The Role of Nutrition on Thyroid Function
Abstract
1. Introduction
2. Methods
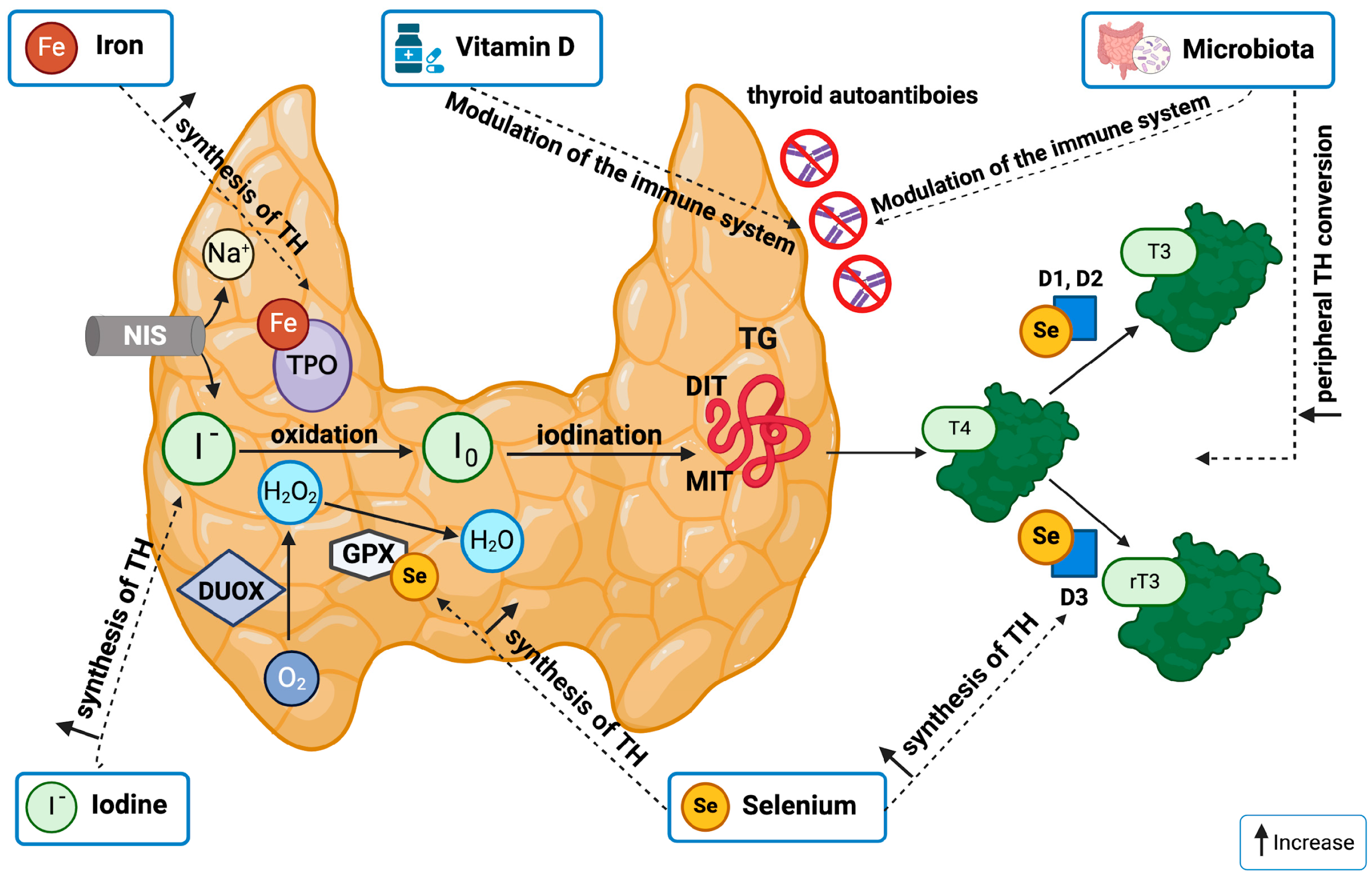
3. Micronutrients and Thyroid Function
3.1. Iodine
3.2. Selenium
3.3. Iron
3.4. Vitamin D
3.5. Zinc
4. Other Micronutrients: Copper, Magnesium, Vitamin A, and Vitamin B12
4.1. Copper
4.2. Magnesium
4.3. Vitamin A
4.4. Vitamin B12
5. Nutrition, Gut Microbiota, and Thyroid Function
6. Conclusions
- -
- iodine is essential for thyroid hormone synthesis and beneficial for the prevention of thyroid disorders;
- -
- selenium may reduce thyroid autoantibodies and improve thyroid function, even though careful monitoring is required;
- -
- hypothyroid individuals should be assessed for anemia, iron, and vitamin B12 deficiency;
- -
- vitamin D supplementation might be beneficial for patients with Hashimoto thyroiditis;
- -
- the evaluation of zinc levels is recommended for subjects with thyroid enlargement; and
- -
- copper and magnesium should be studied for their potential protective role against thyroid cancer, and vitamin A for autoimmune thyroid disease.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Armstrong, M.; Asuka, E.; Fingeret, A. Physiology, Thyroid Function. In StatPearls; [Updated 13 March 2023]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537039/ (accessed on 12 February 2024).
- Knezevic, J.; Starchl, C.; Tmava Berisha, A.; Amrein, K. Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function? Nutrients 2020, 12, 1769. [Google Scholar] [CrossRef] [PubMed]
- Street, M.E.; Shulhai, A.-M.; Petraroli, M.; Patianna, V.; Donini, V.; Giudice, A.; Gnocchi, M.; Masetti, M.; Montani, A.G.; Rotondo, R.; et al. The impact of environmental factors and contaminants on thyroid function and disease from fetal to adult life: Current evidence and future directions. Front. Endocrinol. 2024, 15, 1429884. [Google Scholar] [CrossRef]
- Krishnamurthy, H.K.; Reddy, S.; Jayaraman, V.; Krishna, K.; Song, Q.; Rajasekaran, K.E.; Wang, T.; Bei, K.; Rajasekaran, J.J. Effect of Micronutrients on Thyroid Parameters. J. Thyroid. Res. 2021, 2021, 1865483. [Google Scholar] [CrossRef]
- Rayman, M.P. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc. Nutr. Soc. 2019, 78, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Salas, S.; Gonzalez-Arias, M. Nutrition: Micronutrient Intake, Imbalances, and Interventions. In StatPearls; [Updated 21 September 2023]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK597352/ (accessed on 12 February 2024).
