Brain Perception of Different Oils on Appetite Regulation: An Anorectic Gene Expression Pattern in the Hypothalamus Dependent on the Vagus Nerve
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Supplementation Protocol
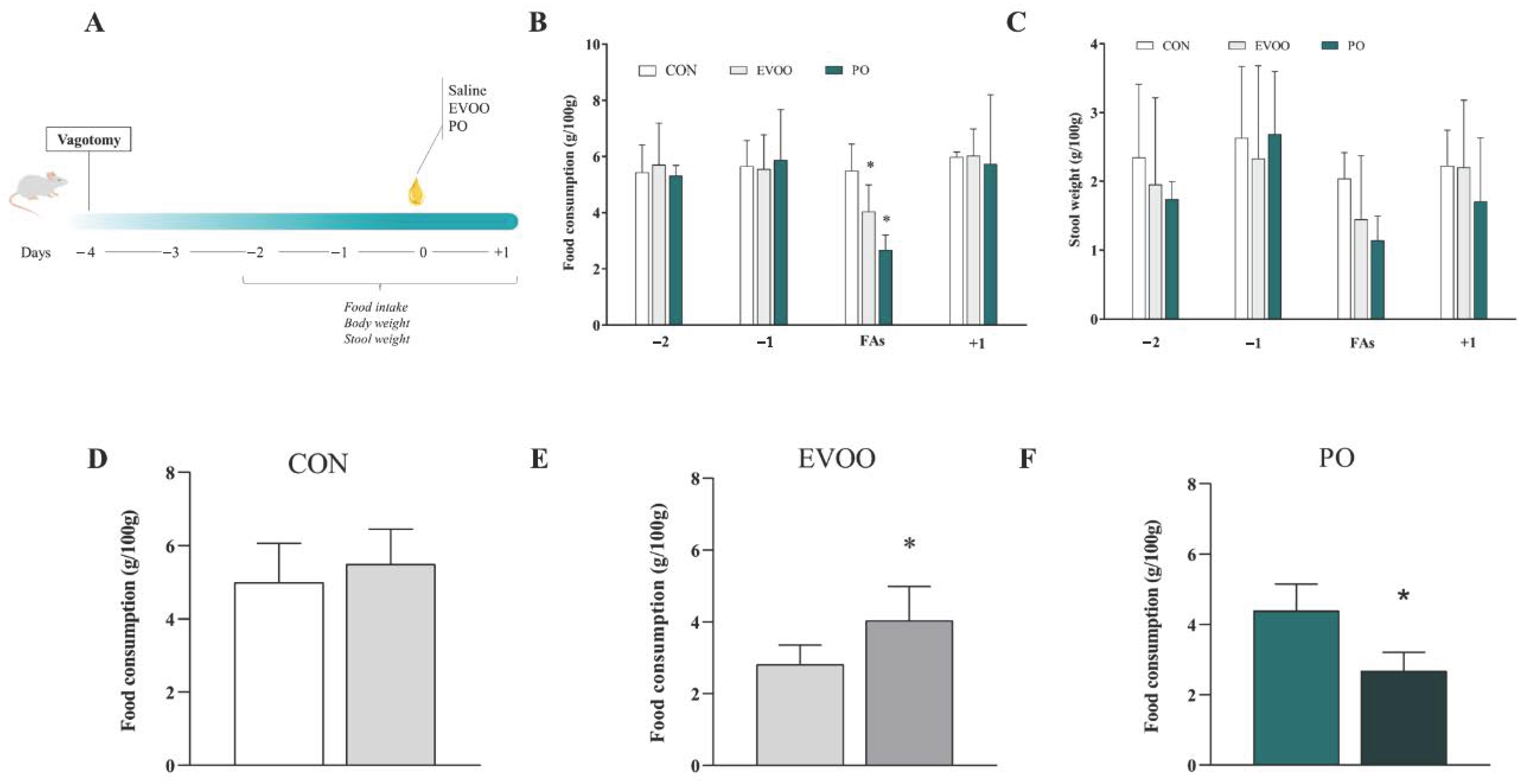
2.2. Experimental Design
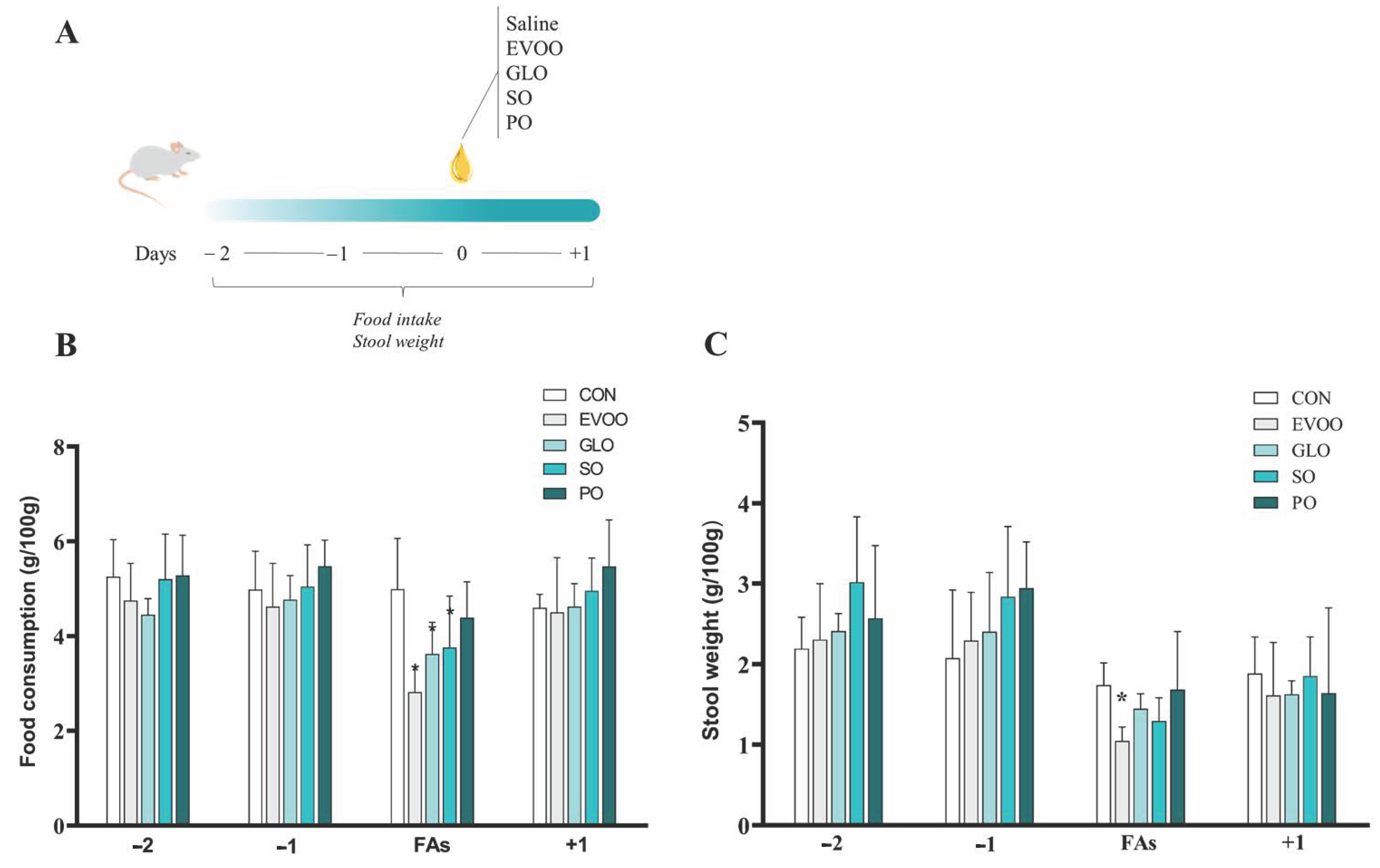
2.3. Assessment of Dietary Intake and Stool Weight
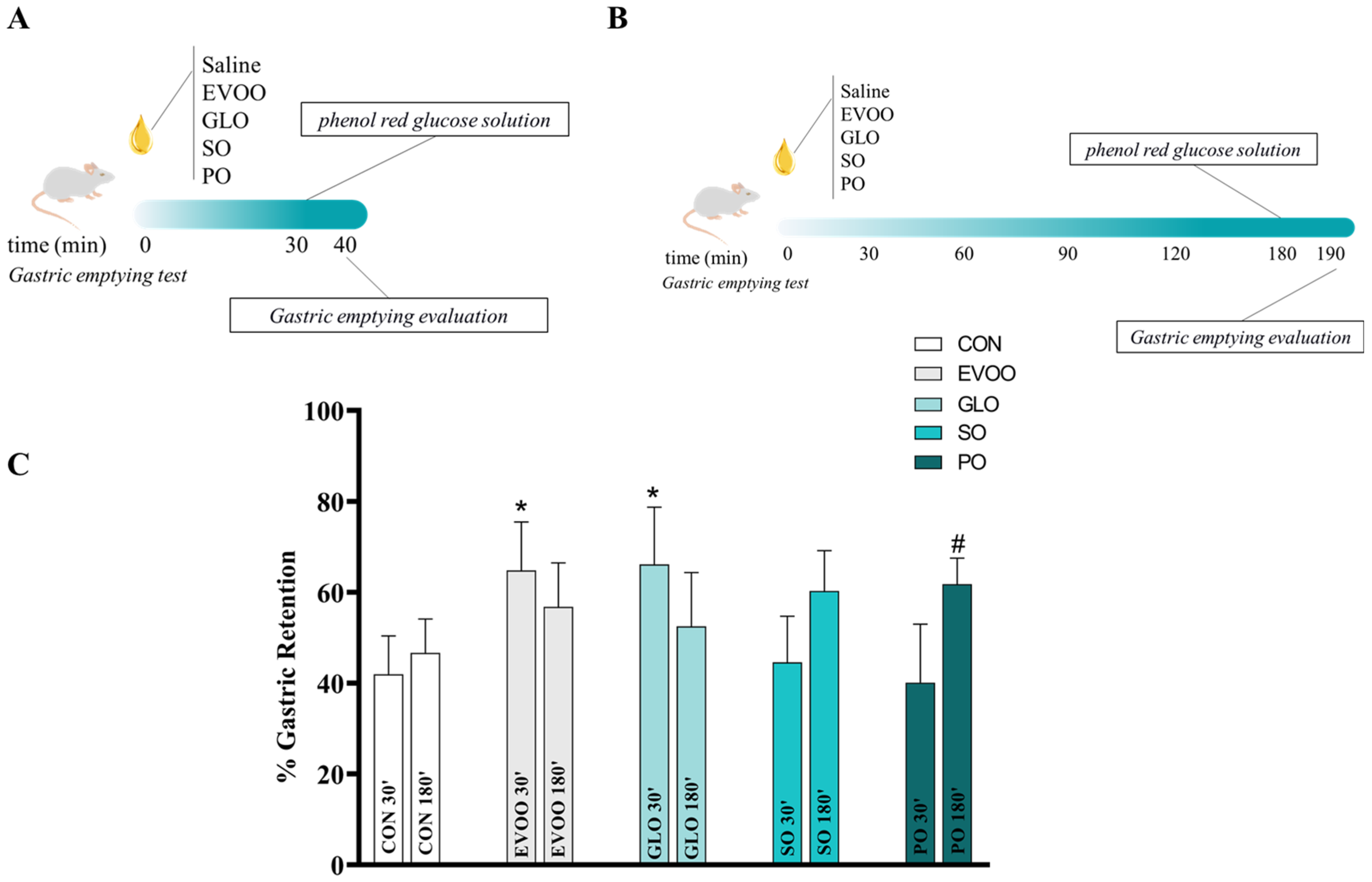
2.4. Gastric Emptying of Liquids
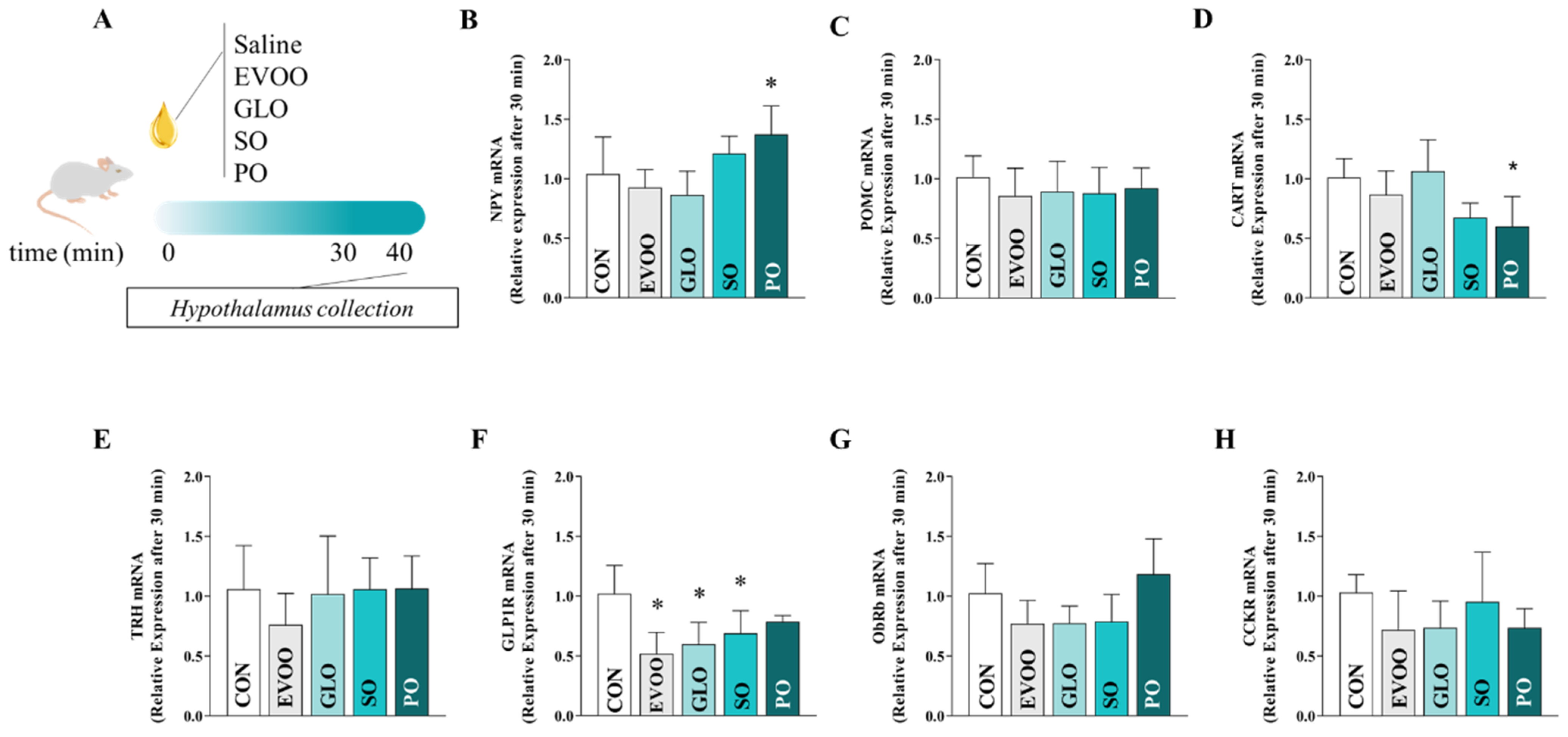
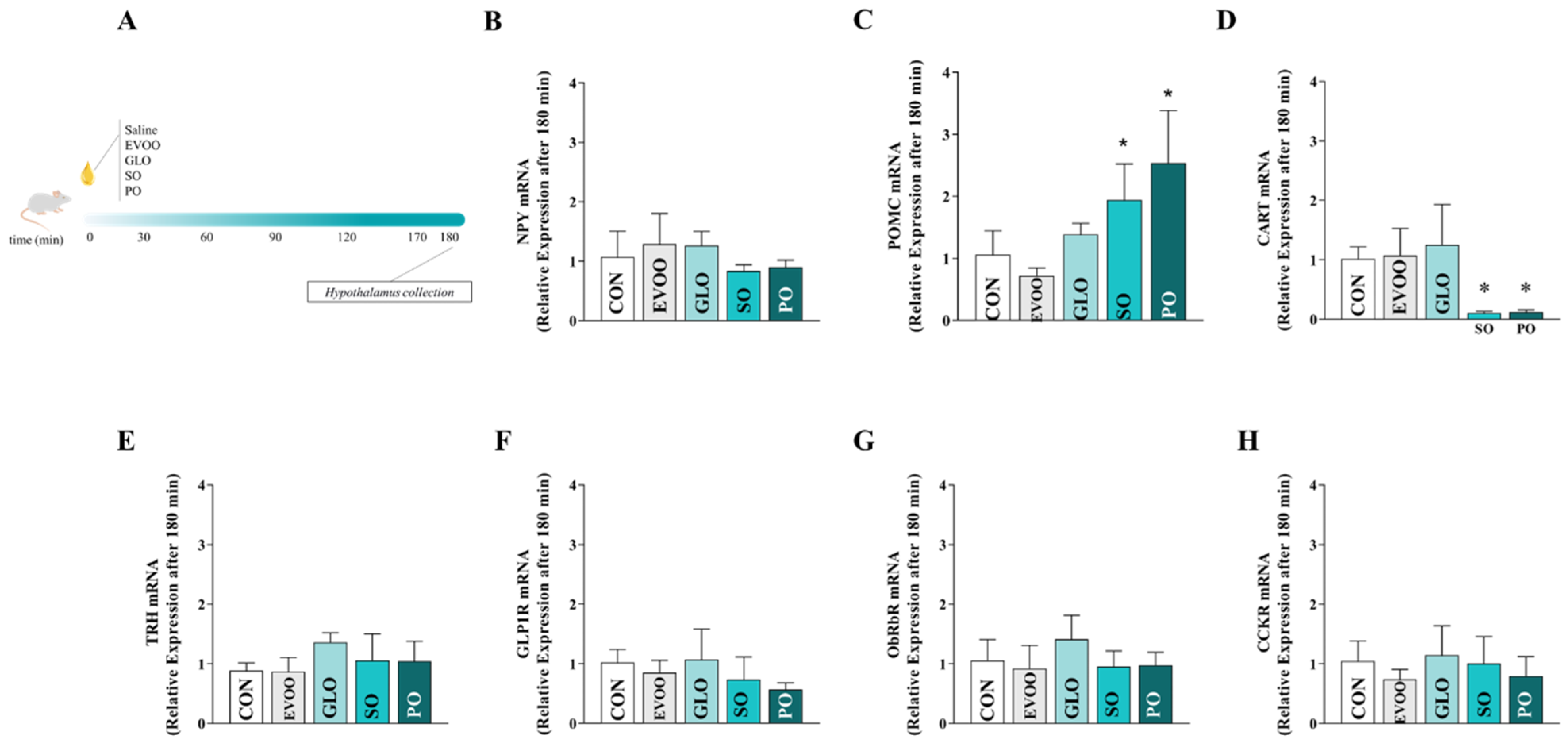
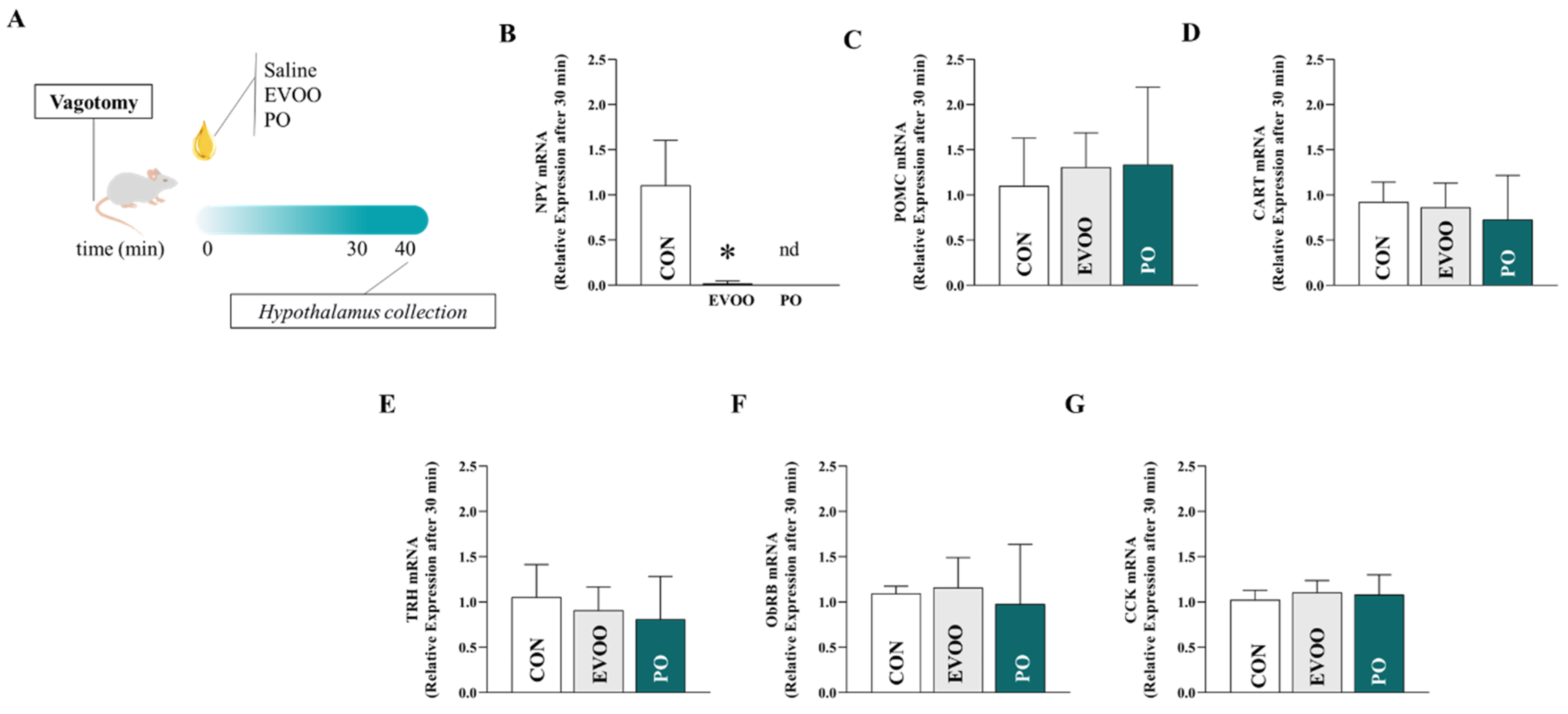
2.5. Procedure for Collecting the Hypothalamus
2.6. RNA Extraction from Hypothalamic Tissue
2.7. Procedure for Performing Subdiaphragmatic Vagotomy
2.8. Outcome Measures
2.9. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, G.A.; Popkin, B.M. Dietary fat intake does affect obesity! Am. J. Clin. Nutr. 1998, 68, 1157–1173. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Welch, I.M.; Sepple, C.P.; Read, N.W. Comparisons of the effects on satiety and eating behavior of infusion of lipid into the different regions of the small intestine. Gut 1988, 29, 306–311. [Google Scholar] [CrossRef]
- Sato, S.; Hokari, R.; Kurihara, C.; Sato, H.; Narimatsu, K.; Hozumi, H.; Ueda, T.; Higashiyama, M.; Okada, Y.; Watanabe, C.; et al. Dietary lipids and sweeteners regulate glucagon-like peptide-2 secretion. Am. J. Physiol.-Gastrointest. Liver Physiol. 2013, 304, G708–G714. [Google Scholar] [CrossRef]
- Haneishi, Y.; Furuya, Y.; Hasegawa, M.; Takemae, H.; Tanioka, Y.; Mizutani, T.; Rossi, M.; Miyamoto, J. Polyunsaturated fatty acids-rich dietary lipid prevents high fat diet-induced obesity in mice. Sci. Rep. 2023, 13, 5556. [Google Scholar] [CrossRef]
- Khan, A.S.; Hichami, A.; Murtaza, B.; Louillat-Habermeyer, M.L.; Ramseyer, C.; Azadi, M.; Yesylevskyy, S.; Mangin, F.; Lirussi, F.; Leemput, J.; et al. Novel Fat Taste Receptor Agonists Curtail Progressive Weight Gain in Obese Male Mice. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 633–663. [Google Scholar] [CrossRef]
