vanden Heuvel, J.P.; Gillies, P.J.; Kris-Etherton, P.M. Effects of a DASH-like diet containing lean beef on vascular health. J. Hum. Hypertens. 2014, 28, 600–605. [Google Scholar] [CrossRef]
- Hill, A.M.; Harris Jackson, K.A.; Roussell, M.A.; West, S.G.; Kris-Etherton, P.M. Type and amount of dietary protein in the treatment of metabolic syndrome: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 757–770. [Google Scholar] [CrossRef]
- Sinclair, A.J.; O’Dea, K.; Dunstan, G.; Ireland, P.D.; Niall, M. Effects on plasma lipids and fatty acid composition of very low fat diets enriched with fish or kangaroo meat. Lipids 1987, 22, 523–529. [Google Scholar] [CrossRef] [PubMed]
- de Mello, V.D.; Zelmanovitz, T.; Perassolo, M.S.; Azevedo, M.J.; Gross, J.L. Withdrawal of red meat from the usual diet reduces albuminuria and improves serum fatty acid profile in type 2 diabetes patients with macroalbuminuria. Am. J. Clin. Nutr. 2006, 83, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Subar, A.F.; Thompson, F.E.; Potischman, N.; Forsyth, B.H.; Buday, R.; Richards, D.; McNutt, S.; Hull, S.G.; Guenther, P.M.; Schatzkin, A.; et al. Formative research of a quick list for an automated self-administered 24-hour dietary recall. J. Am. Diet. Assoc. 2007, 107, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. The Healthy Eating Index—Population Ratio Method. Updated 14 December 2021. Available online: https://epi.grants.cancer.gov/hei/population-ratio-method.html (accessed on 20 November 2020).
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Food Safety Inspection Service Code of Federal Regulations, U.S. Department of Agriculture, Title 9, Part 301, Section 2. Definitions. 2010. Available online: https://www.govinfo.gov/app/details/CFR-2010-title9-vol2/CFR-2010-title9-vol2-sec301-2/context (accessed on 20 November 2020).
- US Department of Agriculture; US Department of Health and Human Services. 2015–2020 Dietary Guidelines for Americans, 8th ed.; US Government Printing Office: Washington, DC, USA, 2015. [Google Scholar]
- Meat, Poultry, and Fish: Picking Healthy Proteins. American Heart Association. Available online: https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/nutrition-basics/meat-poultry-and-fish-picking-healthy-proteins (accessed on 20 November 2020).
- Davis, C.; Hodgson, J.; Bryan, J.; Garg, M.; Woodman, R.; Murphy, K. Older Australians Can Achieve High Adherence to the Mediterranean Diet during a 6 Month Randomised Intervention; Results from the Medley Study. Nutrients 2017, 9, 534. [Google Scholar] [CrossRef] [PubMed]
- Genoni, A.; Lo, J.; Lyons-Wall, P.; Devine, A. Compliance, Palatability and Feasibility of PALEOLITHIC and Australian Guide to Healthy Eating Diets in Healthy Women: A 4-Week Dietary Intervention. Nutrients 2016, 8, 481. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Leidy, H.J.; Campbell, W.W. Regional, but not total, body composition changes in overweight and obese adults consuming a higher protein, energy-restricted diet are sex specific. Nutr. Res. 2013, 33, 629–635. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Y.; Lindemann, S.R.; Cross, T.L.; Tang, M.; Clark, C.M.; Campbell, W.W. Effects of Adding Lean Red Meat to a U.S.-Style Healthy Vegetarian Dietary Pattern on Gut Microbiota and Cardiovascular Risk Factors in Young Adults: A Crossover Randomized Controlled Trial. J. Nutr. 2023, 153, 1439–1452. [Google Scholar] [CrossRef]