- Sorrenti, S.; Baldini, E.; Pironi, D.; Lauro, A.; D’Orazi, V.; Tartaglia, F.; Tripodi, D.; Lori, E.; Gagliardi, F.; Praticò, M.; et al. Iodine: Its Role in Thyroid Hormone Biosynthesis and Beyond. Nutrients 2021, 13, 4469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- European Food Safety Authority Panel 2014. Scientific Opinion on Dietary Reference Values for Iodine. EFSA J. 2014, 12, 3660. [Google Scholar]
- World Health Organization and Food and Agriculture Organization of the United Nations. Vitamin and Mineral Requirements in Human Nutrition: Report of a Joint FAO/WHO Expert Consultation, 2nd ed.; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Iannuzzo, G.; Campanozzi, A.; Trevisani, V.; Rutigliano, I.; Abate, V.; Rendina, D.; De Filippo, G. Iodine Requirements in Pediatrics: From Fetal Life to Adolescence. Front. Endocrinol. 2022, 13, 929176. [Google Scholar] [CrossRef]
- Morreale de Escobar, G.; Obregon, M.J.; Escobar del Rey, F. Role of thyroid hormone during early brain development. Eur. J. Endocrinol. 2004, 151 (Suppl. S3), U25–U37. [Google Scholar] [CrossRef] [PubMed]
- Bath, S.C. Thyroid function and iodine intake: Global recommendations and relevant dietary trends. Nat. Rev. Endocrinol. 2024, 20, 474–486. [Google Scholar] [CrossRef]
- Köhrle, J. Selenium, Iodine and Iron-Essential Trace Elements for Thyroid Hormone Synthesis and Metabolism. Int. J. Mol. Sci. 2023, 24, 3393. [Google Scholar] [CrossRef]
- Dineva, M.; Fishpool, H.; Rayman, M.P.; Mendis, J.; Bath, S.C. Systematic review and meta-analysis of the effects of iodine supplementation on thyroid function and child neurodevelopment in mildly-to-moderately iodine-deficient pregnant women. Am. J. Clin. Nutr. 2020, 112, 389–412. [Google Scholar] [CrossRef] [PubMed]
- Hynes, K.L.; Otahal, P.; Hay, I.; Burgess, J.R. Mild iodine deficiency during pregnancy is associated with reduced educational outcomes in the offspring: 9-year follow-up of the gestational iodine cohort. J. Clin. Endocrinol. Metab. 2013, 98, 1954–1962. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Diaz, E.; Pearce, E.N. Iodine status and supplementation before, during, and after pregnancy. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101430. [Google Scholar] [CrossRef] [PubMed]
- Bath, S.C.; Steer, C.D.; Golding, J.; Emmett, P.; Rayman, M.P. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: Results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 2013, 382, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.; Braverman, L. Consequences of excess iodine. Nat. Rev. Endocrinol. 2014, 10, 136–142. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guideline: Fortification of Food-Grade Salt with Iodine for the Prevention and Control of Iodine Deficiency Disorders; World Health Organization: Geneva, Switzerland, 2014. Available online: https://www.ncbi.nlm.nih.gov/books/NBK254243/ (accessed on 12 February 2024).
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017, 27, 315–389, Erratum in Thyroid 2017, 27, 1212. [Google Scholar] [CrossRef] [PubMed]
- Fallah, R.; Du, L.; Braverman, L.E.; He, X.; Segura-Harrison, M.; Yeh, M.W.; Pearce, E.N.; Chiu, H.K.; Mittelman, S.D.; Leung, A.M. Iodine Nutrition in Weaning Infants in the United States. Thyroid 2019, 29, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Nazeri, P.; Dalili, H.; Mehrabi, Y.; Hedayati, M.; Mirmiran, P.; Azizi, F. Is there any difference between the iodine statuses of breast-fed and formula-fed infants and their mothers in an area with iodine sufficiency? Br. J. Nutr. 2018, 119, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Kawashima, A.; Ishido, Y.; Yoshihara, A.; Oda, K.; Hiroi, N.; Ito, T.; Ishii, N.; Suzuki, K. Iodine excess as an environmental risk factor for autoimmune thyroid disease. Int. J. Mol. Sci. 2014, 15, 12895–12912. [Google Scholar] [CrossRef]
- Duntas, L.H. The Role of Iodine and Selenium in Autoimmune Thyroiditis. Horm. Metab. Res. 2015, 47, 721–726. [Google Scholar] [CrossRef]
- Teng, W.; Shan, Z.; Teng, X.; Guan, H.; Li, Y.; Teng, D.; Jin, Y.; Yu, X.; Fan, C.; Chong, W.; et al. Effect of iodine intake on thyroid diseases in China. N. Engl. J. Med. 2006, 354, 2783–2793. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, I.B.; Knudsen, N.; Carlé, A.; Vejbjerg, P.; Jørgensen, T.; Perrild, H.; Ovesen, L.; Rasmussen, L.B.; Laurberg, P. A cautious iodization programme bringing iodine intake to a low recommended level is associated with an increase in the prevalence of thyroid autoantibodies in the population. Clin. Endocrinol. 2011, 75, 120–169. [Google Scholar] [CrossRef]
- Iacone, R.; Iaccarino Idelson, P.; Campanozzi, A.; Rutigliano, I.; Russo, O.; Formisano, P.; Galeone, D.; Macchia, P.E.; Strazzullo, P.; MINISAL-GIRCSI Study Group. Relationship Between Salt Consumption and Iodine Intake in a Pediatric Population. Eur. J. Nutr. 2021, 60, 2193–2202. [Google Scholar] [CrossRef]
- Rendina, D.; De Palma, D.; De Filippo, G.; De Pascale, F.; Muscariello, R.; Ippolito, R.; Fazio, V.; Fiengo, A.; Benvenuto, D.; Strazzullo, P.; et al. Prevalence of Simple Nodular Goiter and Hashimoto’s Thyroiditis in Current, Previous, and Never Smokers in a Geographical Area with Mild Iodine Deficiency. Horm. Metab. Res. 2015, 47, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Santi, C.; Bagnoli, L. Celebrating Two Centuries of Research in Selenium Chemistry: State of the Art and New Prospective. Molecules 2017, 22, 2124. [Google Scholar] [CrossRef]
