Modulation of the Serum Metabolome by the Short-Chain Fatty Acid Propionate: Potential Implications for Its Cholesterol-Lowering Effect
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Studies
2.2. Metabolite Profiling
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
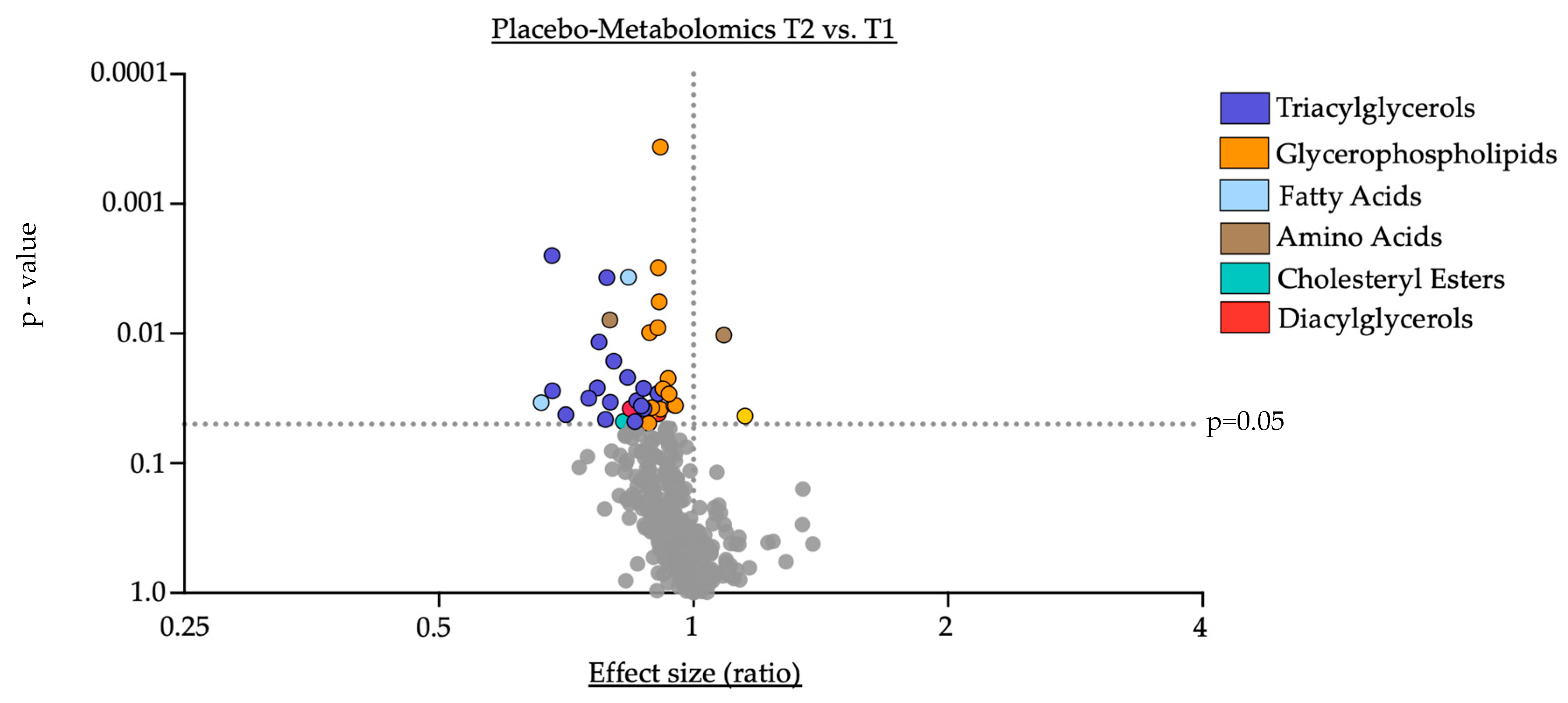
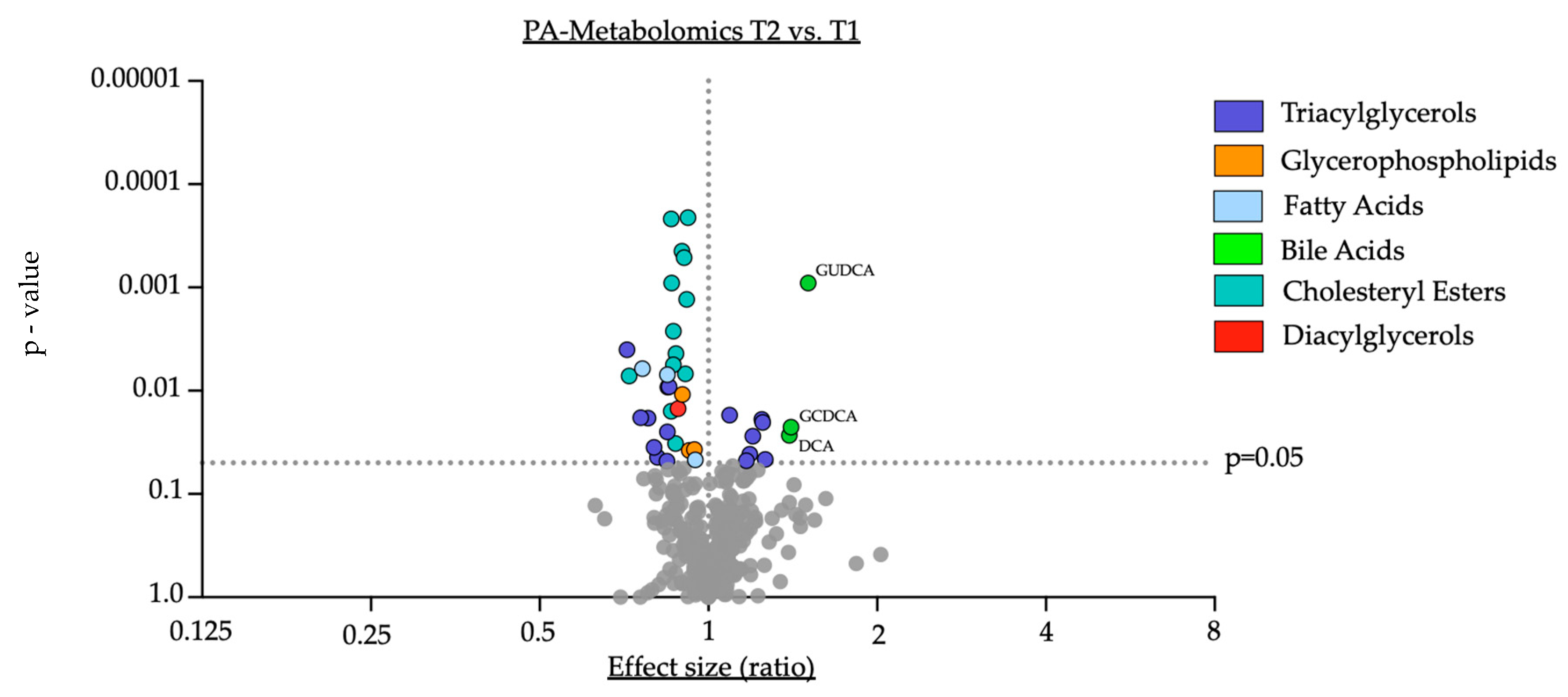
3.2. Impact of Oral Supplementation of PA on the Serum Metabolome in Study Participants
3.3. Correlation of Shifts in Serum Bile Acids upon PA Supplementation with Its Cholesterol-Lowering Effect
4. Discussion
- I.
- Oral supplementation with PA led to a significant downregulation of cholesteryl esters in serum, supporting prior reports of PA-induced downregulation of lipoprotein fractions by PA [2].
- II.
- PA supplementation resulted in significant shifts in the serum metabolome with an increase in distinct bile acids (GCDCA, DCA and GUDCA), indicating a critical role for PA in modulating the circulatory bile acid profile.
- III.