- Maki, K.C.; Van Elswyk, M.E.; Alexander, D.D.; Rains, T.M.; Sohn, E.L.; McNeill, S. A meta-analysis of randomized controlled trials that compare the lipid effects of beef versus poultry and/or fish consumption. J. Clin. Lipidol. 2012, 6, 352–361. [Google Scholar] [CrossRef]
- O’Connor, L.E.; Kim, J.E.; Campbell, W.W. Total red meat intake of ≥0.5 servings/d does not negatively influence cardiovascular disease risk factors: A systemically searched meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2017, 105, 57–69. [Google Scholar] [CrossRef]
- O’Connor, L.E.; Kim, J.E.; Clark, C.M.; Zhu, W.; Campbell, W.W. Effects of Total Red Meat Intake on Glycemic Control and Inflammatory Biomarkers: A Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2021, 12, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Zeraatkar, D.; Johnston, B.C.; Bartoszko, J.; Cheung, K.; Bala, M.M.; Valli, C.; Rabassa, M.; Sit, D.; Milio, K.; Sadeghirad, B.; et al. Effect of Lower Versus Higher Red Meat Intake on Cardiometabolic and Cancer Outcomes: A Systematic Review of Randomized Trials. Ann. Intern. Med. 2019, 171, 721–731. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Satija, A.; Blondin, S.A.; Janiszewski, M.; Emlen, E.; O’Connor, L.E.; Campbell, W.W.; Hu, F.B.; Willett, W.C.; Stampfer, M.J. Meta-Analysis of Randomized Controlled Trials of Red Meat Consumption in Comparison With Various Comparison Diets on Cardiovascular Risk Factors. Circulation 2019, 139, 1828–1845. [Google Scholar] [CrossRef]
- Mahon, A.K.; Flynn, M.G.; Stewart, L.K.; McFarlin, B.K.; Iglay, H.B.; Mattes, R.D.; Lyle, R.M.; Considine, R.V.; Campbell, W.W. Protein intake during energy restriction: Effects on body composition and markers of metabolic and cardiovascular health in postmenopausal women. J. Am. Coll. Nutr. 2007, 26, 182–189. [Google Scholar] [CrossRef]
- Foerster, J.; Maskarinec, G.; Reichardt, N.; Tett, A.; Narbad, A.; Blaut, M.; Boeing, H. The influence of whole grain products and red meat on intestinal microbiota composition in normal weight adults: A randomized crossover intervention trial. PLoS ONE 2014, 9, e109606. [Google Scholar] [CrossRef]
- Thorning, T.K.; Raziani, F.; Bendsen, N.T.; Astrup, A.; Tholstrup, T.; Raben, A. Diets with high-fat cheese, high-fat meat, or carbohydrate on cardiovascular risk markers in overweight postmenopausal women: A randomized crossover trial. Am. J. Clin. Nutr. 2015, 102, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.W. Animal-based and plant-based protein-rich foods and cardiovascular health: A complex conundrum. Am. J. Clin. Nutr. 2019, 110, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Gifford, C.L.; O’Connor, L.E.; Campbell, W.W.; Woerner, D.R.; Belk, K.E. Broad and Inconsistent Muscle Food Classification Is Problematic for Dietary Guidance in the U.S. Nutrients 2017, 9, 1027. [Google Scholar] [CrossRef]