- Godfrey, H.; Rankovic, A.; Grant, C.E.; Shoveller, A.K.; Bakovic, M.; Abood, S.K.; Verbrugghe, A. Dietary choline in gonadectomized kittens improved food intake and body composition but not satiety, serum lipids, or energy expenditure. PLoS ONE 2022, 17, e0264321. [Google Scholar] [CrossRef]
- Lim, W.F.; Nasir, S.M.; Teh, L.K.; James, R.J.; Izhar, M.H.M.; Salleh, M.Z. The methanolic extract of Garcinia atroviridis (MeGa) reduces body weight and food intake, and improves lipid profiles by altering the lipid metabolism: A rat model. Turk. J. Biol. 2020, 44, 437–448. [Google Scholar] [CrossRef]
- Steingoetter, A.; Arnold, M.; Scheuble, N.; Fedele, S.; Bertsch, P.; Liu, D.; Parker, H.L.; Langhans, W.; Fischer, P. A rat model of human lipid emulsion digestion. Front. Nutr. 2019, 6, 170. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Z.; Qi, M.; Zhao, P.; Duan, Y.; Yang, G.; Yuan, L. Brown adipose tissue-derived exosomes mitigate the metabolic syndrome in high fat diet mice. Theranostics 2020, 10, 8197. [Google Scholar] [CrossRef]
- Langsted, A.; Nordestgaard, B.G. Nonfasting versus fasting lipid profile for cardiovascular risk prediction. Pathology 2019, 51, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Corstens, M.N.; Troost, F.J.; Alleleyn, A.M.; Klaassen, T.; Berton-Carabin, C.C.; Schroën, K.; Masclee, A.A. Encapsulation of lipids as emulsion-alginate beads reduces food intake: A randomized placebo-controlled cross-over human trial in overweight adults. Nutr. Res. 2019, 63, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Jais, A.; Brüning, J.C. Arcuate Nucleus-Dependent Regulation of Metabolism-Pathways to Obesity and Diabetes Mellitus. Endocr. Rev. 2022, 43, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Salas, E.; García-Luna, C.; Soberanes-Chávez, P.; de Gortari, P. Role of the thyrotropin-releasing hormone of the limbic system in mood and eating regulation. J. Integr. Neurosci. 2022, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- Hartman, L.; Lago, R.C. Rapid preparation of fatty acid methyl esters from lipids. Lab. Pract. 1973, 22, 475–476. [Google Scholar] [PubMed]
