Effect of Supplementation of a Butyrate-Based Formula in Individuals with Liver Steatosis and Metabolic Syndrome: A Randomized Double-Blind Placebo-Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatment
2.2. Assessments
2.2.1. Clinical Data and Anthropometric Measurements
2.2.2. Laboratory Analyses
2.2.3. Liver Steatosis Assessment
2.2.4. Assessment of Tolerability
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jennison, E.; Byrne, C.D. Recent advances in NAFLD: Current areas of contention. Fac. Rev. 2023, 12, 10. [Google Scholar] [CrossRef]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 223–238. [Google Scholar] [CrossRef]
- Ng, J.J.J.; Loo, W.M.; Siah, K.T.H. Associations between irritable bowel syndrome and non-alcoholic fatty liver disease: A systematic review. World J. Hepatol. 2023, 15, 925–938. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; De Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Ji, Y.; Yin, Y.; Sun, L.; Zhang, W. The molecular and mechanistic insights based on gut-liver Axis: Nutritional target for non-alcoholic fatty liver disease (NAFLD) improvement. Int. J. Mol. Sci. 2020, 21, 3066. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Hanson, P.; Weickert, M.O. Metabolic-Associated Fatty Liver Disease and the Gut Microbiota. Endocrinol. Metab. Clin. N. Am. 2023, 52, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Ferslew, B.C.; Xie, G.; Johnston, C.K.; Su, M.; Stewart, P.W.; Jia, W.; Brouwer, K.L.; Barritt, A.S., IV. Altered Bile Acid Metabolome in Patients with Nonalcoholic Steatohepatitis. Dig. Dis. Sci. 2015, 60, 3318–3328. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.J.; Yao, F.; Lu, W.P.; Xu, H.M. Gut microbiome and metabolic-associated fatty liver disease: Current status and potential applications. World J. Hepatol. 2023, 15, 867–882. [Google Scholar] [CrossRef] [PubMed]
- Kessoku, T.; Imajo, K.; Kobayashi, T.; Ozaki, A.; Iwaki, M.; Honda, Y.; Kato, T.; Ogawa, Y.; Tomeno, W.; Kato, S.; et al. Lubiprostone in patients with non-alcoholic fatty liver disease: A randomised, double-blind, placebo-controlled, phase 2a trial. Lancet Gastroenterol. Hepatol. 2020, 5, 996–1007. [Google Scholar] [CrossRef]