- The increase in the secondary bile acid DCA inversely correlates with the cholesterol-lowering effect of PA in humans, suggesting potential implications for the PA-related regulation of cholesterol metabolism by DCA.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Townsend, N.; Wilson, L.; Bhatnagar, P.; Wickramasinghe, K.; Rayner, M.; Nichols, M. Cardiovascular disease in Europe: Epidemiological update 2016. Eur. Heart J. 2016, 37, 3232–3245. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Zimmermann, F.; Schumann, P.; Jasina, A.; Roessler, J.; Schmidt, D.; Heinze, P.; Kaisler, J.; Nageswaran, V.; Aigner, A.; et al. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. Eur. Heart J. 2021, 43, 518–533. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Knauf, C. How gut microbes talk to organs: The role of endocrine and nervous routes. Mol. Metab. 2016, 5, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Roessler, J.; Leistner, D.M.; Landmesser, U.; Haghikia, A. Modulatory role of gut microbiota in cholesterol and glucose metabolism: Potential implications for atherosclerotic cardiovascular disease. Atherosclerosis 2022, 359, 1–12. [Google Scholar] [CrossRef] [PubMed]
- de Aguiar Vallim, T.Q.; Tarling, E.J.; Edwards, P.A. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013, 17, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Pushpass, R.G.; Alzoufairi, S.; Jackson, K.G.; Lovegrove, J.A. Circulating bile acids as a link between the gut microbiota and cardiovascular health: Impact of prebiotics, probiotics and polyphenol-rich foods. Nutr. Res. Rev. 2022, 35, 161–180. [Google Scholar] [CrossRef]
- de Boer, J.F.; Bloks, V.W.; Verkade, E.; Heiner-Fokkema, M.R.; Kuipers, F. New insights in the multiple roles of bile acids and their signaling pathways in metabolic control. Curr. Opin. Lipidol. 2018, 29, 194–202. [Google Scholar] [CrossRef]
- Ringseis, R.; Grundmann, S.M.; Schuchardt, S.; Most, E.; Eder, K. Limited Impact of Pivalate-Induced Secondary Carnitine Deficiency on Hepatic Transcriptome and Hepatic and Plasma Metabolome in Nursery Pigs. Metabolites 2021, 11, 573. [Google Scholar] [CrossRef] [PubMed]
- Chong Nguyen, C.; Duboc, D.; Rainteau, D.; Sokol, H.; Humbert, L.; Seksik, P.; Bellino, A.; Abdoul, H.; Bouazza, N.; Treluyer, J.M.; et al. Circulating bile acids concentration is predictive of coronary artery disease in human. Sci. Rep. 2021, 11, 22661. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jones, P.J.; Woollett, L.A.; Buckley, D.D.; Yao, L.; Granholm, N.A.; Tolley, E.A.; Heubi, J.E. Effects of chenodeoxycholic acid and deoxycholic acid on cholesterol absorption and metabolism in humans. Transl. Res. 2006, 148, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Houten, S.M.; Mataki, C.; Christoffolete, M.A.; Kim, B.W.; Sato, H.; Messaddeq, N.; Harney, J.W.; Ezaki, O.; Kodama, T.; et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006, 439, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Zambad, S.P.; Tuli, D.; Mathur, A.; Ghalsasi, S.A.; Chaudhary, A.R.; Deshpande, S.; Gupta, R.C.; Chauthaiwale, V.; Dutt, C. TRC210258, a novel TGR5 agonist, reduces glycemic and dyslipidemic cardiovascular risk in animal models of diabesity. Diabetes Metab. Syndr. Obes. 2013, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Houten, S.M.; Wang, L.; Moschetta, A.; Mangelsdorf, D.J.; Heyman, R.A.; Moore, D.D.; Auwerx, J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Investig. 