- Cintra, D.E.; Costa, A.V.; Maria do Carmo, G.P.; Matta, S.L.; Silva, M.T.C.; Costa, N.M. Lipid profile of rats fed high-fat diets based on flaxseed, peanut, trout, or chicken skin. Nutrition 2006, 22, 197–205. [Google Scholar] [CrossRef] [PubMed]
- McConnell, E.L.; Basit, A.W.; Murdan, S. Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in-vivo experiments. J. Pharm. Pharmacol. 2008, 60, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Giezenaar, C.; Lange, K.; Hausken, T.; Jones, K.L.; Horowitz, M.; Chapman, I.; Soenen, S. Acute Effects of Substitution, and Addition, of Carbohydrates and Fat to Protein on Gastric Emptying, Blood Glucose, Gut Hormones, Appetite, and Energy Intake. Nutrients 2018, 10, 1451. [Google Scholar] [CrossRef] [PubMed]
- Porsgaard, T.; Straarup, E.M.; Høy, C.E. Gastric emptying in rats following administration of a range of different fats measured as acetaminophen concentration in plasma. Ann. Nutr. Metab. 2003, 47, 132–138. [Google Scholar] [CrossRef]
- De Sousa Cavalcante, M.L.; Silva, M.S.; Cavalcante, A.K.M.; de Oliveira Santos, R.; Nunes, D.D.T.; Busquets, S.; Argiles, J.M.; Seelaender, M.; de Matos Neto, E.M.; dos Santos, A.A.; et al. Win 55,212-2, atenolol and subdiaphragmatic vagotomy prevent acceleration of gastric emptying induced by cachexia via Yoshida-AH-130 cells in rats. Eur. J. Pharmacol. 2020, 877, 173087. [Google Scholar] [CrossRef]
- Reynell, P.C.; Spray, G.H. The simultaneous measurement of absorption and transit in the gastrointestinal tract of the rat. J. Physiol. 1956, 131, 452. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.S.; de Andrade Gomes, Y.; de Sousa Cavalcante, M.L.; Telles, P.V.N.; da Silva, A.C.A.; Severo, J.S.; de Oliveira Santos, R.; dos Santos, B.L.B.; Cavalcante, G.L.; Lima Rocha, C.H.; et al. Exercise and pyridostigmine prevents gastric emptying delay and increase blood pressure and cisplatin-induced baroreflex sensitivity in rats. Life Sci. 2021, 267, 118972. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.J.; Pavia, J.M. Increased endogenous noradrenaline and neuropeptide Y release from the hypothalamus of streptozotocin diabetic rats. Brain Res. 2004, 1006, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, T.T.; Torres-Leal, F.L.; Campaña, A.B.; Lima, F.B.; Donato, J., Jr. l-Leucine Supplementation Worsens the Adiposity of Already Obese Rats by Promoting a Hypothalamic Pattern of Gene Expression that Favors Fat Accumulation. Nutrients 2014, 6, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, M.; Al-Massadi, O.; Folgueira, C.; Bremser, S.; Gallego, R.; Torres-Leal, L.; Haddad-Tóvolli, R.; García-Caceres, C.; Hernandez-Bautista, R.; Lam, B.Y.H.; et al. p53 in AgRP neurons is required for protection against diet-induced obesity via JNK1. Nat. Commun. 2018, 9, 3432. [Google Scholar] [CrossRef] [PubMed]
- Da Graça, J.R.V.; Parente, C.C.; Fiúza, R.F.; da Silva, P.A.F.; Mota, B.T.; Salles, L.D.; de Souza Silva, C.M.; da Silva, M.T.B.; de Oliveira, R.B.; dos Santos, A.A. Subtotal nephrectomy inhibits the gastric emptying of liquid in awake rats. Physiol. Rep. 2015, 3, e12291. [Google Scholar] [CrossRef]
- Kalananthan, T.; Murashita, K.; Rønnestad, I.; Ishigaki, M.; Takahashi, K.; Silva, M.S.; Wakabayashi, Y.; Lai, F.; Shimizu, M.; Nilsen, T.O.; et al. Hypothalamic agrp and pomc mRNA responses to gastrointestinal fullness and fasting in Atlantic Salmon (Salmo salar L.). Front. Physiol. 2020, 11, 61. [Google Scholar] [CrossRef]
- Page, A.J.; Kentish, S.J. Plasticity of gastrointestinal vagal afferent satiety signals. Neurogastroenterol. Motil. 2017, 29, e12973. [Google Scholar] [CrossRef]
- Wang, G.J.; Tomasi, D.; Backus, W.; Wang, R.; Telang, F.; Geliebter, A.; Korner, J.; Bauman, A.; Fowler, J.S.; Thanos, P.K.; et al. Gastric distention activates satiety circuitry in the human brain. Neuroimage 2008, 39, 1824–1831. [Google Scholar] [CrossRef]
- Ritter, R.C. Gastrointestinal mechanisms of satiation for food. Physiol. Behav. 2004, 81, 249–273. [Google Scholar] [CrossRef]