- Cao, C.; Shi, M.; Wang, X.; Yao, Y.; Zeng, R. Effects of probiotics on non-alcoholic fatty liver disease: A review of human clinical trials. Front. Nutr. 2023, 10, 1155306. [Google Scholar] [CrossRef]
- Hu, H.; Lin, A.; Kong, M.; Yao, X.; Yin, M.; Xia, H.; Ma, J.; Liu, H. Intestinal microbiome and NAFLD: Molecular insights and therapeutic perspectives. J. Gastroenterol. 2020, 55, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Agostini, A.; Brolli, M.; Sista, M.T. Modulation of bowel inflammation with Dibuzin®. Pharmanutr. Funct. Foods 2021, 6, 58. [Google Scholar]
- Cicero, A.F.G.; Caliceti, C.; Fogacci, F.; Giovannini, M.; Calabria, D.; Colletti, A.; Veronesi, M.; Roda, A.; Borghi, C. Effect of apple polyphenols on vascular oxidative stress and endothelium function: A translational study. Mol. Nutr. Food Res. 2017, 61, 1700373. [Google Scholar] [CrossRef] [PubMed]
- Fogacci, F.; Giovannini, M.; Di Micoli, A.; Fiorini, G.; Grandi, E.; Borghi, C.; Cicero, A.F.G. A Randomized, Double-Blind, Placebo-Controlled Clinical Trial on the Effect of a Dietary Supplement Containing Dry Artichoke and Bergamot Extracts on Metabolic and Vascular Risk Factors in Individuals with Suboptimal Cholesterol Levels. Nutrients 2024, 16, 1587. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.; Mohan, V.; Joshi, S. Should waist circumference be replaced by index of central obesity (ICO) in definition of metabolic syndrome? Diabetes Metab. Res. Rev. 2012, 28, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Tocci, G.; D’Addato, S.; Grandi, E.; Banach, M.; Borghi, C. Three arms, double-blind, non-inferiority, randomized clinical study testing the lipid-lowering effect of a novel dietary supplement containing red yeast rice and artichoke extracts compared to Armolipid Plus® and placebo. Arch. Med. Sci. 2023, 19, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Fogacci, F.; Di Micoli, V.; Veronesi, M.; Cicero, A.F.G. Comparative effect of a nutraceutical compound based on a flavonoid complex from bergamot on plasma lipids, glucose metabolism, and liver enzymes: A 3-arm, double-blind, placebo-controlled, randomized clinical trial. Arch. Med. Sci. 2022, 19, 1180–1185. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Miridi, M.; Galluzzo, A.; AlkaMesy Study Group. Visceral adiposity index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, D.; Kim, H.J.; Lee, C.O.; In Yang, J.; Kim, W.; Kim, Y.J.; Yoon, J.H.; Cho, S.H.; Sung, M.W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- Bedogni, G.; Kahn, H.S.; Bellentani, S.; Tiribelli, C. A simple index of lipid overaccumulation is a good marker of liver steatosis. BMC Gastroenterol. 2010, 10, 98. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.E.; Saverymuttu, S.H.; al-Sam, S.; Cook, M.G.; Maxwell, J.D. Comparison of liver histology with ultrasonography in assessing diffuse parenchymal liver disease. Clin. Radiol. 1991, 43, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Veronesi, M.; Strocchi, E.; Grandi, E.; Rizzoli, E.; Poli, A.; Marangoni, F.; Borghi, C. A randomized Placebo-Controlled Clinical Trial to Evaluate the Medium-Term Effects of Oat Fibers on Human Health: The Beta-Glucan Effects on Lipid Profile, Glycemia and inTestinal Health (BELT) Study. Nutrients 2020, 12, 686. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Fact. 2020, 19, 168. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Arioli, S.; Behare, P.; Belzer, C.; Berni Canani, R.; Chatel, J.M.; D’Auria, E.; de Freitas, M.Q.; Elinav, E.; Esmerino, E.A.; et al. Postbiotics—When simplification fails to clarify. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 825–826. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat. Rev. Immunol. 2024, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.C.; Huang, S.C. The combined beneficial effects of postbiotic butyrate on active vitamin D3-orchestrated innate immunity to Salmonella colitis. Biomedicines 2021, 9, 1296. [Google Scholar] [CrossRef]
- Hodgkinson, K.; El Abbar, F.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin. Nutr. 2023, 42, 61–75. [Google Scholar] [CrossRef]