2004, 113, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lee, F.Y.; Barrera, G.; Lee, H.; Vales, C.; Gonzalez, F.J.; Willson, T.M.; Edwards, P.A. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc. Natl. Acad. Sci. USA 2006, 103, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Sinal, C.J.; Tohkin, M.; Miyata, M.; Ward, J.M.; Lambert, G.; Gonzalez, F.J. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 2000, 102, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Sun, L.; Hu, X.; Wang, X.; Xu, F.; Chen, B.; Liang, X.; Xia, J.; Wang, P.; Aibara, D.; et al. Suppressing the intestinal farnesoid X receptor/sphingomyelin phosphodiesterase 3 axis decreases atherosclerosis. J. Clin. Investig. 2021, 131, e142865. [Google Scholar] [CrossRef]
- Huang, K.; Liu, C.; Peng, M.; Su, Q.; Liu, R.; Guo, Z.; Chen, S.; Li, Z.; Chang, G. Glycoursodeoxycholic Acid Ameliorates Atherosclerosis and Alters Gut Microbiota in Apolipoprotein E–Deficient Mice. J. Am. Heart Assoc. 2021, 10, e019820. [Google Scholar] [CrossRef]
- Farr, S.; Stankovic, B.; Hoffman, S.; Masoudpoor, H.; Baker, C.; Taher, J.; Dean, A.E.; Anakk, S.; Adeli, K. Bile acid treatment and FXR agonism lower postprandial lipemia in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G682–G693. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151. [Google Scholar] [CrossRef]
- Biagioli, M.; Marchianò, S.; Carino, A.; Di Giorgio, C.; Santucci, L.; Distrutti, E.; Fiorucci, S. Bile Acids Activated Receptors in Inflammatory Bowel Disease. Cells 2021, 10, 1281. [Google Scholar] [CrossRef]
- Zhou, T.; Yang, K.; Huang, J.; Fu, W.; Yan, C.; Wang, Y. Effect of Short-Chain Fatty Acids and Polyunsaturated Fatty Acids on Metabolites in H460 Lung Cancer Cells. Molecules 2023, 28, 2357. [Google Scholar] [CrossRef]
- Sinha, S.R.; Haileselassie, Y.; Nguyen, L.P.; Tropini, C.; Wang, M.; Becker, L.S.; Sim, D.; Jarr, K.; Spear, E.T.; Singh, G.; et al. Dysbiosis-Induced Secondary Bile Acid Deficiency Promotes Intestinal Inflammation. Cell Host Microbe 2020, 27, 659–670. [Google Scholar] [CrossRef]
- Lambert, J.E.; Parks, E.J. Postprandial metabolism of meal triglyceride in humans. Biochim. Biophys. Acta 2012, 1821, 721–726. [Google Scholar] [CrossRef]
- Guido, M.E.; Monjes, N.M.; Wagner, P.M.; Salvador, G.A. Circadian Regulation and Clock-Controlled Mechanisms of Glycerophospholipid Metabolism from Neuronal Cells and Tissues to Fibroblasts. Mol. Neurobiol. 2022, 59, 326–353. [Google Scholar] [CrossRef]
- Yuan, R.K.; Zitting, K.M.; Wang, W.; Buxton, O.M.; Williams, J.S.; Duffy, J.F.; Czeisler, C.A. Fasting blood triglycerides vary with circadian phase in both young and older people. Physiol. Rep. 2020, 8, e14453. [Google Scholar] [CrossRef]