- Qi, Y.; Lee, N.J.; Ip, C.K.; Enriquez, R.; Tasan, R.; Zhang, L.; Herzog, H. Agrp-negative arcuate NPY neurons drive feeding under positive energy balance via altering leptin responsiveness in POMC neurons. Cell Metab. 2023, 35, 979–995. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.; Peng, H.; Yu, N.; Zhang, Y.; Lin, X.; Lu, Y. Involvement of POMC neurons in LEAP2 regulation of food intake and body weight. Front. Endocrinol. 2022, 13, 932761. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Lee, N.J.; Ip, C.K.; Enriquez, R.; Tasan, R.; Zhang, L.; Herzog, H. NPY derived from AGRP neurons controls feeding via Y1 and energy expenditure and food foraging behaviour via Y2 signalling. Mol. Metab. 2022, 59, 101455. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Liang, X.F.; He, S.; Xu, J. Role of NPY receptor 8 in regulating of food intake in Chinese perch (Siniperca chuatsi). Aquac. Int. 2021, 29, 2619–2634. [Google Scholar] [CrossRef]
- Kaviani, S.; Cooper, J.A. Appetite responses to high-fat meals or diets of varying fatty acid composition: A comprehensive review. Eur. J. Clin. Nutr. 2017, 71, 1154–1165. [Google Scholar] [CrossRef] [PubMed]
- Prater, M.C.; Scheurell, A.R.; Paton, C.M.; Cooper, J.A. Hunger and satiety responses to diets enriched with cottonseed oil vs. olive oil. Physiol. Behav. 2023, 259, 114041. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Goh, H.J.; Govindharajulu, P.; Khee-Shing Leow, M.; Henry, C.J. Differential effects of monounsaturated and polyunsaturated fats on satiety and gut hormone responses in healthy subjects. Food 2019, 8, 634. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, N.; McKnight, A.D.; Carty, J.R.; Arnold, M.; Betley, J.N.; Alhadeff, A.L. Hypothalamic detection of macronutrients via multiple gut-brain pathways. Cell Metab. 2021, 33, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Rabail, R.; Shabbir, M.A.; Sahar, A.; Miecznikowski, A.; Kieliszek, M.; Aadil, R.M. An intricate review on nutritional and analytical profiling of coconut, flaxseed, olive, and sunflower oil blends. Molecules 2021, 26, 7187. [Google Scholar] [CrossRef]