- Chada, S.; Whitney, C.; Newburger, P.E. Post-transcriptional regulation of GPx gene expression by selenium in the HL-60 human myeloid cell line. Blood 1989, 74, 2535–2541. [Google Scholar] [CrossRef]
- Flohe, L.; Günzler, W.A.; Schock, H.H. Glutathione peroxidase: A selenoenzyme. FEBS Lett. 1973, 32, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Wildberger, E.; Kohler, H.; Jenzer, H.; Kämpf, J.; Studer, H. Inactivation of peroxidase and glucose oxidase by H2O2 and iodide during in vitro thyroglobulin iodination. Mol. Cell Endocrinol. 1986, 46, 149–154. [Google Scholar] [CrossRef]
- Negro, R.; Greco, G.; Mangieri, T.; Pezzarossa, A.; Dazzi, D.; Hassan, H. The influence of selenium supplementation on postpartum thyroid status in pregnant women with thyroid peroxidase autoantibodies. J. Clin. Endocrinol. Metab. 2007, 92, 1263–1268. [Google Scholar] [CrossRef]
- Mao, J.; Pop, V.J.; Bath, S.C.; Vader, H.L.; Redman, C.W.; Rayman, M.P. Effect of low-dose selenium on thyroid autoimmunity and thyroid function in UK pregnant women with mild-to-moderate iodine deficiency. Eur. J. Nutr. 2016, 55, 55–61. [Google Scholar] [CrossRef]
- Chanoine, J.P. Selenium and thyroid function in infants, children and adolescents. Biofactors 2003, 19, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, C.; Li, S.; Cui, L.; Zhao, J.; Liao, L. Selenium and thyroid diseases. Front. Endocrinol. 2023, 14, 1133000. [Google Scholar] [CrossRef] [PubMed]
- van Bakel, M.M.; Printzen, G.; Wermuth, B.; Wiesmann, U.N. Antioxidant and thyroid hormone status in selenium-deficient phenylketonuric and hyperphenylalaninemic patients. Am. J. Clin. Nutr. 2000, 72, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Calomme, M.R.; Vanderpas, J.B.; François, B.; Van Caillie-Bertrand, M.; Herchuelz, A.; Vanovervelt, N.; Van Hoorebeke, C.; Vanden Berghe, D.A. Thyroid function parameters during a selenium repletion/depletion study in phenylketonuric subjects. Experientia 1995, 51, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Chanoine, J.P.; Veronikis, I.; Alex, S.; Stone, S.; Fang, S.L.; Leonard, J.L.; Braverman, L.E. The postnatal serum 3,5,3′-triiodothyronine (T3) surge in the rat is largely independent of extrathyroidal 5′-deiodination of thyroxine to T3. Endocrinology 1993, 133, 2604–2609. [Google Scholar] [CrossRef] [PubMed]
- Chanoine, J.P.; Alex, S.; Stone, S.; Fang, S.L.; Veronikis, I.; Leonard, J.L.; Braverman, L.E. Placental 5-deiodinase activity and fetal thyroid hormone economy are unaffected by selenium deficiency in the rat. Pediatr. Res. 1993, 34, 288–292. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Contempré, B.; Duale, N.L.; Dumont, J.E.; Ngo, B.; Diplock, A.T.; Vanderpas, J. Effect of selenium supplementation on thyroid hormone metabolism in an iodine and selenium deficient population. Clin. Endocrinol. 1992, 36, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, Y.; Chen, P.; Wei, J.; Lv, H.; Wang, S.; Wu, Y.; Zhao, X.; Peng, X.; Rijntjes, E.; et al. Increased Incidence of Hashimoto Thyroiditis in Selenium Deficiency: A Prospective 6-Year Cohort Study. J. Clin. Endocrinol. Metab. 2022, 107, e3603–e3611. [Google Scholar] [CrossRef]
- Wichman, J.; Winther, K.H.; Bonnema, S.J.; Hegedüs, L. Selenium Supplementation Significantly Reduces Thyroid Autoantibody Levels in Patients with Chronic Autoimmune Thyroiditis: A Systematic Review and Meta-Analysis. Thyroid 2016, 26, 1681–1692. [Google Scholar] [CrossRef]
- Winther, K.H.; Wichman, J.E.; Bonnema, S.J.; Hegedüs, L. Insufficient documentation for clinical efficacy of selenium supplementation in chronic autoimmune thyroiditis, based on a systematic review and meta-analysis. Endocrine 2017, 55, 376–385. [Google Scholar] [CrossRef]
- Huwiler, V.V.; Maissen-Abgottspon, S.; Stanga, Z.; Mühlebach, S.; Trepp, R.; Bally, L.; Bano, A. Selenium Supplementation in Patients with Hashimoto Thyroiditis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Thyroid 2024, 34, 295–313. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kong, X.Q.; Qiu, G.Y.; Yang, Z.B.; Tan, Z.X.; Quan, X.Q. Clinical efficacy of selenium supplementation in patients with Hashimoto thyroiditis: A systematic review and meta-analysis. Medicine 2023, 102, e33791. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Godlewska, M.; Banga, P.J. Thyroid peroxidase as a dual active site enzyme: Focus on biosynthesis, hormonogenesis and thyroid disorders of autoimmunity and cancer. Biochimie 2019, 160, 34–45. [Google Scholar] [CrossRef]