- Fu, Z.D.; Cui, J.Y.; Klaassen, C.D. Atorvastatin induces bile acid-synthetic enzyme Cyp7a1 by suppressing FXR signaling in both liver and intestine in mice. J. Lipid Res. 2014, 55, 2576–2586. [Google Scholar] [CrossRef]
- Imaizumi, K.; Hirata, K.; Yasni, S.; Sugano, M. Propionate Enhances Synthesis and Secretion of Bile Acids in Primary Cultured Rat Hepatocytes via Succinyl CoA. Biosci. Biotechnol. Biochem. 1992, 56, 1894–1896. [Google Scholar] [CrossRef]

| All Patients (n = 55) | Placebo (n = 28) | PA (n = 27) | p-Value | |
|---|---|---|---|---|
| Age, y | 50.4 (±11.6) | 51.8 (±11.1) | 49.1 (±11.9) | 0.395 |
| Females | 39 (70.1%) | 21 (75%) | 18 (66.7%) | 0.778 |
| Body Mass Index * | 27.1 (±4.3) | 26.4 (±3.7) | 27.7 (±4.7) | 0.268 |
| Medical history | ||||
| Diabetes | 1 | 0 | 1 | 0.313 |
| Hypertension | 11 | 4 | 7 | 0.378 |
| Cholesterol levels (mg/dL) | ||||
| Total (mg/dL) | 256.9 (±43.3) | 263.1 (±36.1) | 250.4 (±48.7) | 0.284 |
| LDL (mg/dL) | 184.7 (±41) | 188.3 (±34.9) | 181.2 (±46.3) | 0.53 |
| HDL (mg/dL) | 67.2 (±20.8) | 72.8 (±20.1) | 61.3 (±19.8) | 0.047 |
| Metabolite | Placebo_T1 | Placebo_T2 | p-Value |
|---|---|---|---|
| Cholesteryl Esters | |||
| CE(14:0) | 35.9 (±11.5) | 35.7 (±9.5) | 0.899 |
| CE(14:1) | 0.9 (±0.8) | 0.9 (±0.8) | 0.866 |
| CE(15:0) | 14.2 (±5.8) | 14.1 (±4.9) | 0.39 |
| CE(15:1) | 0.8 (±0.3) | 0.7 (±0.2) | 0.054 |
| CE(16:0) | 282.5 (±62.3) | 275.5 (±50.4) | 0.587 |
| CE(16:1) | 93.6 (±43.9) | 89.9 (±41.2) | 0.476 |
| CE(17:0) | 10.6 (±3.3) | 10.5 (±3.1) | 0.567 |
| CE(17:1) | 8.2 (±4.2) | 7.9 (±4.3) | 0.55 |
| CE(18:0) | 22.8 (±5.6) | 22.5 (±6.8) | 0.789 |
| CE(18:1) | 576.6 (±160.1) | 565 (±180.7) | 0.641 |
| CE(18:2) | 1630 (±341.5) | 1592.5 (±313) | 0.471 |
| CE(18:3) | 100.2 (±44.1) | 90.6 (±34.7) | 0.124 |
| CE(20:0) | 2 (±0.6) | 1.6 (±0.5) | 0.048 |
| CE(20:1) | 0.8 (±0.2) | 0.7 (±0.2) | 0.175 |
| CE(20:3) | 38.5 (±12.8) | 39.8 (±13.7) | 0.497 |
| CE(20:4) | 336.3 (±111.9) | 347.8 (±130.8) | 0.504 |
| CE(20:5) | 126.3 (±66) | 127.9 (±66.3) | 0.65 |
| CE(22:2) | 0.2 (±0) | 0.2 (±0.1) | 0.117 |
| CE(22:5) | 3.4 (±0.9) | 3.4 (±1) | 0.959 |
| CE(22:6) | 53.3 (±22.6) | 53.1 (±19) | 0.526 |
| Bile Acids | |||
| Cholic acid | 0.1 (±0.1) | 0.4 (±0.4) | 0.999 |
| Deoxycholic acid | 0.3 (±0.3) | 0.3 (±0.3) | 0.772 |
| Glycocholic acid | 0.2 (±0.2) | 0.2 (±0.4) | 0.419 |
| Glycochenodeoxycholic acid | 0.4 (±0.3) | 0.5 (±0.6) | 0.399 |
| Glycodeoxycholic acid | 0.2 (±0.3) | 0.3 (±0.3) | 0.417 |
| Glycolithocholic acid | 0.01 (±0) | 0.01 (±0) | 0.999 |
| Glycolithocholic acid sulfate | 0.2 (±0.1) | 0.2 (±0.2) | 0.915 |
| Glycoursodeoxycholic acid | 0.1 (±0.1) | 0.1 (±0.1) | 0.241 |
| Taurocholic acid | 0.1 (±0.1) | 0.1 (±0.2) | 0.296 |
| Taurochenodeoxycholic acid | 0.1 (±0.1) | 0.1 (±0.1) | 0.827 |
| Taurodeoxycholic acid | 0.1 (±0.1) | 0.1 (±0.1) | 0.768 |
| Metabolite | Placebo_T1 | Placebo_T2 | p-Value |
|---|---|---|---|
| Cholesteryl Esters | |||
| CE(14:0) | 35.4 (±12.9) | 30.4 (±12.7) | 0.016 |