- Sun, E.W.; Martin, A.M.; Young, R.L.; Keating, D.J. The regulation of peripheral metabolism by gut-derived hormones. Front. Endocrinol. 2019, 9, 433815. [Google Scholar] [CrossRef]
- Djohan, Y.F.; Camara-Cissé, M.; Fouret, G.; Bonafos, B.; Jover, B.; Cristol, J.P.; Coudray, C.; Feillet-Coudray, C.; Badia, E. Diets rich in olive oil, palm oil, or lard alter mitochondrial biogenesis and mitochondrial membrane composition in rat liver. Biochem. Res. Int. 2022, 2022, 9394356. [Google Scholar] [CrossRef] [PubMed]
- Klop, G.; Hatew, B.; Bannink, A.; Dijkstra, J. Feeding nitrate and docosahexaenoic acid affects enteric methane production and milk fatty acid composition in lactating dairy cows. J. Dairy Sci. 2016, 99, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Behrouz, V.; Yari, Z. A review on differential effects of dietary fatty acids on weight, appetite and energy expenditure. Crit. Rev. Food Sci. Nutr. 2022, 62, 2235–2249. [Google Scholar] [CrossRef] [PubMed]
- Raka, F.; Farr, S.; Kelly, J.; Stoianov, A.; Adeli, K. Metabolic control via nutrient-sensing mechanisms: Role of taste receptors and the gut-brain neuroendocrine axis. Am. J. Physiol.-Endocrinol. Metab. 2019, 317, E559–E572. [Google Scholar] [CrossRef] [PubMed]
- Michael, N.J.; Watt, M.J. Long chain fatty acids differentially regulate sub-populations of arcuate POMC and NPY neurons. Neuroscience 2020, 451, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Bajdak-Rusinek, K. The effect of palmitic acid on inflammatory response in macrophages: An overview of molecular mechanisms. Inflamm. Res. 2019, 68, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.T.; Oh, H.H.; Nguyen, A.K.; Choi, C.S.; Youn, J.H. Circulating free fatty acids inhibit food intake in an oleate-specific manner in rats. Physiol. Behav. 2016, 167, 194–201. [Google Scholar] [CrossRef]