- Moreno-Reyes, R.; Corvilain, B.; Daelemans, C.; Wolff, F.; Fuentes Peña, C.; Vandevijvere, S. Iron Deficiency Is a Risk Factor for Thyroid Dysfunction During Pregnancy: A Population-Based Study in Belgium. Thyroid 2021, 31, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Brigham, D.E.; Beard, J.L. Effect of thyroid hormone replacement in iron-deficient rats. Am. J. Physiol. 1995, 269 Pt 2, R1140–R1147. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Araque, C.; Valdés, S.; Lago-Sampedro, A.; Lillo-Muñoz, J.A.; Garcia-Fuentes, E.; Perez-Valero, V.; Gutierrez-Repiso, C.; Goday, A.; Urrutia, I.; Peláez, L.; et al. Iron deficiency is associated with Hypothyroxinemia and Hypotriiodothyroninemia in the Spanish general adult population: Di@bet.es study. Sci. Rep. 2018, 8, 6571. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Burgi, H.; Hurrell, R.F. Iron deficiency predicts poor maternal thyroid status during pregnancy. J. Clin. Endocrinol. Metab. 2007, 92, 3436–3440. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, S.; Gao, X.; Wei, Y.; Zhu, G.; Yang, C. The Relationship between Iron Deficiency and Thyroid Function in Chinese Women during Early Pregnancy. J. Nutr. Sci. Vitaminol. 2016, 62, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; O’Brien, K.O. Pregnancy and iron homeostasis: An update. Nutr. Rev. 2013, 71, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, X.; Yuan, L.; Guo, L. Iron Deficiency, a Risk Factor of Thyroid Disorders in Reproductive-Age and Pregnant Women: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 629831. [Google Scholar] [CrossRef]
- van Gucht, A.L.M.; Meima, M.E.; Moran, C.; Agostini, M.; Tylki-Szymanska, A.; Krajewska, M.W.; Chrzanowska, K.; Efthymiadou, A.; Chrysis, D.; Demir, K.; et al. Anemia in Patients with Resistance to Thyroid Hormone α: A Role for Thyroid Hormone Receptor α in Human Erythropoiesis. J. Clin. Endocrinol. Metab. 2017, 102, 3517–3525. [Google Scholar] [CrossRef] [PubMed]
- Pastori, V.; Pozzi, S.; Labedz, A.; Ahmed, S.; Ronchi, A.E. Role of Nuclear Receptors in Controlling Erythropoiesis. Int. J. Mol. Sci. 2022, 23, 2800. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Köhrle, J. The impact of iron and selenium deficiencies on iodine and thyroid metabolism: Biochemistry and relevance to public health. Thyroid 2002, 12, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, H.; Korwutthikulrangsri, M.; Fu, J.; Liao, X.H.; Dumitrescu, A.M. Role of the Thyroid Gland in Expression of the Thyroid Phenotype of Sbp2-Deficient Mice. Endocrinology 2020, 161, bqz032. [Google Scholar] [CrossRef]
- Koç, Ş.; Güngör, K.; Güngör, N.D.; Uzunlulu, M. Iron Deficiency in Women with Thyroid-Specific Autoantibodies: A Case Control Study. J. Exp. Clin. Med. 2022, 39, 194–198. [Google Scholar] [CrossRef]
- Ravanbod, M.; Asadipooya, K.; Kalantarhormozi, M.; Nabipour, I.; Omrani, G.R. Treatment of iron-deficiency anemia in patients with subclinical hypothyroidism. Am. J. Med. 2013, 126, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Czarnywojtek, A.; Florek, E.; Pietrończyk, K.; Sawicka-Gutaj, N.; Ruchała, M.; Ronen, O.; Nixon, I.J.; Shaha, A.R.; Rodrigo, J.P.; Tufano, R.P.; et al. The Role of Vitamin D in Autoimmune Thyroid Diseases: A Narrative Review. J. Clin. Med. 2023, 12, 1452. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D. Nonclassic actions of vitamin D. J. Clin. Endocrinol. Metab. 2009, 94, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Ginde, A.A.; Sullivan, A.F.; Mansbach, J.M.; Camargo, C.A., Jr. Vitamin D insufficiency in pregnant and nonpregnant women of childbearing age in the United States. Am. J. Obstet. Gynecol. 2010, 202, 436.e1–436.e8. [Google Scholar] [CrossRef]
- van der Meer, I.M.; Karamali, N.S.; Boeke, A.J.; Lips, P.; Middelkoop, B.J.; Verhoeven, I.; Wuister, J.D. High prevalence of vitamin D deficiency in pregnant non-Western women in The Hague, Netherlands. Am. J. Clin. Nutr. 2006, 84, 350–353, quiz 468–469. [Google Scholar] [CrossRef]
- Mulligan, M.L.; Felton, S.K.; Riek, A.E.; Bernal-Mizrachi, C. Implications of vitamin D deficiency in pregnancy and lactation. Am. J. Obstet. Gynecol. 2010, 202, 429.e1–429.e9. [Google Scholar] [CrossRef] [PubMed]
- Smyth, P.P.; Wijeyaratne, C.N.; Kaluarachi, W.N.; Smith, D.F.; Premawardhana, L.D.; Parkes, A.B.; Jayasinghe, A.; de Silva, D.G.; Lazarus, J.H. Sequential studies on thyroid antibodies during pregnancy. Thyroid 2005, 15, 474–477. [Google Scholar] [CrossRef]
- Panesar, N.S.; Chan, K.W.; Li, C.Y.; Rogers, M.S. Status of anti-thyroid peroxidase during normal pregnancy and in patients with hyperemesis gravidarum. Thyroid 2006, 16, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Evliyaoğlu, O.; Acar, M.; Özcabı, B.; Erginöz, E.; Bucak, F.; Ercan, O.; Kucur, M. Vitamin D Deficiency and Hashimoto’s Thyroiditis in Children and Adolescents: A Critical Vitamin D Level for This Association? J. Clin. Res. Pediatr. Endocrinol. 2015, 7, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Cvek, M.; Kaličanin, D.; Barić, A.; Vuletić, M.; Gunjača, I.; Torlak Lovrić, V.; Škrabić, V.; Punda, A.; Boraska Perica, V. Vitamin D and Hashimoto’s Thyroiditis: Observations from CROHT Biobank. Nutrients 2021, 13, 2793. [Google Scholar] [CrossRef]
- Hanna, H.W.Z.; Rizzo, C.; Abdel Halim, R.M.; El Haddad, H.E.; Salam, R.; El-Sayed Abou-Youssef, H. Vitamin D status in Hashimoto’s thyroiditis and its association with vitamin D receptor genetic variants. J. Steroid Biochem. Mol. Biol. 2021, 212, 105922. [Google Scholar] [CrossRef]
- Filipova, L.; Lazurova, Z.; Fulop, P.; Lazurova, I. Vitamin D insufficiency is not associated with thyroid autoimmunity in Slovak women with Hashimoto’s disease. Bratisl. Lek. Listy 2023, 124, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Metwalley, K.A.; Farghaly, H.S.; Sherief, T.; Hussein, A. Vitamin D status in children and adolescents with autoimmune thyroiditis. J. Endocrinol. Investig. 2016, 39, 793–797. [Google Scholar] [CrossRef]
