Efficacy and Safety of Honey Dressings in the Management of Chronic Wounds: An Updated Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategies
2.2. Eligibility Criteria
2.3. Study Selection and Data Extraction
2.4. Risk of Bias Assessment
2.5. Certainty of the Evidence
2.6. Statistical Analysis
3. Results
3.1. Search and Selection of Studies
3.2. Study Characteristics
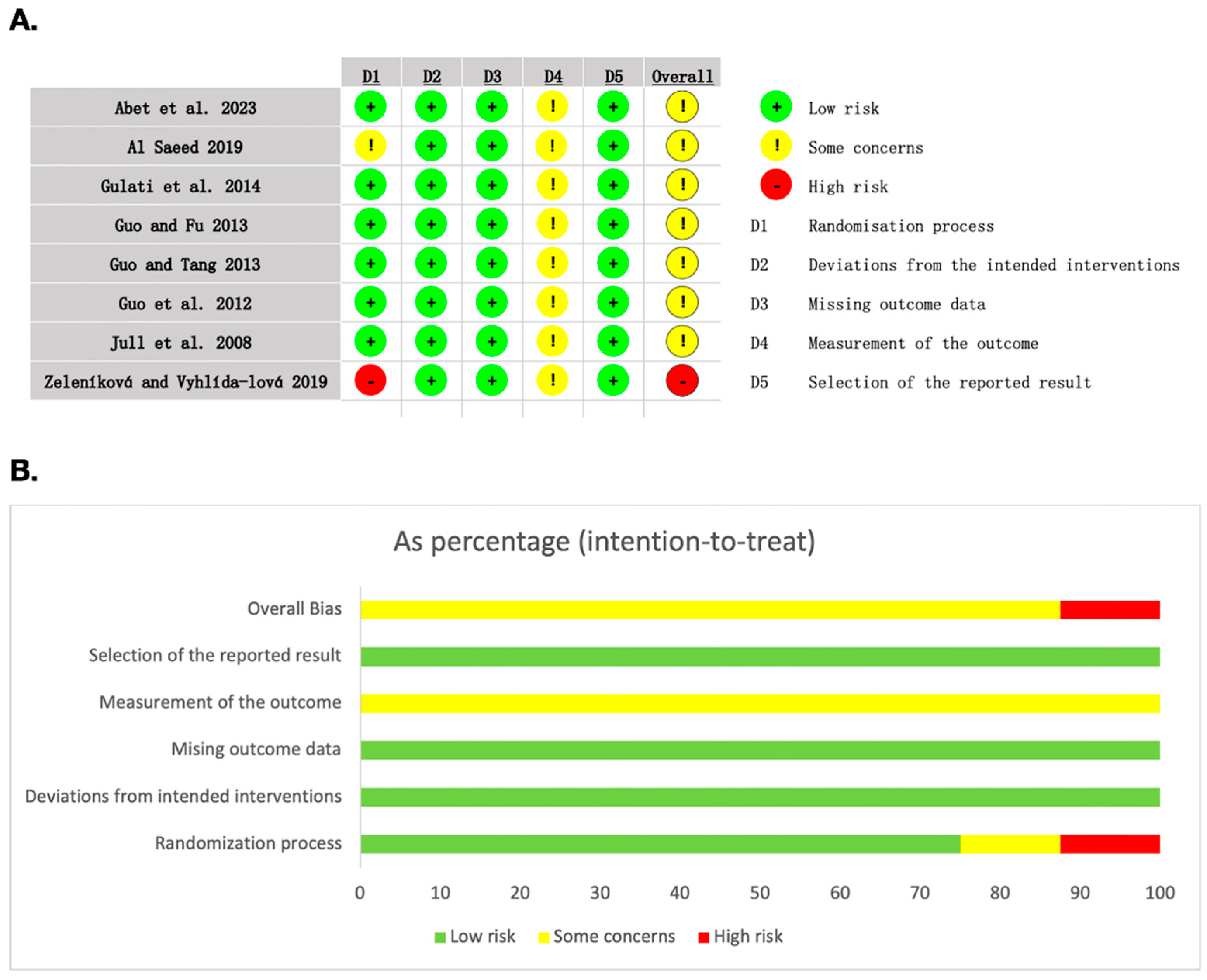
3.3. Risk of Bias Included Studies
3.4. Analyses of Outcomes
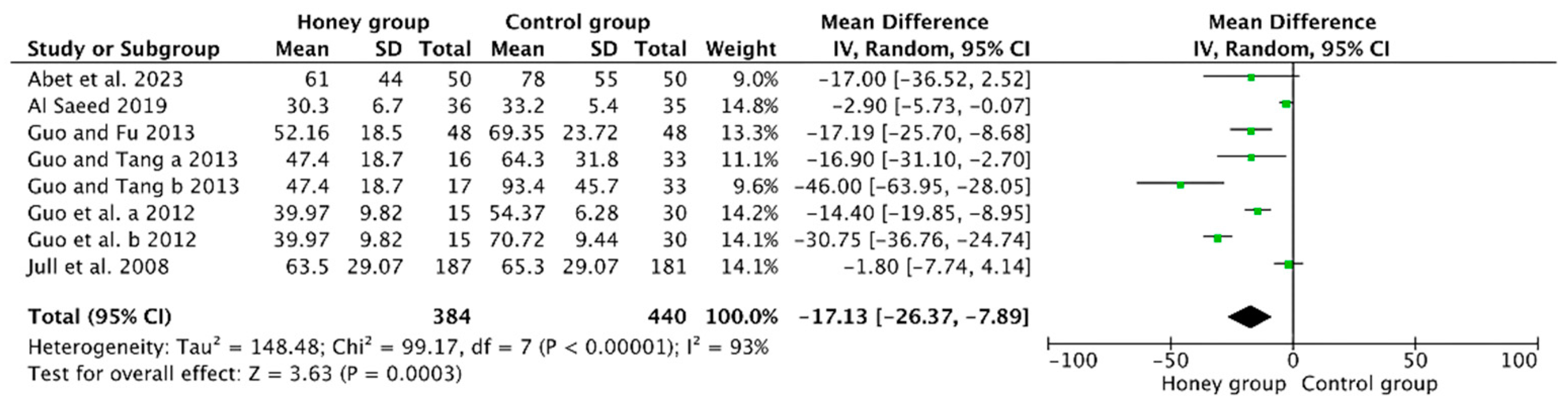
3.4.1. Mean Time to Achieve Wound Healing
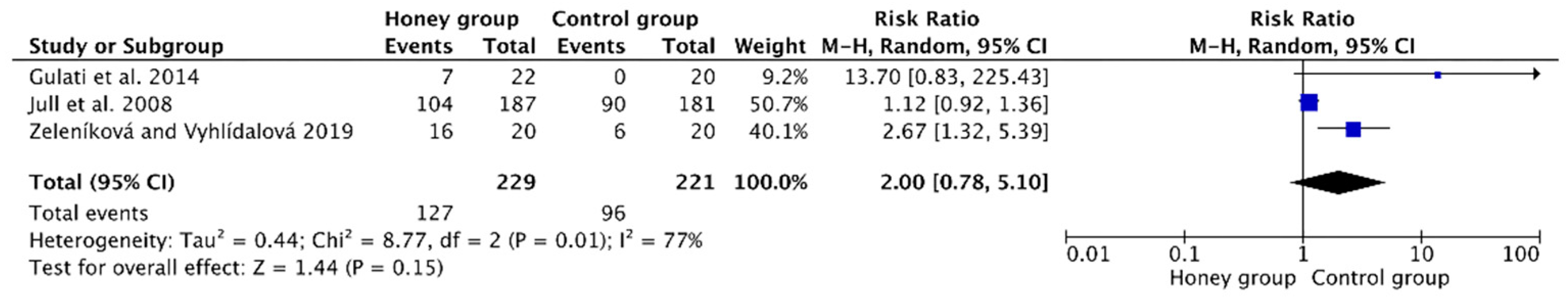
3.4.2. Rate of Complete Wound Healing
3.4.3. Incidence of Adverse Events
3.4.4. Percentage of Wound Healing (%)
3.4.5. The Degree of Pain during Treatment (VAS Scores)
3.4.6. Bacterial Clearance Time of Wounds
3.4.7. LOS
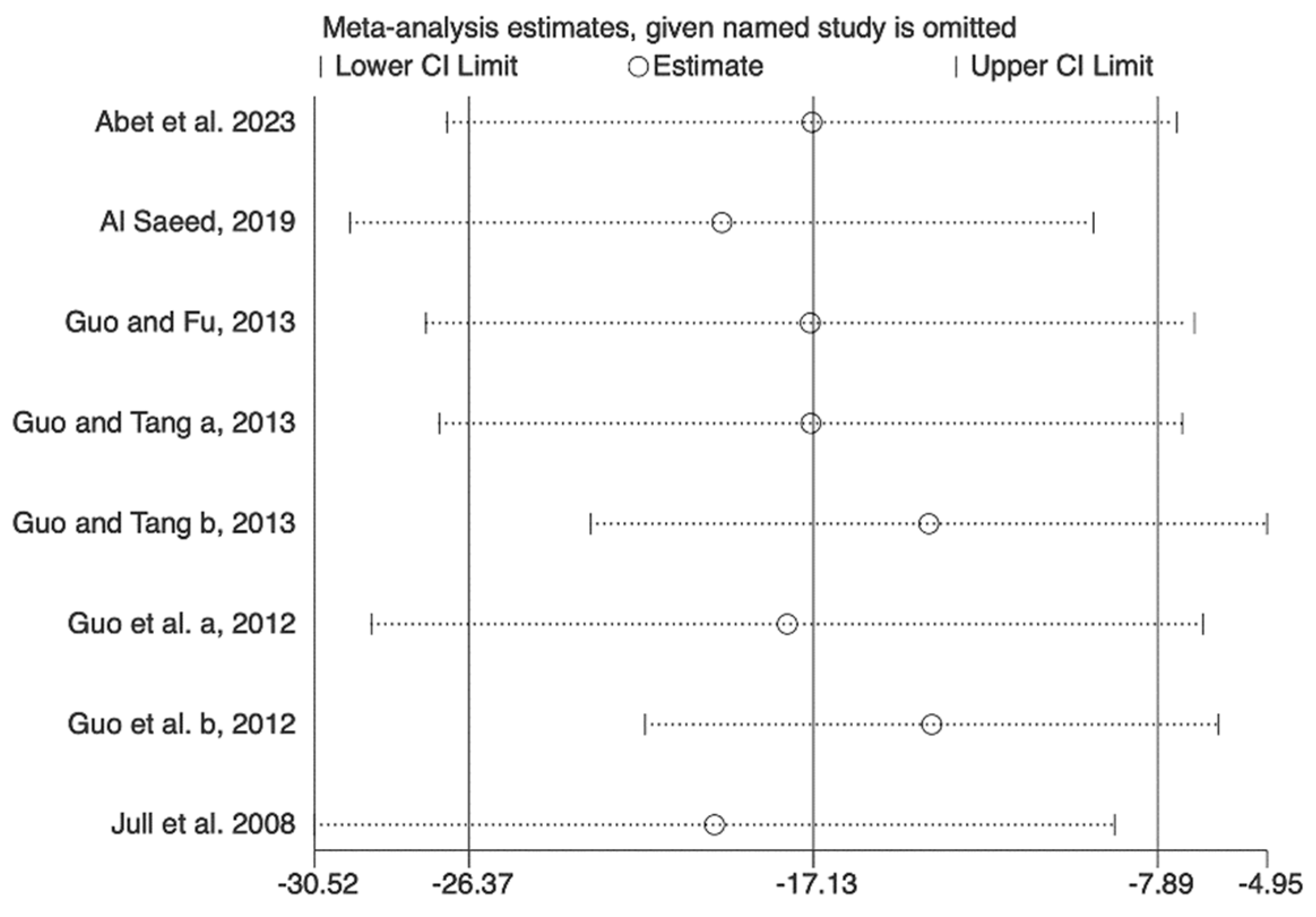
3.5. Sensitivity Analysis
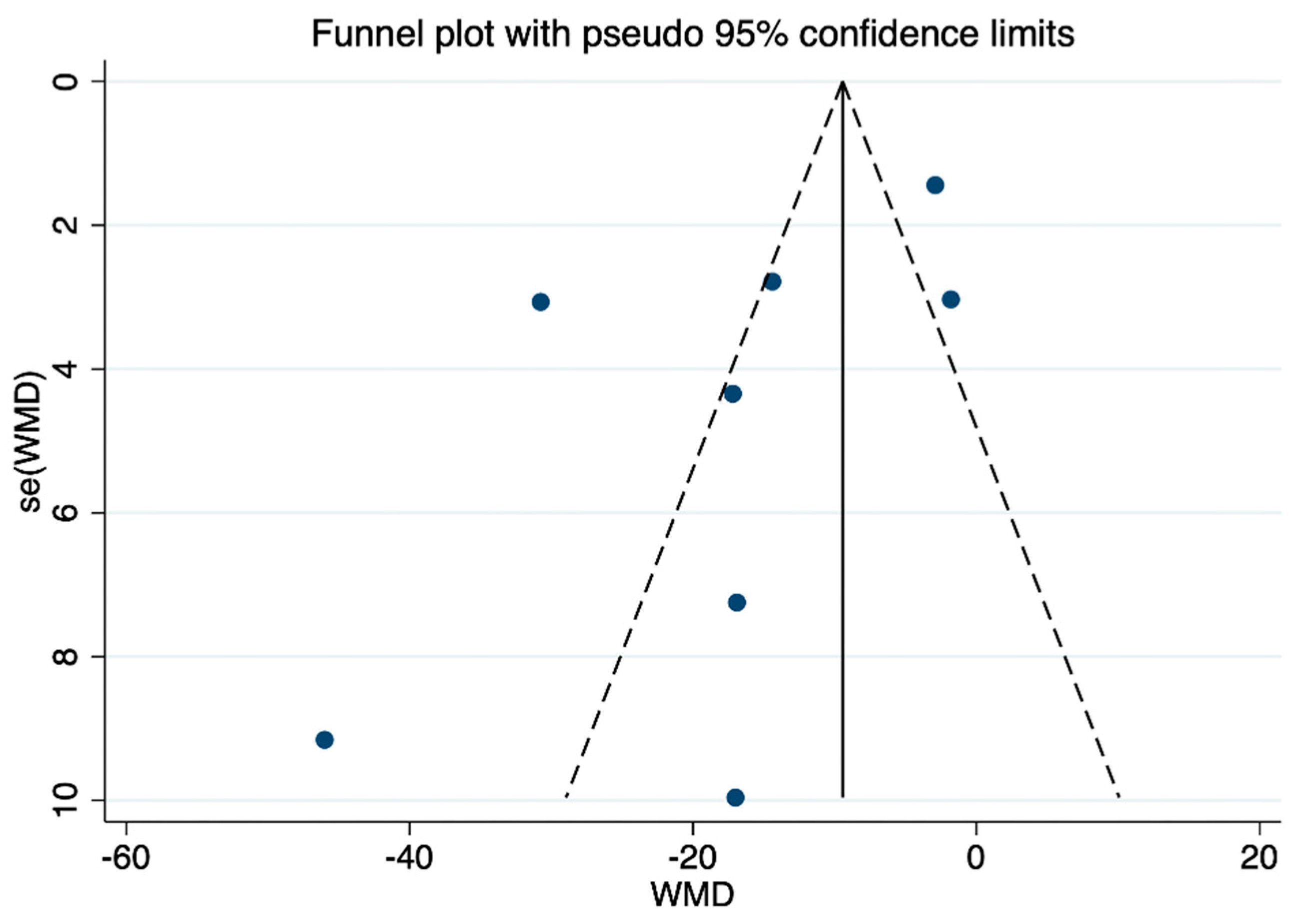
3.6. Publication Bias
3.7. Certainty of the Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mustoe, T.A.; O’Shaughnessy, K.; Kloeters, O. Chronic wound pathogenesis and current treatment strategies: A unifying hypothesis. Plast. Reconstr. Surg. 2006, 117 (Suppl. S7), 35s–41s. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Mohamedi, A.H.; Senkowsky, J.; Nair, A.; Tang, L. Imaging in Chronic Wound Diagnostics. Adv. Wound Care 2020, 9, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.J.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D.; Fife, C.E. Chronic wound prevalence and the associated cost of treatment in Medicare beneficiaries: Changes between 2014 and 2019. J. Med. Econ. 2023, 26, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Miyata, G. The nutraceutical benefit, part iii: Honey. Nutrition 2000, 16, 468–469. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Qureshi, A.; Srivastava, A.; Kataria, K.; Kumar, P.; Ji, A.B. A Prospective Randomized Study to Compare the Effectiveness of Honey Dressing vs. Povidone Iodine Dressing in Chronic Wound Healing. Indian J. Surg. 2014, 76, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Kamaratos, A.V.; Tzirogiannis, K.N.; Iraklianou, S.A.; Panoutsopoulos, G.I.; Kanellos, I.E.; Melidonis, A.I. Manuka honey-impregnated dressings in the treatment of neuropathic diabetic foot ulcers. Int. Wound J. 2014, 11, 259–263. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Memon, I.; Rajpar, Z.H.; Asim, M.; Anwar, A.; Elahi, W. Management of Fournier’s Gangrene; a randomized controlled trial at high volume center comparing the efficacy of honey and Eusol dressing in wound healing. J. Liaquat Univ. Med. Health Sci. 2019, 18, 6–11. [Google Scholar]
- Imran, M.; Hussain, M.B.; Baig, M. A randomized, controlled clinical trial of honey-impregnated dressing for treating diabetic foot ulcer. J. Coll. Physicians Surg. Pak. 2015, 25, 721–725. [Google Scholar]
- Salehi, V.; Barhaghtalab, M.J.Y.; Mehrabi, S.; Iraji, A.; Sadat, S.A.; Yusefi, S.H.; Malekzadeh, J.M. Does application of honey improve surgical outcome in pilonidal cyst excision with secondary intention healing? A prospective randomized placebo-controlled clinical trial. Perioper. Med. 2022, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Molan, P.C. Potential of honey in the treatment of wounds and burns. Am. J. Clin. Dermatol. 2001, 2, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Saikaly, S.K.; Khachemoune, A. Honey and Wound Healing: An Update. Am. J. Clin. Dermatol. 2017, 18, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Jull, A.B.; Cullum, N.; Dumville, J.C.; Westby, M.J.; Deshpande, S.; Walker, N. Honey as a topical treatment for wounds. Cochrane Database Syst. Rev. 2015, 2015, CD005083. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Chapter 10: Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (Updated August 2023); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane, 2023; Available online: www.training.cochrane.org/handbook (accessed on 22 March 2024).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Abet, E.; Jean, M.-H.; Greilsamer, T.; Planche, L.; Maurice, F.; Brau-Weber, A.G.; Denimal, F. The value of honey dressings in pilonidal cyst healing: A prospective randomized single-center trial. Tech. Coloproctol. 2023, 27, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Al Saeed, M. Prospective randomized comparison of controlled release ionic silver hydrophilic dressings and medicated honey-impregnated dressings in treating neuropathic diabetic foot ulcer. Saudi J. Health Sci. 2019, 8, 25. [Google Scholar] [CrossRef]
- Guo, C.L.; Fu, X.Y. Observation on effect of different dressings applied in treatment of patients with chronic wound. Chin. Gen. Pract. Nurs. 2013, 11, 1253–1255. [Google Scholar]
- Guo, C.L.; Tang, L.H. Effects and safty of honey dressing in the treatment of diabetes chronic wound. Chin. Gen. Pract. 2013, 16, 1765–1768. [Google Scholar]
- Guo, C.L.; Tian, Y.F.; Zhang, W.L.; Zhou, J. Effects of honey dressing on the traumatic skin ulcers. Chin. J. Nurs. 2012, 47, 919–922. [Google Scholar]
- Jull, A.; Walker, N.; Parag, V.; Molan, P.; Rodgers, A. Randomized clinical trial of honey-impregnated dressings for venous leg ulcers. Br. J. Surg. 2008, 95, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Zeleníková, R.; Vyhlídalová, D. Applying honey dressings to non-healing wounds in elderly persons receiving home care. J. Tissue Viability 2019, 28, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Hermanns, R.; Mateescu, C.; Thrasyvoulou, A.; Tananaki, C.; Wagener, F.A.; Cremers, N.A. Defining the standards for medical grade honey. J. Apic. Res. 2019, 59, 125–135. [Google Scholar] [CrossRef]