- Laing, B.T.; Li, P.; Schmidt, C.A.; Bunner, W.; Yuan, Y.; Landry, T.; Prete, A.; McClung, J.M.; Huang, H. AgRP/NPY neuron excitability is modulated by metabotropic glutamate receptor 1 during fasting. Front. Cell. Neurosci. 2018, 12, 276. [Google Scholar] [CrossRef]
- Hoffman, G.E.; Murphy, A.Z. Anatomical markers of activity in hypothalamic systems. In Neuroendocrinology in Physiology and Medicine; Humana Press: Totowa, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Kiss, D.S.; Toth, I.; Jocsak, G.; Barany, Z.; Bartha, T.; Frenyo, L.V.; Horvath, T.L.; Zsarnovszky, A. Functional aspects of hypothalamic asymmetry. Brain Sci. 2020, 10, 389. [Google Scholar] [CrossRef]
- Oh, E.Y.; Park, B.S.; Yang, H.R.; Lee, H.G.; Tu, T.H.; Yang, S.; Han, M.-R.; Kim, J.G. Whole Transcriptome Analysis of Hypothalamus in Mice during Short-Term Starvation. Int. J. Mol. Sci. 2023, 24, 3204. [Google Scholar] [CrossRef]
- Moffitt, J.R.; Bambah-Mukku, D.; Eichhorn, S.W.; Vaughn, E.; Shekhar, K.; Perez, J.D.; Rubinstein, N.D.; Hao, J.; Regev, A.; Dulac, C.; et al. Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science 2018, 362, eaau5324. [Google Scholar] [CrossRef] [PubMed]
| FAs | Nomenclature | EVOO | GLO | SO | PO |
|---|---|---|---|---|---|
| C12:0 | Lauric | 0.04 | 0.04 | 0.03 | 0.07 |
| C14:0 | Myristic | 0.07 | 0.09 | 0.09 | 0.08 |
| C15:0 | Pentadecanoic | 0.08 | 0.04 | 0.03 | - |
| C16:0 | Palmitic | 5.65 | 6.20 | 10.85 | 40.47 |
| C16:1 | Palmitoleic | 0.81 | 0.10 | 0.11 | - |
| C17:0 | Margaric | 0.10 | 0.07 | 0.08 | - |
| C17:1 | Heptadecanoic | 0.08 | 0.06 | 0.06 | - |
| C18:0 | Stearic | 2.19 | 4.93 | 3.39 | 5.02 |
| C18:1 Trans | Elaidic | - | - | - | 0.07 |
| C18:1 (w9) | Oleic | 82.51 | 19.80 | 24.82 | 40.38 |
| C18:2 Trans | Linoelaidic | 0.09 | 0.08 | 0.28 | 0.12 |
| C18:2 (w6) | Linoleic | 6.39 | 15.28 | 52.72 | 10.16 |
| C18:3 Trans | - | 0.00 | 0.23 | 0.75 | - |
| C18:3 (w3) | Linolenic | 0.79 | 52.5 | 5.69 | 1.26 |
| C20:0 | Araquidic | 0.53 | 0.16 | 0.32 | 1.06 |
| C20:1 (w9) | Eicosenoic | 0.34 | 0.12 | 0.21 | 0.34 |
| C22:0 | Docosanoic | 0.19 | 0.16 | 0.40 | 0.68 |