- Mazokopakis, E.E.; Papadomanolaki, M.G.; Tsekouras, K.C.; Evangelopoulos, A.D.; Kotsiris, D.A.; Tzortzinis, A.A. Is vitamin D related to pathogenesis and treatment of Hashimoto’s thyroiditis? Hell. J. Nucl. Med. 2015, 18, 222–227. [Google Scholar]
- Chaudhary, S.; Dutta, D.; Kumar, M.; Saha, S.; Mondal, S.A.; Kumar, A.; Mukhopadhyay, S. Vitamin D supplementation reduces thyroid peroxidase antibody levels in patients with autoimmune thyroid disease: An open-labeled randomized controlled trial. Indian. J. Endocrinol. Metab. 2016, 20, 391–398. [Google Scholar]
- Taheriniya, S.; Arab, A.; Hadi, A.; Fadel, A.; Askari, G. Vitamin D and thyroid disorders: A systematic review and Meta-analysis of observational studies. BMC Endocr. Disord. 2021, 21, 171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bizzaro, G.; Shoenfeld, Y. Vitamin D and thyroid autoimmune diseases: The known and the obscure. Immunol. Res. 2015, 61, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Bui, L.; Zhu, Z.; Hawkins, S.; Cortez-Resendiz, A.; Bellon, A. Vitamin D regulation of the immune system and its implications for COVID-19: A mini review. SAGE Open Med. 2021, 9, 20503121211014073. [Google Scholar] [CrossRef] [PubMed]
- Palanca, A.; Ampudia-Blasco, F.J.; Real, J.T. The Controversial Role of Vitamin D in Thyroid Cancer Prevention. Nutrients 2022, 14, 2593. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xue, C.; Ren, S.; Dong, L.; Gao, J.; Li, X. Association between vitamin D status and thyroid cancer: A meta-analysis. Front. Nutr. 2024, 11, 1423305. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lebiedziński, F.; Lisowska, K.A. Impact of Vitamin D on Immunopathology of Hashimoto’s Thyroiditis: From Theory to Practice. Nutrients 2023, 15, 3174. [Google Scholar] [CrossRef]
- Tang, J.; Shan, S.; Li, F.; Yun, P. Effects of vitamin D supplementation on autoantibodies and thyroid function in patients with Hashimoto’s thyroiditis: A systematic review and meta-analysis. Medicine 2023, 102, e36759. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Costa, M.I.; Sarmento-Ribeiro, A.B.; Gonçalves, A.C. Zinc: From Biological Functions to Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 4822. [Google Scholar] [CrossRef] [PubMed]
- O’Kane, S.M.; Mulhern, M.S.; Pourshahidi, L.K.; Strain, J.J.; Yeates, A.J. Micronutrients, iodine status and concentrations of thyroid hormones: A systematic review. Nutr. Rev. 2018, 76, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Severo, J.S.; Morais, J.B.S.; de Freitas, T.E.C.; Andrade, A.L.P.; Feitosa, M.M.; Fontenelle, L.C.; de Oliveira, A.R.S.; Cruz, K.J.C.; do Nascimento Marreiro, D. The Role of Zinc in Thyroid Hormones Metabolism. Int. J. Vitam. Nutr. Res. 2019, 89, 80–88. [Google Scholar] [CrossRef]
- Prasad, A.S.; Bao, B. Molecular mechanisms of zinc as a pro-antioxidant mediator: Clinical therapeutic implications. Antioxidants 2019, 8, 164. [Google Scholar] [CrossRef] [PubMed]
- Maywald, M.; Rink, L. Zinc homeostasis and immunosenescence. J. Trace Elem. Med. Biol. 2015, 29, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Maywald, M.; Wang, F.; Rink, L. The intracellular free zinc level is vital for treg function and a feasible tool to discriminate between treg and activated th cells. Int. J. Mol. Sci. 2018, 19, 3575. [Google Scholar] [CrossRef] [PubMed]
- Kravchenko, V.; Zakharchenko, T. Thyroid hormones and minerals in immunocorrection of disorders in autoimmune thyroid diseases. Front. Endocrinol. 2023, 14, 1225494. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xue, S.; Zhang, L.; Chen, G. Trace elements and the thyroid. Front. Endocrinol. 2022, 13, 904889. [Google Scholar] [CrossRef] [PubMed]
- Betsy, A.; Binitha, M.; Sarita, S. Zinc deficiency associated with hypothyroidism: An overlooked cause of severe alopecia. Int. J. Trichology 2013, 5, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Ertek, S.; Cicero, A.F.; Caglar, O.; Erdogan, G. Relationship between serum zinc levels, thyroid hormones and thyroid volume following successful iodine supplementation. Hormones 2010, 9, 263–268. [Google Scholar] [CrossRef]
- Rezaei, M.; Javadmoosavi, S.Y.; Mansouri, B.; Azadi, N.A.; Mehrpour, O.; Nakhaee, S. Thyroid dysfunction: How concentration of toxic and essential elements contribute to risk of hypothyroidism, hyperthyroidism, and thyroid cancer. Environ. Sci. Pollut. Res. 2019, 26, 35787–35796. [Google Scholar] [CrossRef] [PubMed]
- Regmi, D.; Gautam, N.; Shahi, A.; Bohara, S.; Subedi, S.; Jayan, A. Status of Serum Zinc Level in Hypothyroid Patients with Normal Serum Albumin Level: A Case Control Study. J. Univers. Coll. Med. Sci. 2019, 7, 34–38. [Google Scholar] [CrossRef]
- Talebi, S.; Ghaedi, E.; Sadeghi, E.; Mohammadi, H.; Hadi, A.; Clark, C.C.T.; Askari, G. Trace Element Status and Hypothyroidism: A Systematic Review and Meta-analysis. Biol. Trace Elem. Res. 2020, 197, 1–14. [Google Scholar] [CrossRef]