- Molan, P. The role of honey in the management of wounds. J. Wound Care 1999, 8, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Nikhat, S.; Fazil, M. History, phytochemistry, experimental pharmacology and clinical uses of honey: A comprehensive review with special reference to Unani medicine. J. Ethnopharmacol. 2022, 282, 114614. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Biological properties and therapeutic activities of honey in wound healing: A narrative review and meta-analysis. J. Tissue Viability 2016, 25, 98–118. [Google Scholar] [CrossRef]
- Brudzynski, K. Effect of hydrogen peroxide on antibacterial activities of Canadian honeys. Can. J. Microbiol. 2006, 52, 1228–1237. [Google Scholar] [CrossRef]
- Brudzynski, K.; Sjaarda, C. Honey glycoproteins containing antimicrobial peptides, Jelleins of the Major Royal Jelly Protein 1, are responsible for the cell wall lytic and bactericidal activities of honey. PLoS ONE 2015, 10, e0120238. [Google Scholar] [CrossRef]
- Rabie, E.; Serem, J.C.; Oberholzer, H.M.; Gaspar, A.R.M.; Bester, M.J. How methylglyoxal kills bacteria: An ultrastructural study. Ultrastruct. Pathol. 2016, 40, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Abubaker, K.; St-Martin, L.; Castle, A. Re-examining the role of hydrogen peroxide in bacteriostatic and bactericidal activities of honey. Front. Microbiol. 2011, 2, 213. [Google Scholar] [CrossRef] [PubMed]
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef]
- Scepankova, H.; Combarros-Fuertes, P.; Fresno, J.M.; Tornadijo, M.E.; Dias, M.S.; Pinto, C.A.; Saraiva, J.A.; Estevinho, L.M. Role of Honey in Advanced Wound Care. Molecules 2021, 26, 4784. [Google Scholar] [CrossRef]
- Stewart, J.A.; McGrane, O.L.; Wedmore, I.S. Wound care in the wilderness: Is there evidence for honey? Wilderness Environ. Med. 2014, 25, 103–110. [Google Scholar] [CrossRef]