- Pérez-Reytor, D.; Puebla, C.; Karahanian, E.; García, K. Use of short-chain fatty acids for the recovery of the intestinal epithelial barrier affected by bacterial toxins. Front. Physiol. 2021, 12, 650313. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, L.E.; Koetsier, M.A.; van Deventer, S.J.; van Tol, E.A. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut 2003, 52, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- Spooner, H.C.; Derrick, S.A.; Maj, M.; Manjarín, R.; Hernandez, G.V.; Tailor, D.S.; Bastani, P.S.; Fanter, R.K.; Fiorotto, M.L.; Burrin, D.G.; et al. High-Fructose, High-Fat Diet Alters Muscle Composition and Fuel Utilization in a Juvenile Iberian Pig Model of Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 4195. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The influence of probiotics on the Firmicutes/Bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk, A.A.; Zheng, D.; Shibolet, O.; Elinav, E. The role of themicrobiome in NAFLD and NASH. EMBO Mol. Med. 2019, 11, e9302. [Google Scholar] [CrossRef] [PubMed]
- Amiri, P.; Arefhosseini, S.; Bakhshimoghaddam, F.; Jamshidi Gurvan, H.; Hosseini, S.A. Mechanistic insights into the pleiotropic effects of butyrate as a potential therapeutic agent on NAFLD management: A systematic review. Front. Nutr. 2022, 9, 1037696. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.H.; Wang, Z.X.; Zhou, D.; Han, Y.; Ma, F.; Hu, Z.; Xin, F.Z.; Liu, X.L.; Ren, T.Y.; Zhang, F.; et al. Sodium Butyrate Supplementation Inhibits Hepatic Steatosis by Stimulating Liver Kinase B1 and Insulin-Induced Gene. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Chen, Y.W.; Zhao, Z.H.; Yang, R.X.; Xin, F.Z.; Liu, X.L.; Pan, Q.; Zhou, H.; Fan, J.G. Sodium butyrate reduces high-fat diet-induced non-alcoholic steatohepatitis through upregulation of hepatic GLP-1R expression. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef]
- Liu, L.; Fu, Q.; Li, T.; Shao, K.; Zhu, X.; Cong, Y.; Zhao, X. Gut microbiota and butyrate contribute to nonalcoholic fatty liver disease in premenopause due to estrogen deficiency. PLoS ONE 2022, 17, e0262855. [Google Scholar] [CrossRef]