- Chang, H.C.; Chang, Y.S. Association between serum zinc levels and androgenetic alopecia: A systematic review and meta-analysis. J. Cosmet. Dermatol. 2022, 21, 1774–1777. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zheng, H.; Shan, B.; Wu, Y. Changes of serum trace elements level in patients with alopecia areata: A meta-analysis. J. Dermatol. 2017, 44, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Manisha, A.; Roshan, K.M.; Sudeep, K.; Imran, M.; Sumesh, P.S. Study of Trace Elements in Patients of Hypothyroidism with Special Reference to Zinc and Copper. Biomed. J. Sci. Tech. Res. 2018, 6, 5190–5194. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, H.B.; Cheng, M.X.; Wang, L.; Gao, C.B.; Huang, F. Association of exposure to multiple metals with papillary thyroid cancer risk in China. Environ. Sci. Pollut. Res. Int. 2019, 26, 20560–20572. [Google Scholar] [CrossRef] [PubMed]
- Baltaci, A.K.; Dundar, T.K.; Aksoy, F.; Mogulkoc, R. Changes in the serum levels of trace elements before and after the operation in thyroid cancer patients. Biol. Trace Elem. Res. 2017, 175, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, H.; Jiang, H.; Lin, H.; He, J.; Fan, S.; Yu, D.; Yang, L.; Tang, H.; Lin, E.; et al. The effect of micronutrient on thyroid cancer risk: A Mendelian randomization study. Front. Nutr. 2024, 11, 1331172. [Google Scholar] [CrossRef]
- El-Fadeli, S.; Bouhouch, S.; Skalny, A.V.; Barkouch, Y.; Pineau, A.; Cherkaoui, M.; Sedki, A. Effects of Imbalance in Trace Element on Thyroid Gland from Moroccan Children. Biol. Trace Elem. Res. 2016, 170, 288–293. [Google Scholar] [CrossRef]
- Turan, E.; Turksoy, V.A. Selenium, Zinc, and Copper Status in Euthyroid Nodular Goiter: A Cross-Sectional Study. Int. J. Prev. Med. 2021, 12, 46. [Google Scholar] [CrossRef]
- Szczepanik, J.; Podgórski, T.; Domaszewska, K. The Level of Zinc, Copper and Antioxidant Status in the Blood Serum of Women with Hashimoto’s Thyroiditis. Int. J. Environ. Res. Public Health 2021, 18, 7805. [Google Scholar] [CrossRef]
- Sivakumar, R.R.; Chinnaiah Govindareddy, D.; Sahoo, J.; Bobby, Z.; Chinnakali, P. Effect of daily zinc supplementation for 12 weeks on serum thyroid auto-antibody levels in children and adolescents with autoimmune thyroiditis—A randomized controlled trial. J. Pediatr. Endocrinol. Metab. 2024, 37, 137–143. [Google Scholar] [CrossRef]
- Lu, L.; Huang, Z.; Wang, X.; Chen, J. Interaction Between Dietary Selenium and Zinc Intakes on Hypothyroidism. Biol. Trace Elem. Res. 2023, 201, 4667–4676. [Google Scholar] [CrossRef] [PubMed]
- Wróblewski, M.; Wróblewska, J.; Nuszkiewicz, J.; Pawłowska, M.; Wesołowski, R.; Woźniak, A. The Role of Selected Trace Elements in Oxidoreductive Homeostasis in Patients with Thyroid Diseases. Int. J. Mol. Sci. 2023, 24, 4840. [Google Scholar] [CrossRef] [PubMed]
- Georgieva Bacelova, M.; Dimitrova Gatseva, P.; Ivanova Deneva, T.; Miteva Davcheva, D.; Veselinova Bivolarska, A. Are the elements zinc, copper, magnesium, and rubidium related to nutrition and iodine deficiency in pregnant Bulgarian women from iodine deficient region? Cent. Eur. J. Public Health 2024, 32, 31–38. [Google Scholar] [CrossRef]
- Jain, R.B. Thyroid Function and Serum Copper, Selenium, and Zinc in General U.S. Population. Biol. Trace Elem. Res. 2014, 159, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Rasic-Milutinovic, Z.; Jovanovic, D.; Bogdanovic, G.; Trifunovic, J.; Mutic, J. Potential Influence of Selenium, Copper, Zinc and Cadmium on L-Thyroxine Substitution in Patients with Hashimoto Thyroiditis and Hypothyroidism. Exp. Clin. Endocrinol. Diabetes 2017, 125, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Cai, W.S.; Li, J.L.; Feng, Z.; Cao, J.; Xu, B. The Association Between Serum Levels of Selenium, Copper, and Magnesium with Thyroid Cancer: A Meta-analysis. Biol. Trace Elem. Res. 2015, 167, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Brady, D.C.; Crowe, M.S.; Greenberg, D.N.; Counter, C.M. Copper Chelation Inhibits BRAFV600E-Driven Melanomagenesis and Counters Resistance to BRAFV600E and MEK1/2 Inhibitors. Cancer Res. 2017, 77, 6240–6252. [Google Scholar] [CrossRef]
- Kolanu, B.R.; Vadakedath, S.; Boddula, V.; Kandi, V. Activities of Serum Magnesium and Thyroid Hormones in Pre-, Peri-, and Post-menopausal Women. Cureus 2020, 12, e6554. [Google Scholar] [CrossRef] [PubMed]
- Moncayo, R.; Moncayo, H. The WOMED model of benign thyroid disease: Acquired magnesium deficiency due to physical and psychological stressors relates to dysfunction of oxidative phosphorylation. BBA Clin. 2014, 3, 44–64. [Google Scholar] [CrossRef]
- Ige, A.O.; Chidi, R.N.; Egbeluya, E.E.; Jubreel, R.O.; Adele, B.O.; Adewoye, E.O. Amelioration of thyroid dysfunction by magnesium in experimental diabetes may also prevent diabetes-induced renal impairment. Heliyon 2019, 5, e01660. [Google Scholar] [CrossRef]
- Yanko, R.V.; Chaka, E.G.; Levashov, M.I. Histomorphological changes in the thyroid gland ofrats after magnesium chloride ingestion. Clin. Exp. Morphology. 2019, 8, 41–47. [Google Scholar] [CrossRef]
- Wang, K.; Wei, H.; Zhang, W.; Li, Z.; Ding, L.; Yu, T.; Tan, L.; Liu, Y.; Liu, T.; Wang, H.; et al. Severely low serum magnesium is associated with increased risks of positive anti-thyroglobulin antibody and hypothyroidism: A cross-sectional study. Sci. Rep. 2018, 8, 9904. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hu, X.; Li, J.; Nie, Z.; Kang, S.; Liu, H.; Wang, Y.; Jia, X.; Lyu, Z. The Inverse Association of Serum Magnesium with Papillary Thyroid Cancer in Thyroid Nodules: A Cross-Sectional Survey Based on Thyroidectomy Population. Biol. Trace Elem. Res. 2023, 201, 3279–3289. [Google Scholar] [CrossRef]