- Molan, P.C.; Rhodes, T. Honey: A biologic wound dressing. Wounds 2015, 27, 141–151. [Google Scholar]

| Author (Year) | Country | Type of Honey | Sample Size (E/C) | Wound Etiology | Interventions, Frequency and Treatment Duration | Control | Follow-Up Period or Time | Outcome Measure |
|---|---|---|---|---|---|---|---|---|
| Abet et al., 2023 [19] | France | NA | 50/50 | Pilonidal cyst | Honey + alginate dressing; not mention the specific usage | Alginate dressing | 180 days | (a) |
| Al Saeed, 2019 [20] | Saudi Arabia | Manuka honey | 36/35 | Diabetic foot ulcer | Manuka honey dressing covered with an occlusive secondary one; changed daily or more frequently if the dressing was markedly soaked, until the infection was eradicated and healthy granulations were formed | Controlled release silver hydrophilic dressing | Until healed | (a), (f), (g) |
| Gulati et al., 2014 [7] | India | Azadericta indica honey | 22/20 | Various etiology a | Honey was applied to fill the wound cavity sufficiently (1–2 mL) and then covered with film dressing (Tegaderm). Patients with venous leg ulcers were reinforced by elastic compression garments, changed on alternate days for 6 weeks. | Povidone iodine dressing | 6 weeks | (b), (c), (d), (e) |
| Guo and Fu, 2013 [21] | China | Wild native honey in Shennongjia | 48/48 | Various etiology b | Honey dressing covered the wound (apply directly to the wound up to 0.5 cm thick appropriately). Change the dressing when the outer layer dressing is permeated by seepage > 1/2, until healed or ready for surgical closure | Functional dressing | Until healed or ready for surgical closure | (a), (d) |