- Cherubini, A.; Della Torre, S.; Pelusi, S.; Valenti, L. Sexual dimorphism of metabolic dysfunction-associated steatotic liver disease. Trends Mol. Med. 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A.; et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016, 63, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Michail, S.; Lin, M.; Frey, M.R.; Fanter, R.; Paliy, O.; Hilbush, B.; Reo, N.V. Altered gut microbial energy and metabolism in children with nonalcoholic fatty liver disease. FEMS Microbiol. Ecol. 2015, 91, 1–9. [Google Scholar] [CrossRef] [PubMed]
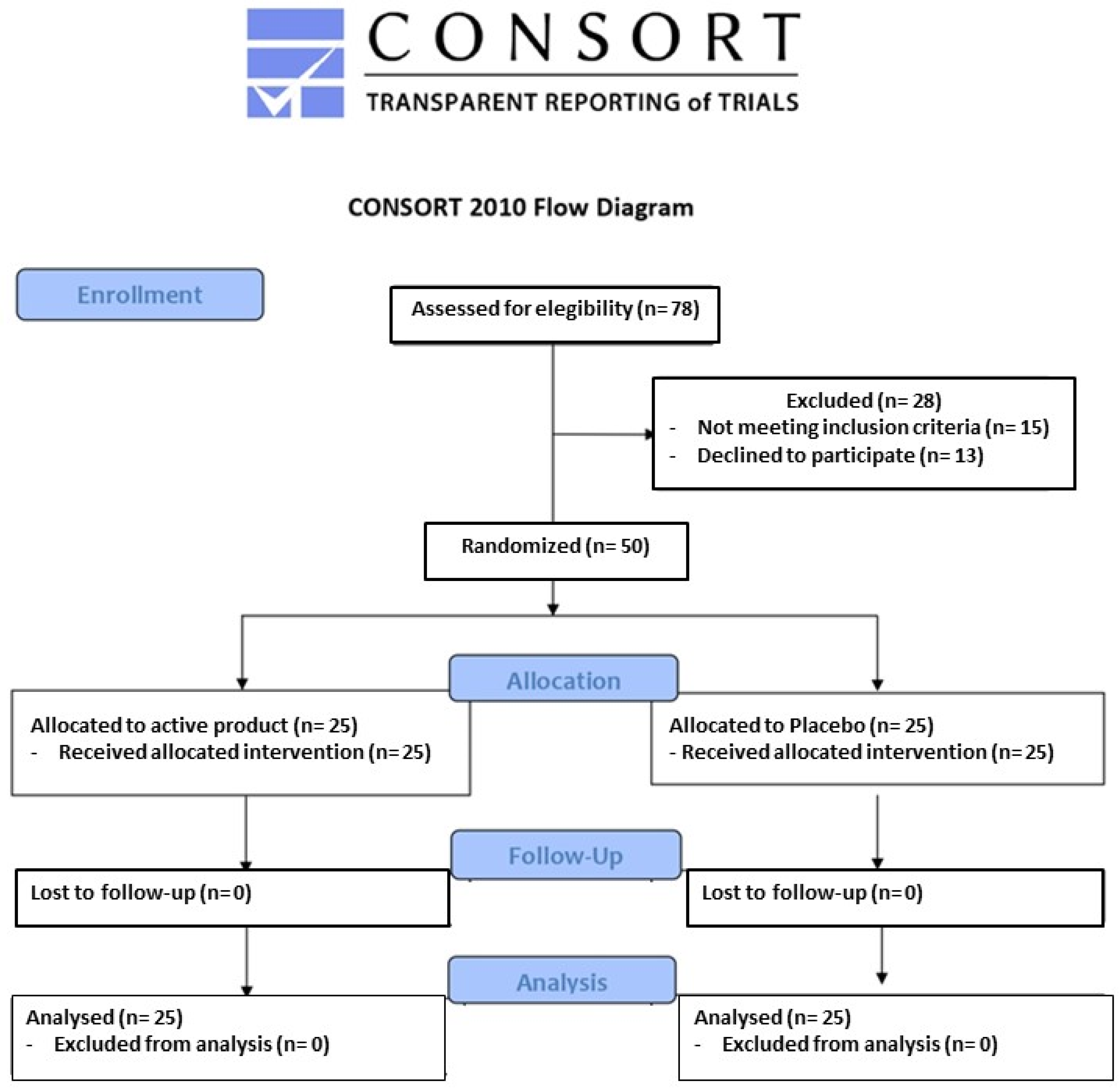
| Parameter | Pre-Run-In Visit (day −28) | Randomization Visit (day 0) | End of Study Visit (day 84) |
|---|---|---|---|
| Age (years) | 61 ± 4 | ||
| Body mass index (kg/m2) | 27.3 ± 2.1 | 27.1 ± 2.2 | 27.0 ± 1.9 |
| Waist circumference (cm) | 92 ± 5 | 90 ± 5 | 90 ± 6 |
| Visceral adiposity index | 2.9 ± 1.5 | 2.7 ± 1.1 | 2.6 ± 1.6 |
| Steatosis severity | — | N. 17 Mild N. 8 Moderate | N. 2 Zero N. 17 Mild N. 6 Moderate |
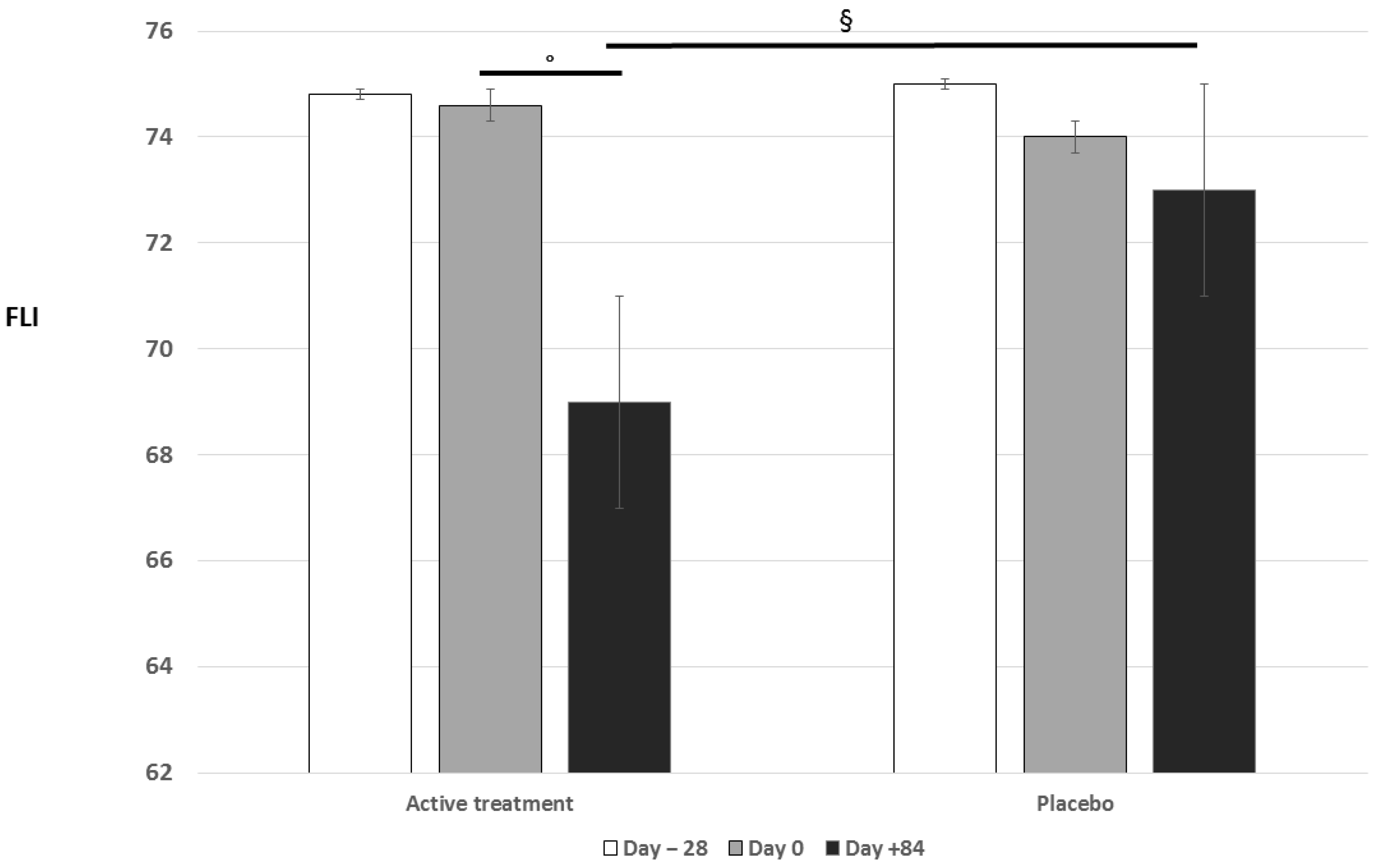
| Fatty liver index | 74.9 ± 17.7 | 74.6 ± 20.9 | 69.5 ± 14.7 °§ |
| Hepatic steatosis index | 47.5 ± 7.8 | 49.4 ± 8.8 | 43.1 ± 7.4 ° |
| Lipid accumulation product | 75.0 ± 37.2 | 69.8 ± 35.4 | 65.3 ± 33.9 |
| Total cholesterol (mg/dL) | 234 ± 12 | 228 ± 13 * | 221 ± 11 °§ |
| HDL-cholesterol (mg/dL) | 46 ± 3 | 45 ± 4 | 46 ± 4 |
| LDL-cholesterol (mg/dL) | 137 ± 9 | 139 ± 9 | 134 ± 8 |
| Triglycerides (mg/dL) | 254 ± 23 | 221 ± 24 * | 207 ± 23 °§ |
| FPG (mg/dL) | 98 ± 9 | 96 ± 10 | 96 ± 11 |
| GOT (U/L) | 23 ± 6 | 23 ± 5 | 22 ± 3 |
| GPT (U/L) | 25 ± 7 | 26 ± 7 | 23 ± 6 |
| Gamma-GT (U/L) | 31 ± 8 | 30 ± 9 | 26 ± 4 ° |
| hs-C reactive protein (mg/L) | 1.5 ± 0.3 | 1.4 ± 0.5 | 1.4 ± 0.4 |
| Tolerability (VAS) | — | — | 8 ± 1 |
| Parameter | Pre-Run-In Visit (day −28) | Randomization Visit (day 0) | End of Study Visit (day 84) |
|---|---|---|---|
| Age (years) | 60 ± 5 | ||
| Body mass index (kg/m2) | 27.1 ± 2.2 | 27.0 ± 1.9 | 26.9 ± 2.0 |
| Waist circumference (cm) | 93 ± 6 | 91 ± 6 | 90 ± 7 |
| Visceral adiposity index | 2.9 ± 1.6 | 2.8 ± 1.3 | 2.6 ± 1.8 |
| Steatosis severity | — | N. 16 Mild N. 9 Moderate | N. 1 Zero N. 16 Mild N. 8 Moderate |