| CE(14:1) | 1.1 (±0.9) | 0.9 (±0.8) | 0.192 |
| CE(15:0) | 14.8 (±5.4) | 12.5 (±4.8) | 0.009 |
| CE(15:1) | 0.8 (±0.2) | 0.7 (±0.3) | 0.134 |
| CE(16:0) | 270.5 (±51.4) | 242.8 (±50.7) | <0.001 |
| CE(16:1) | 95.9 (±42.3) | 82.5 (±38.1) | 0.001 |
| CE(17:0) | 10.5 (±3.9) | 9.2 (±3) | 0.004 |
| CE(17:1) | 8 (±3.5) | 7 (±3.1) | 0.032 |
| CE(18:0) | 22.1 (±6.3) | 20.2 (±6.8) | 0.001 |
| CE(18:1) | 540.3 (±150) | 491.7 (±149.8) | 0.007 |
| CE(18:2) | 1597 (±322.4) | 1468.9 (±313.1) | <0.001 |
| CE(18:3) | 96 (±36.2) | 82.3 (±36.9) | <0.001 |
| CE(20:0) | 2 (±0.6) | 1.5 (±0.5) | 0.007 |
| CE(20:1) | 0.8 (±0.2) | 0.7 (±0.3) | 0.211 |
| CE(20:3) | 37.8 (±15.6) | 32.7 (±12.8) | 0.006 |
| CE(20:4) | 318.3 (±112.7) | 288.1 (±105) | 0.001 |
| CE(20:5) | 131.1 (±64.4) | 99 (±51.1) | <0.001 |
| CE(22:2) | 0.2 (±0.1) | 0.1 (±0) | 0.184 |
| CE(22:5) | 3.1 (±1) | 2.9 (±1) | 0.08 |
| CE(22:6) | 51.5 (±20.8) | 44.6 (±15.9) | 0.003 |
| Bile Acids | |||
| Cholic acid | 0.3 (±0.3) | 0.3 (±0.2) | 0.61 |
| Deoxycholic acid | 0.2 (±0.2) | 0.3 (±0.2) | 0.027 |
| Glycocholic acid | 0.1 (±0.2) | 0.2 (±0.1) | 0.059 |
| Glycochenodeoxycholic acid | 0.3 (±0.3) | 0.4 (±0.3) | 0.023 |
| Glycodeoxycholic acid | 0.1 (±0.2) | 0.2 (±0.2) | 0.111 |
| Glycolithocholic acid | 0.01 (±0) | 0.01 (±0) | 0.707 |
| Glycolithocholic acid sulfate | 0.2 (±0.2) | 0.2 (±0.2) | 0.312 |
| Glycoursodeoxycholic acid | 0.01 (+0) | 0.1 (±0) | <0.001 |
| Taurocholic acid | 0.01 (±0) | 0.01 (±0) | 0.242 |
| Taurochenodeoxycholic acid | 0.01 (±0) | 0.1 (±0.1) | 0.158 |
| Taurodeoxycholic acid | 0.01 (±0) | 0.01 (±0.1) | 0.469 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roessler, J.; Zimmermann, F.; Schumann, P.; Nageswaran, V.; Ramezani Rad, P.; Schuchardt, S.; Leistner, D.M.; Landmesser, U.; Haghikia, A. Modulation of the Serum Metabolome by the Short-Chain Fatty Acid Propionate: Potential Implications for Its Cholesterol-Lowering Effect. Nutrients 2024, 16, 2368. https://doi.org/10.3390/nu16142368
Roessler J, Zimmermann F, Schumann P, Nageswaran V, Ramezani Rad P, Schuchardt S, Leistner DM, Landmesser U, Haghikia A. Modulation of the Serum Metabolome by the Short-Chain Fatty Acid Propionate: Potential Implications for Its Cholesterol-Lowering Effect. Nutrients. 2024; 16(14):2368. https://doi.org/10.3390/nu16142368
Chicago/Turabian StyleRoessler, Johann, Friederike Zimmermann, Paul Schumann, Vanasa Nageswaran, Pegah Ramezani Rad, Sven Schuchardt, David M. Leistner, Ulf Landmesser, and Arash Haghikia. 2024. "Modulation of the Serum Metabolome by the Short-Chain Fatty Acid Propionate: Potential Implications for Its Cholesterol-Lowering Effect" Nutrients 16, no. 14: 2368. https://doi.org/10.3390/nu16142368
APA StyleRoessler, J., Zimmermann, F., Schumann, P., Nageswaran, V., Ramezani Rad, P., Schuchardt, S., Leistner, D. M., Landmesser, U., & Haghikia, A. (2024). Modulation of the Serum Metabolome by the Short-Chain Fatty Acid Propionate: Potential Implications for Its Cholesterol-Lowering Effect. Nutrients, 16(14), 2368. https://doi.org/10.3390/nu16142368