- Chandra, A.K.; Goswami, H.; Sengupta, P. Effects of magnesium on cytomorphology and enzyme activities in thyroid of rats. Indian. J. Exp. Biol. 2014, 52, 787–792. [Google Scholar] [PubMed]
- Carazo, A.; Macáková, K.; Matoušová, K.; Krčmová, L.K.; Protti, M.; Mladěnka, P. Vitamin A Update: Forms, Sources, Kinetics, Detection, Function, Deficiency, Therapeutic Use and Toxicity. Nutrients 2021, 13, 1703. [Google Scholar] [CrossRef]
- Brossaud, J.; Pallet, V.; Corcuff, J.B. Vitamin A, endocrine tissues and hormones: Interplay and interactions. Endocr. Connect. 2017, 6, R121–R130. [Google Scholar] [CrossRef]
- Grignard, E.; Håkansson, H.; Munn, S. Regulatory needs and activities to address the retinoid system in the context of endocrine disruption: The European viewpoint. Reprod. Toxicol. 2020, 93, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Biebinger, R.; Arnold, M.; Koss, M.; Kloeckener-Gruissem, B.; Langhans, W.; Hurrell, R.F.; Zimmermann, M.B. Effect of concurrent vitamin A and iodine deficiencies on the thyroid-pituitary axis in rats. Thyroid 2006, 16, 961–965. [Google Scholar] [CrossRef]
- Wolf, G. The regulation of the thyroid-stimulating hormone of the anterior pituitary gland by thyroid hormone and by 9-cis-retinoic acid. Nutr. Rev. 2002, 60, 374–377. [Google Scholar] [CrossRef]
- Zimmermann, M.B. Interactions of vitamin A and iodine deficiencies: Effects on the pituitary-thyroid axis. Int. J. Vitam. Nutr. Res. 2007, 77, 236–240. [Google Scholar] [CrossRef]
- van Bennekum, A.M.; Wei, S.; Gamble, M.V.; Vogel, S.; Piantedosi, R.; Gottesman, M.; Episkopou, V.; Blaner, W.S. Biochemical basis for depressed serum retinol levels in transthyretin-deficient mice. J. Biol. Chem. 2001, 276, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Ravaglia, G.; Forti, P.; Maioli, F.; Nesi, B.; Pratelli, L.; Savarino, L.; Cucinotta, D.; Cavalli, G. Blood micronutrient and thyroid hormone concentrations in the oldest-old. J. Clin. Endocrinol. Metab. 2000, 85, 2260–2265. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.S.; Biondi, B. Subclinical thyroid disease. Lancet 2012, 379, 1142–1154. [Google Scholar] [CrossRef] [PubMed]
- Homma, H.; Watanabe, M.; Inoue, N.; Isono, M.; Hidaka, Y.; Iwatani, Y. Polymorphisms in Vitamin A-Related Genes and Their Functions in Autoimmune Thyroid Disease. Thyroid 2021, 31, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Virili, C.; Fallahi, P.; Antonelli, A.; Benvenga, S.; Centanni, M. Gut microbiota and Hashimoto’s thyroiditis. Rev. Endocr. Metab. Disord. 2018, 19, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; Kenny, D.J.; Xavier, R.J. Gut Microbiota Regulation of T Cells During Inflammation and Autoimmunity. Annu. Rev. Immunol. 2019, 37, 599–624. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Heil, S.G.; Verhoeven, M.M.A.; van den Heuvel, E.G.H.M.; de Groot, L.C.P.G.M.; Eussen, S.J.P.M. Vitamin B12 Intake From Animal Foods, Biomarkers, and Health Aspects. Front. Nutr. 2019, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Kacharava, T.; Giorgadze, E.; Janjgava, S.; Lomtadze, N.; Taboridze, I. Correlation Between Vitamin B12 Deficiency and Autoimmune Thyroid Diseases. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Szczepanek-Parulska, E.; Hernik, A.; Ruchała, M. Anemia in Thyroid Diseases. Pol. Arch. Intern. Med. 2017, 127, 352–360. [Google Scholar] [CrossRef]
- Aktaş, H.Ş. Vitamin B12 and Vitamin D Levels in Patients with Autoimmune Hypothyroidism and Their Correlation with Anti-Thyroid Peroxidase Antibodies. Med. Princ. Pract. 2020, 29, 364–370. [Google Scholar] [CrossRef]
- Jaya Kumari, S.; Bantwal, G.; Devanath, A.; Aiyyar, V.; Patil, M. Evaluation of serum vitamin B12 levels and its correlation with anti-thyroperoxidase antibody in patients with autoimmune thyroid disorders. Indian. J. Clin. Biochem. 2015, 30, 217–220. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Y.P.; Lin, H.P.; Chen, H.M.; Kuo, Y.S.; Lang, M.J.; Sun, A. Hemoglobin, iron, and vitamin B12 deficiencies and high blood homocysteine levels in patients with anti-thyroid autoantibodies. J. Formos. Med. Assoc. 2014, 113, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, A.A.; Karaźniewicz-Łada, M.; Filipowicz, D.; Ruchała, M.; Główka, F.K. Metabolic Characteristics of Hashimoto’s Thyroiditis Patients and the Role of Microelements and Diet in the Disease Management-An Overview. Int. J. Mol. Sci. 2022, 23, 6580. [Google Scholar] [CrossRef] [PubMed]
- Benites-Zapata, V.A.; Ignacio-Cconchoy, F.L.; Ulloque-Badaracco, J.R.; Hernandez-Bustamante, E.A.; Alarcón-Braga, E.A.; Al-Kassab-Córdova, A.; Herrera-Añazco, P. Vitamin B12 levels in thyroid disorders: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1070592. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petraroli, M.; Castellone, E.; Patianna, V.; Esposito, S. Gut Microbiota and Obesity in Adults and Children: The State of the Art. Front. Pediatr. 2021, 9, 657020. [Google Scholar] [CrossRef] [PubMed]
- Raspini, B.; Porri, D.; De Giuseppe, R.; Chieppa, M.; Liso, M.; Cerbo, R.M.; Civardi, E.; Garofoli, F.; Monti, M.C.; Vacca, M.; et al. Prenatal and postnatal determinants in shaping offspring’s microbiome in the first 1000 days: Study protocol and preliminary results at one month of life. Ital. J. Pediatr. 2020, 46, 45. [Google Scholar] [CrossRef] [PubMed]