| C24:0 | Tetracosanoic | 0.14 | 0.14 | 0.17 | - |
| Total SAT | 8.99 | 11.83 | 15.36 | 47.38 | |
| Total MONO | 83.74 | 20.08 | 25.2 | 40.79 | |
| Total POLI | 7.27 | 68.09 | 59.44 | 11.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, G.d.C.A.; Miranda, B.C.R.; Lima, J.O.P.F.; Martins, J.A.; de Sousa, A.A.; Nobre, T.A.; Severo, J.S.; da Silva, T.E.O.; Afonso, M.d.S.; Lima, J.D.C.C.; et al. Brain Perception of Different Oils on Appetite Regulation: An Anorectic Gene Expression Pattern in the Hypothalamus Dependent on the Vagus Nerve. Nutrients 2024, 16, 2397. https://doi.org/10.3390/nu16152397
Lopes GdCA, Miranda BCR, Lima JOPF, Martins JA, de Sousa AA, Nobre TA, Severo JS, da Silva TEO, Afonso MdS, Lima JDCC, et al. Brain Perception of Different Oils on Appetite Regulation: An Anorectic Gene Expression Pattern in the Hypothalamus Dependent on the Vagus Nerve. Nutrients. 2024; 16(15):2397. https://doi.org/10.3390/nu16152397
Chicago/Turabian StyleLopes, Gele de Carvalho Araújo, Brenda Caroline Rodrigues Miranda, João Orlando Piauilino Ferreira Lima, Jorddam Almondes Martins, Athanara Alves de Sousa, Taline Alves Nobre, Juliana Soares Severo, Tiago Eugênio Oliveira da Silva, Milessa da Silva Afonso, Joana Darc Carola Correia Lima, and et al. 2024. "Brain Perception of Different Oils on Appetite Regulation: An Anorectic Gene Expression Pattern in the Hypothalamus Dependent on the Vagus Nerve" Nutrients 16, no. 15: 2397. https://doi.org/10.3390/nu16152397
APA StyleLopes, G. d. C. A., Miranda, B. C. R., Lima, J. O. P. F., Martins, J. A., de Sousa, A. A., Nobre, T. A., Severo, J. S., da Silva, T. E. O., Afonso, M. d. S., Lima, J. D. C. C., de Matos Neto, E. M., Torres, L. R. d. O., Cintra, D. E., Lottenberg, A. M., Seelaender, M., Silva, M. T. B. d., & Torres-Leal, F. L. (2024). Brain Perception of Different Oils on Appetite Regulation: An Anorectic Gene Expression Pattern in the Hypothalamus Dependent on the Vagus Nerve. Nutrients, 16(15), 2397. https://doi.org/10.3390/nu16152397