- O’Connor, L.E.; Gifford, C.L.; Woerner, D.R.; Sharp, J.L.; Belk, K.E.; Campbell, W.W. Dietary Meat Categories and Descriptions in Chronic Disease Research Are Substantively Different within and between Experimental and Observational Studies: A Systematic Review and Landscape Analysis. Adv. Nutr. 2020, 11, 41–51. [Google Scholar] [CrossRef]
- Abete, I.; Romaguera, D.; Vieira, A.R.; Lopez de Munain, A.; Norat, T. Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: A meta-analysis of cohort studies. Br. J. Nutr. 2014, 112, 762–775. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Wang, C.; Mao, Z.; Zhou, W.; Zhang, L.; Fan, M.; Cui, S.; Li, L. Meat and fish intake and type 2 diabetes: Dose-response meta-analysis of prospective cohort studies. Diabetes Metab. 2020, 46, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Hyeon, J.; Lee, S.A.; Kwon, S.O.; Lee, H.; Keum, N.; Lee, J.K.; Park, S.M. Role of Total, Red, Processed, and White Meat Consumption in Stroke Incidence and Mortality: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. J. Am. Heart Assoc. 2017, 6, e005983. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hill, E.R.; Campbell, W.W.; O’Connor, L.E. Plant- and Animal-Based Protein-Rich Foods and Cardiovascular Health. Curr. Atheroscler. Rep. 2022, 24, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Petersen, K.F.; Shulman, G.I.; Alwarith, J.; Rembert, E.; Tura, A.; Hill, M.; Holubkov, R.; Barnard, N.D. Effect of a Low-Fat Vegan Diet on Body Weight, Insulin Sensitivity, Postprandial Metabolism, and Intramyocellular and Hepatocellular Lipid Levels in Overweight Adults: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2025454. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.E.; Warziski, M.; Styn, M.A.; Music, E.; Hudson, A.G.; Sereika, S.M. A randomized clinical trial of a standard versus vegetarian diet for weight loss: The impact of treatment preference. Int. J. Obes. 2008, 32, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, L.; Lee, D.; Ahmed, A.; Cheung, A.; Khan, T.A.; Blanco, S.; Mejia; Mirrahimi, A.; Jenkins, D.J.A.; Livesey, G.; et al. Effect of low glycaemic index or load dietary patterns on glycaemic control and cardiometabolic risk factors in diabetes: Systematic review and meta-analysis of randomised controlled trials. BMJ 2021, 374, n1651. [Google Scholar] [CrossRef] [PubMed]
- Gayoso-Diz, P.; Otero-González, A.; Rodriguez-Alvarez, M.X.; Gude, F.; García, F.; De Francisco, A.; Quintela, A.G. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: Effect of gender and age: EPIRCE cross-sectional study. BMC Endocr. Disord. 2013, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Sanders, L.M.; Wilcox, M.L.; Maki, K.C. Red meat consumption and risk factors for type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2022, 77, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Roussell, M.A.; Hill, A.M.; Kris-Etherton, P.M.; Walzem, R.L. Baseline Insulin Resistance Is a Determinant of the Small, Dense Low-Density Lipoprotein Response to Diets Differing in Saturated Fat, Protein, and Carbohydrate Contents. Nutrients 2021, 13, 4328. [Google Scholar] [CrossRef]