| Guo and Tang, 2013 [22] | China | Dandelion honey | 33/66 | Diabetic chronic ulcers | Honey dressing covered 3–4 layers (apply directly to the wound up to 0.5 cm thick appropriately). Change the dressing when the outer layer dressing is permeated by seepage > 1/2, until healed or ready for surgical closure | C1: Functional dressing C2: Povidone iodine dressing | Until healed or ready for surgical closure | (a), (f) |
| Guo et al., 2012 [23] | China | Wild native honey in Shennongjia | 30/60 | Traumatic skin chronic ulcers | Honey dressing covers 3–4 layers (apply directly to the wound up to 0.5 cm thick appropriately); once daily application initially and then, frequency determined by clinical need, until healed or ready for surgical closure | C1: Functional dressing C2: Conventional dressing | Until healed or ready for surgical closure | (a), (d) |
| Jull et al., 2008 [24] | New Zealand | Manuka honey | 187/181 | Venous ulcers | Manuka honey impregnated into calcium alginate dressing + compression bandaging; frequency determined by clinical need | Usual care (received dressings that the district nurse deemed appropriate at the time of each visit) | 12 weeks | (a), (b), (c), (d) |
| Zeleníko vá and Vyhlídalo vá, 2019 [25] | Czech Republic | Manuka honey | 20/20 | Various etiology c | Honey dressing; not mention the specific usage | Povidone iodine, nanocrystalline silver, or hydrogel | 90 days | (b), (e) |
| Quality Assessment | No. of Patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of Studies | Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Honey Dressing | Control | Relative (95% CI) | Absolute | ||
| Mean time to achieve wound healing (Better indicated by lower values) | ||||||||||||
| 6 | randomized trials | serious 1 | serious 2 | no serious indirectness | no serious imprecision | reporting bias 3 | 384 | 440 | - | MD 17.13 lower (from 26.37 to 7.89 and less) | ⊕OOO VERY LOW | CRITICAL |
| Complete wound healing rate | ||||||||||||