- Street, M.E.; Shulhai, A.M.; Rotondo, R.; Giannì, G.; Caffarelli, C. Current knowledge on the effects of environmental contaminants in early life nutrition. Front. Nutr. 2023, 10, 1120293. [Google Scholar] [CrossRef]
- Biagi, E.; Quercia, S.; Aceti, A.; Beghetti, I.; Rampelli, S.; Turroni, S.; Faldella, G.; Candela, M.; Brigidi, P.; Corvaglia, L. The Bacterial Ecosystem of Mother’s Milk and Infant’s Mouth and Gut. Front. Microbiol. 2017, 8, 1214. [Google Scholar] [CrossRef] [PubMed]
- Ercan, O.; Tarcin, G. Overview on Endocrine disruptors in food and their effects on infant’s health. Glob. Pediatrics 2022, 2, 100019. [Google Scholar] [CrossRef]
- Mendonca, K.; Hauser, R.; Calafat, A.M.; Arbuckle, T.E.; Duty, S.M. Bisphenol A concentrations in maternal breast milk and infant urine. Int. Arch. Occup. Environ. Health 2014, 87, 13–20. [Google Scholar] [CrossRef]
- Witczak, A.; Pohoryło, A.; Abdel-Gawad, H. Endocrine-Disrupting Organochlorine Pesticides in Human Breast Milk: Changes during Lactation. Nutrients 2021, 13, 229. [Google Scholar] [CrossRef] [PubMed]
- Di Napoli, I.; Tagliaferri, S.; Sommella, E.; Salviati, E.; Porri, D.; Raspini, B.; Cena, H.; Campiglia, P.; La Rocca, C.; Cerbo, R.M.; et al. Lifestyle Habits and Exposure to BPA and Phthalates in Women of Childbearing Age from Northern Italy: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 9710. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Calabrese, F.M.; Loperfido, F.; Maccarini, B.; Cerbo, R.M.; Sommella, E.; Salviati, E.; Voto, L.; De Angelis, M.; Ceccarelli, G.; et al. Maternal Exposure to Endocrine-Disrupting Chemicals: Analysis of Their Impact on Infant Gut Microbiota Composition. Biomedicines 2024, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Virili, C.; Stramazzo, I.; Bagaglini, M.F.; Carretti, A.L.; Capriello, S.; Romanelli, F.; Trimboli, P.; Centanni, M. The relationship between thyroid and human-associated microbiota: A systematic review of reviews. Rev. Endocr. Metab. Disord. 2024, 25, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wang, X.; Wang, L.; Sun, X.; Tan, G.; Wei, W.; Zheng, G.; Ma, X.; Tian, D.; Yu, H. Genetics, Epigenetics, Cellular Immunology, and Gut Microbiota: Emerging Links with Graves’ Disease. Front. Cell Dev. Biol. 2022, 9, 794912. [Google Scholar] [CrossRef] [PubMed]
- Sibuh, B.Z.; Quazi, S.; Panday, H.; Parashar, R.; Jha, N.K.; Mathur, R.; Jha, S.K.; Taneja, P.; Jha, A.K. The Emerging Role of Epigenetics in Metabolism and Endocrinology. Biology 2023, 12, 256. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-León, M.J.; Mangalam, A.K.; Regaldiz, A.; González-Madrid, E.; Rangel-Ramírez, M.A.; Álvarez-Mardonez, O.; Vallejos, O.P.; Méndez, C.; Bueno, S.M.; Melo-González, F.; et al. Gut microbiota short-chain fatty acids and their impact on the host thyroid function and diseases. Front. Endocrinol. 2023, 14, 1192216. [Google Scholar] [CrossRef] [PubMed]
- Poursalehi, D.; Lotfi, K.; Mirzaei, S.; Asadi, A.; Akhlaghi, M.; Saneei, P. Association between methyl donor nutrients and metabolic health status in overweight and obese adolescents. Sci. Rep. 2022, 12, 17045. [Google Scholar] [CrossRef]
- Habza-Kowalska, E.; Kaczor, A.A.; Bartuzi, D.; Piłat, J.; Gawlik-Dziki, U. Some Dietary Phenolic Compounds Can Activate Thyroid Peroxidase and Inhibit Lipoxygenase-Preliminary Study in the Model Systems. Int. J. Mol. Sci. 2021, 22, 5108. [Google Scholar] [CrossRef]
- Jiang, W.; Lu, G.; Gao, D.; Lv, Z.; Li, D. The relationships between the gut microbiota and its metabolites with thyroid diseases. Front. Endocrinol. 2022, 13, 943408. [Google Scholar] [CrossRef]
- Calcaterra, V.; Mameli, C.; Rossi, V.; Magenes, V.C.; Massini, G.; Perazzi, C.; Verduci, E.; Zuccotti, G. What we know about the relationship between autoimmune thyroid diseases and gut microbiota: A perspective on the role of probiotics on pediatric endocrinology. Minerva Pediatr. 2022, 74, 650–671. [Google Scholar] [CrossRef] [PubMed]
| Age | Adequate Intake (ug/Day) REF * for EFSA | Recommended Iodine Intake (ug/Day) REF ** for WHO |
|---|---|---|
| 0–6 months old | - | 90 |
| 7–12 months old | 70 | 90 |
| 1–6 years old | 90 | 90 |
| 7–10 years old | 90 | 120 |
| 11–14 years old | 120 | 120–150 |
| 15–17 years old | 130 | 150 |
| ≥18 years old | 150 | 150 |
| During pregnancy | 200 | 250 |
| During lactation | 200 | 250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shulhai, A.-M.; Rotondo, R.; Petraroli, M.; Patianna, V.; Predieri, B.; Iughetti, L.; Esposito, S.; Street, M.E. The Role of Nutrition on Thyroid Function. Nutrients 2024, 16, 2496. https://doi.org/10.3390/nu16152496
Shulhai A-M, Rotondo R, Petraroli M, Patianna V, Predieri B, Iughetti L, Esposito S, Street ME. The Role of Nutrition on Thyroid Function. Nutrients. 2024; 16(15):2496. https://doi.org/10.3390/nu16152496
Chicago/Turabian StyleShulhai, Anna-Mariia, Roberta Rotondo, Maddalena Petraroli, Viviana Patianna, Barbara Predieri, Lorenzo Iughetti, Susanna Esposito, and Maria Elisabeth Street. 2024. "The Role of Nutrition on Thyroid Function" Nutrients 16, no. 15: 2496. https://doi.org/10.3390/nu16152496
APA StyleShulhai, A.-M., Rotondo, R., Petraroli, M., Patianna, V., Predieri, B., Iughetti, L., Esposito, S., & Street, M. E. (2024). The Role of Nutrition on Thyroid Function. Nutrients, 16(15), 2496. https://doi.org/10.3390/nu16152496