- Mangravite, L.M.; Chiu, S.; Wojnoonski, K.; Rawlings, R.S.; Bergeron, N.; Krauss, R.M. Changes in atherogenic dyslipidemia induced by carbohydrate restriction in men are dependent on dietary protein source. J. Nutr. 2011, 141, 2180–2185. [Google Scholar] [CrossRef]
- Fleming, J.A.; Kris-Etherton, P.M.; Petersen, K.S.; Baer, D.J. Effect of varying quantities of lean beef as part of a Mediterranean-style dietary pattern on lipids and lipoproteins: A randomized crossover controlled feeding trial. Am. J. Clin. Nutr. 2021, 113, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [CrossRef]
- O’Connor, L.E.; Li, J.; Sayer, R.D.; Hennessy, J.E.; Campbell, W.W. Short-Term Effects of Healthy Eating Pattern Cycling on Cardiovascular Disease Risk Factors: Pooled Results from Two Randomized Controlled Trials. Nutrients 2018, 10, 1725. [Google Scholar] [CrossRef] [PubMed]

| VEG | BEEF | |
|---|---|---|
| Energy (kcal/d) | 2546 ± 150 | 2436 ± 79 |
| Total Fat (g/d) | 84 ± 13 | 91 ± 11 |
| Total Carbohydrate (g/d) | 361 ± 30 | 286 ± 28 a |
| Total Protein (g/d) | 105 ± 15 | 134 ± 9 a |
| Animal Protein (g/d) | 36 ± 12 | 81 ± 11 a |
| Vegetable Protein (g/d) | 69 ± 15 | 54 ± 10 a |
| Cholesterol (mg/d) | 200 ± 170 | 325 ± 158 a |
| Total Saturated Fatty Acids (SFA) (g/d) | 22 ± 5 | 25 ± 4 a |
| Total Monounsaturated Fatty Acids (MUFA) (g/d) | 37 ± 6 | 40 ± 6 a |
| Total Polyunsaturated Fatty Acids (PUFA) (g/d) | 19 ± 5 | 17 ± 4 a |
| Glucose (g/d) | 20 ± 6 | 19 ± 5 |
| Total Dietary Fiber (g/d) | 50 ± 7 | 41 ± 5 a |
| Soluble Dietary Fiber (g/d) | 9 ± 1 | 7 ± 1 a |
| Insoluble Dietary Fiber (g/d) | 40 ± 6 | 34 ± 5 a |
| % Energy from Fat | 29 ± 3 | 33 ± 3 a |
| % Energy from Carbohydrate | 56 ± 5 | 46 ± 4 a |
| % Energy from Protein | 15 ± 2 | 22 ± 2 a |
| % Energy from SFA | 7 ± 2 | 9 ± 2 a |
| % Energy from MUFA | 12 ± 2 | 14 ± 2 a |
| % Energy from PUFA | 6 ± 1 | 6 ± 1 |
| Added Sugars (by Available Carbohydrate) (g/d) | 18 ± 7 | 18 ± 7 |
| Available Carbohydrate (g/d) | 311 ± 26 | 244 ± 25 a |
| Glycemic Index (glucose reference) | 52 ± 2 | 51 ± 2 |
| Glycemic Index (bread reference) | 74 ± 4 | 73 ± 3 |
| Glycemic Load (glucose reference) | 161 ± 13 | 125 ± 13 a |
| Glycemic Load (bread reference) | 230 ± 19 | 179 ± 19 a |
| Magnesium (mg/d) | 573 ± 85 | 516 ± 53 a |
| Sodium (mg/d) | 3758 ± 442 | 3180 ± 381 a |
| Potassium (mg/d) | 4556 ± 230 | 4488 ± 260 |
| Outcome | Baseline 1 |
|---|---|
| Age at enrollment, y | 40 ± 8.1 |
| Female, n (%) | 22 (55) |
| Caucasian, n (%) | 35 (85) |
| BMI, kg/m2 | 29.6 ± 3.3 |
| Total cholesterol, mg/dL | 179.5 ± 4.0 |
| LDL, mg/dL | 121.5 ± 3.4 |
| HDL, mg/dL | 48.1 ± 1.1 |
| Triglycerides, mg/dL | 98.1 ± 5.8 |
| Glucose, mg/dL | 93.3 ± 1.0 |
| Insulin, µIU/mL | 8.1 ± 0.5 |
| SBP/DBP, mmHg | 115 ± 1.2/76 ± 1.0 |
| Outcome | VEG HDP | BEEF HDP | p Values | |||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Change | Pre | Post | Change | Time | Time × Diet | |