| 3 | randomized trials | serious 1 | serious 2 | no serious indirectness | serious 4 | reporting bias 3 | 127/229 (55.5%) | 96/221 (43.4%) | RR 2 (0.78 to 5.1) | 434 more per 1000 (from 96 and less to 1000 and more) | ⊕OOO VERY LOW | CRITICAL |
| 30% | 300 more per 1000 (from 66 and less to 1000 and more) | |||||||||||
| Percentage of wound healing (%) (Better indicated by lower values) | ||||||||||||
| 3 | randomized trials | serious 1 | serious 2 | no serious indirectness | no serious imprecision | reporting bias 3 | 265 | 289 | - | MD 18.31 higher (from 8.86 to 27.76 and higher) | ⊕OOO VERY LOW | CRITICAL |
| Bacterial clearance time of wounds (Better indicated by lower values) | ||||||||||||
| 2 | randomized trials | serious 1 | serious 2 | no serious indirectness | no serious imprecision | reporting bias 3 | 69 | 101 | - | MD 11.36 lower (from 25.91 and lower to 3.18 and higher) | ⊕OOO VERY LOW | IMPORTANT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Chen, L.; Ran, X. Efficacy and Safety of Honey Dressings in the Management of Chronic Wounds: An Updated Systematic Review and Meta-Analysis. Nutrients 2024, 16, 2455. https://doi.org/10.3390/nu16152455
Tang Y, Chen L, Ran X. Efficacy and Safety of Honey Dressings in the Management of Chronic Wounds: An Updated Systematic Review and Meta-Analysis. Nutrients. 2024; 16(15):2455. https://doi.org/10.3390/nu16152455
Chicago/Turabian StyleTang, Ying, Lihong Chen, and Xingwu Ran. 2024. "Efficacy and Safety of Honey Dressings in the Management of Chronic Wounds: An Updated Systematic Review and Meta-Analysis" Nutrients 16, no. 15: 2455. https://doi.org/10.3390/nu16152455
APA StyleTang, Y., Chen, L., & Ran, X. (2024). Efficacy and Safety of Honey Dressings in the Management of Chronic Wounds: An Updated Systematic Review and Meta-Analysis. Nutrients, 16(15), 2455. https://doi.org/10.3390/nu16152455