| Fatty liver index | 75.3 ± 16.9 | 74.1 ± 18.4 | 73.3 ± 15.6 |
| Hepatic steatosis index | 48.9 ± 7.1 | 48.1 ± 8.2 | 45.5 ± 7.8 |
| Lipid accumulation product | 74.7 ± 31.3 | 71.6 ± 30.7 | 68.8 ± 28.7 |
| Total cholesterol (mg/dL) | 237 ± 13 | 230 ± 11 * | 228 ± 12 |
| HDL-cholesterol (mg/dL) | 45 ± 5 | 44 ± 5 | 45 ± 4 |
| LDL-cholesterol (mg/dL) | 142 ± 9 | 140 ± 10 | 139 ± 9 |
| Triglycerides (mg/dL) | 248 ± 19 | 229 ± 22 * | 218 ± 23 ° |
| FPG (mg/dL) | 99 ± 10 | 97 ± 9 | 98 ± 10 |
| GOT (U/L) | 25 ± 5 | 24 ± 5 | 25 ± 6 |
| GPT (U/L) | 26 ± 6 | 25 ± 7 | 25 ± 6 |
| Gamma-GT (U/L) | 30 ± 9 | 28 ± 9 | 28 ± 7 |
| hs-C reactive protein (mg/L) | 1.3 ± 0.4 | 1.5 ± 0.4 | 1.4 ± 0.5 |
| Tolerability (VAS) | — | — | 8 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fogacci, F.; Giovannini, M.; Di Micoli, V.; Grandi, E.; Borghi, C.; Cicero, A.F.G. Effect of Supplementation of a Butyrate-Based Formula in Individuals with Liver Steatosis and Metabolic Syndrome: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Nutrients 2024, 16, 2454. https://doi.org/10.3390/nu16152454
Fogacci F, Giovannini M, Di Micoli V, Grandi E, Borghi C, Cicero AFG. Effect of Supplementation of a Butyrate-Based Formula in Individuals with Liver Steatosis and Metabolic Syndrome: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Nutrients. 2024; 16(15):2454. https://doi.org/10.3390/nu16152454
Chicago/Turabian StyleFogacci, Federica, Marina Giovannini, Valentina Di Micoli, Elisa Grandi, Claudio Borghi, and Arrigo Francesco Giuseppe Cicero. 2024. "Effect of Supplementation of a Butyrate-Based Formula in Individuals with Liver Steatosis and Metabolic Syndrome: A Randomized Double-Blind Placebo-Controlled Clinical Trial" Nutrients 16, no. 15: 2454. https://doi.org/10.3390/nu16152454
APA StyleFogacci, F., Giovannini, M., Di Micoli, V., Grandi, E., Borghi, C., & Cicero, A. F. G. (2024). Effect of Supplementation of a Butyrate-Based Formula in Individuals with Liver Steatosis and Metabolic Syndrome: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Nutrients, 16(15), 2454. https://doi.org/10.3390/nu16152454