| Total cholesterol (mg/dL) | 180.5 ± 4 | 166.0 ± 4 | −14.5 ± 3.5 * | 178.8 ± 4 | 168.5 ± 4 | −10.3 ± 3.5 * | 0.001 | 0.166 |
| LDL (mg/dL) | 120.9 ± 3.5 | 110.3 ± 3.4 | −10.6 ± 2.9 * | 119.4 ± 3.4 | 113.7 ± 3.4 | −5.8 ± 2.9 | 0.005 | 0.036 |
| Total LDL particles (nmol/L) | 870.3 ± 21.8 | 792.9 ± 21.8 | −77.4 ± 19.8 * | 853 ± 21.7 | 791.5 ± 21.8 | −61.5 ± 19.7 * | <0.001 | 0.366 |
| Dense LDL III (nmol/L) | 288.3 ± 15.4 | 266.3 ± 15.3 | −22 ± 16.1 | 273.5 ± 15.3 | 243.4 ± 15.4 | −30.1 ± 16 | 0.063 | 0.636 |
| Dense LDL IV (nmol/L) | 77.6 ± 2.9 | 70.3 ± 2.9 | −7.4 ± 3 | 75.8 ± 2.9 | 70.6 ± 2.9 | −5.2 ± 3 | 0.010 | 0.577 |
| HDL (mg/dL) | 48.1 ± 1.2 | 42 ± 1.2 | −6.1 ± 1 * | 47.4 ± 1.2 | 42.1 ± 1.2 | −5.4 ± 1 * | <0.001 | 0.415 |
| Total HDL particles (nmol/L) | 7036.8 ± 88.5 | 6687.3 ± 88.5 | −349.5 ± 95 * | 6998.7 ± 88.4 | 6663 ± 88.4 | −335.7 ± 94.6 * | <0.001 | 0.916 |
| Buoyant HDL2b (nmol/L) | 2135 ± 59 | 1889.3 ± 58.8 | −245.6 ± 51.6 * | 2128 ± 58.7 | 1863.1 ± 58.9 | −264.9 ± 51.1 * | <0.001 | 0.657 |
| Apolipoprotein B (mg/dL) | 88.5 ± 2.2 | 84.3 ± 2.2 | −4.2 ± 2 | 87 ± 2.2 | 85.1 ± 2.2 | −1.8 ± 2 | 0.100 | 0.158 |
| Apolipoprotein A1(mg/dL) | 135.7 ± 2.4 | 120.7 ± 2.4 | −15.1 ± 2.2 * | 134.5 ± 2.4 | 121.3 ± 2.4 | −13.2 ± 2.2 * | <0.001 | 0.424 |
| Triglycerides (mg/dL) | 103.6 ± 5.7 | 104.9 ± 5.7 | 1.3 ± 5.3 | 98.3 ± 5.7 | 97.4 ± 5.7 | −0.9 ± 5.2 | 0.967 | 0.681 |
| Glucose (mg/dL) | 94.7 ± 1.1 | 92.1 ± 1.1 | −2.7 ± 1.1 | 93.3 ± 1.1 | 93.9 ± 1.1 | 0.6 ± 1.1 | 0.317 | 0.001 |
| Insulin (µIU/mL) | 9.2 ± 0.7 | 7.8 ± 0.7 | −1.4 ± 0.5 * | 7.8 ± 0.7 | 7.7 ± 0.7 | −0.1 ± 0.5 | 0.100 | 0.020 |
| HOMA-IR | 2.4 ± 0.4 | 1.8 ± 0.5 | −0.6 ± 0.5 | 1.8 ± 0.4 | 1.9 ± 0.5 | 0.1 ± 0.5 | 0.618 | 0.140 |
| SBP (mmHg) | 114.9 ± 1.2 | 112.2 ± 1.2 | −2.7 ± 1.2 | 114 ± 1.2 | 112.5 ± 1.2 | −1.5 ± 1.2 | 0.044 | 0.265 |
| DBP (mmHg) | 75.8 ± 1.1 | 74.4 ± 1.1 | −1.4 ± 0.9 | 76.2 ± 1.1 | 74.4 ± 1.1 | −1.8 ± 0.9 | 0.065 | 0.634 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hill, E.R.; Wang, Y.; Davis, E.M.; Campbell, W.W. Healthy Dietary Patterns with and without Meat Improved Cardiometabolic Disease Risk Factors in Adults: A Randomized Crossover Controlled Feeding Trial. Nutrients 2024, 16, 2542. https://doi.org/10.3390/nu16152542
Hill ER, Wang Y, Davis EM, Campbell WW. Healthy Dietary Patterns with and without Meat Improved Cardiometabolic Disease Risk Factors in Adults: A Randomized Crossover Controlled Feeding Trial. Nutrients. 2024; 16(15):2542. https://doi.org/10.3390/nu16152542
Chicago/Turabian StyleHill, Erica R, Yu Wang, Eric M Davis, and Wayne W Campbell. 2024. "Healthy Dietary Patterns with and without Meat Improved Cardiometabolic Disease Risk Factors in Adults: A Randomized Crossover Controlled Feeding Trial" Nutrients 16, no. 15: 2542. https://doi.org/10.3390/nu16152542
APA StyleHill, E. R., Wang, Y., Davis, E. M., & Campbell, W. W. (2024). Healthy Dietary Patterns with and without Meat Improved Cardiometabolic Disease Risk Factors in Adults: A Randomized Crossover Controlled Feeding Trial. Nutrients, 16(15), 2542. https://doi.org/10.3390/nu16152542






