Plant-Based Dietary Protein Is Associated with Lower Metabolic Syndrome Risk in Division III Female Athletes: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Dietary Intake Assessment
2.4. Anthropometric and Hemodynamic Assessments
2.5. Blood Lipid Profile and Glucose
2.6. Body Composition
2.7. siMS Score and siMS Risk Score
2.8. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Nutritional Intake Analysis
3.2.1. Energy, Fiber, and Macronutrient Intake
3.2.2. Micronutrient Intake
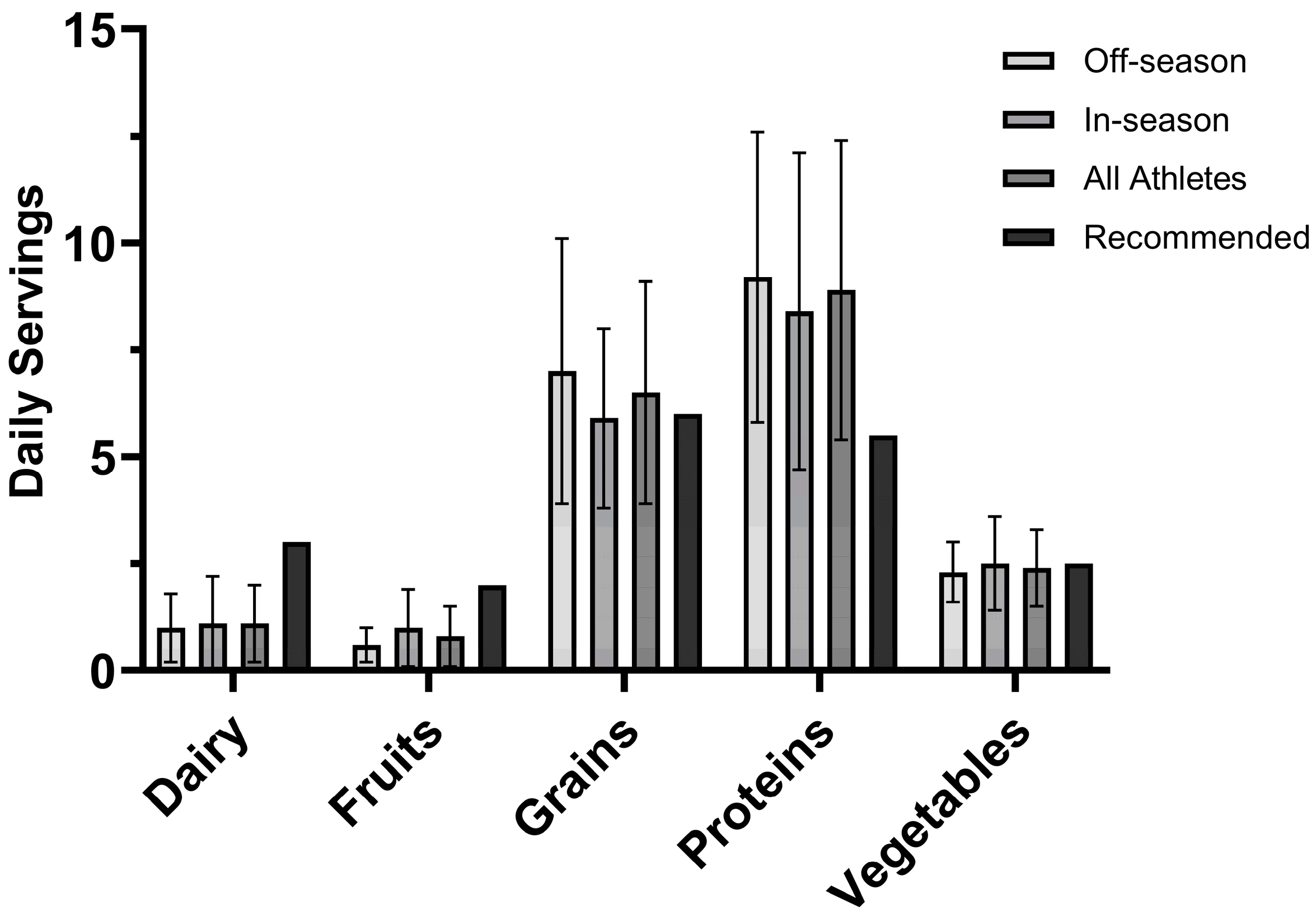
3.2.3. USDA MyPlate Recommendations
3.2.4. Protein Pacing
3.3. Body Composition
3.4. Blood Glucose and Lipid Profiles
3.5. siMS Score and siMS Risk Score
3.6. Regression Analyses
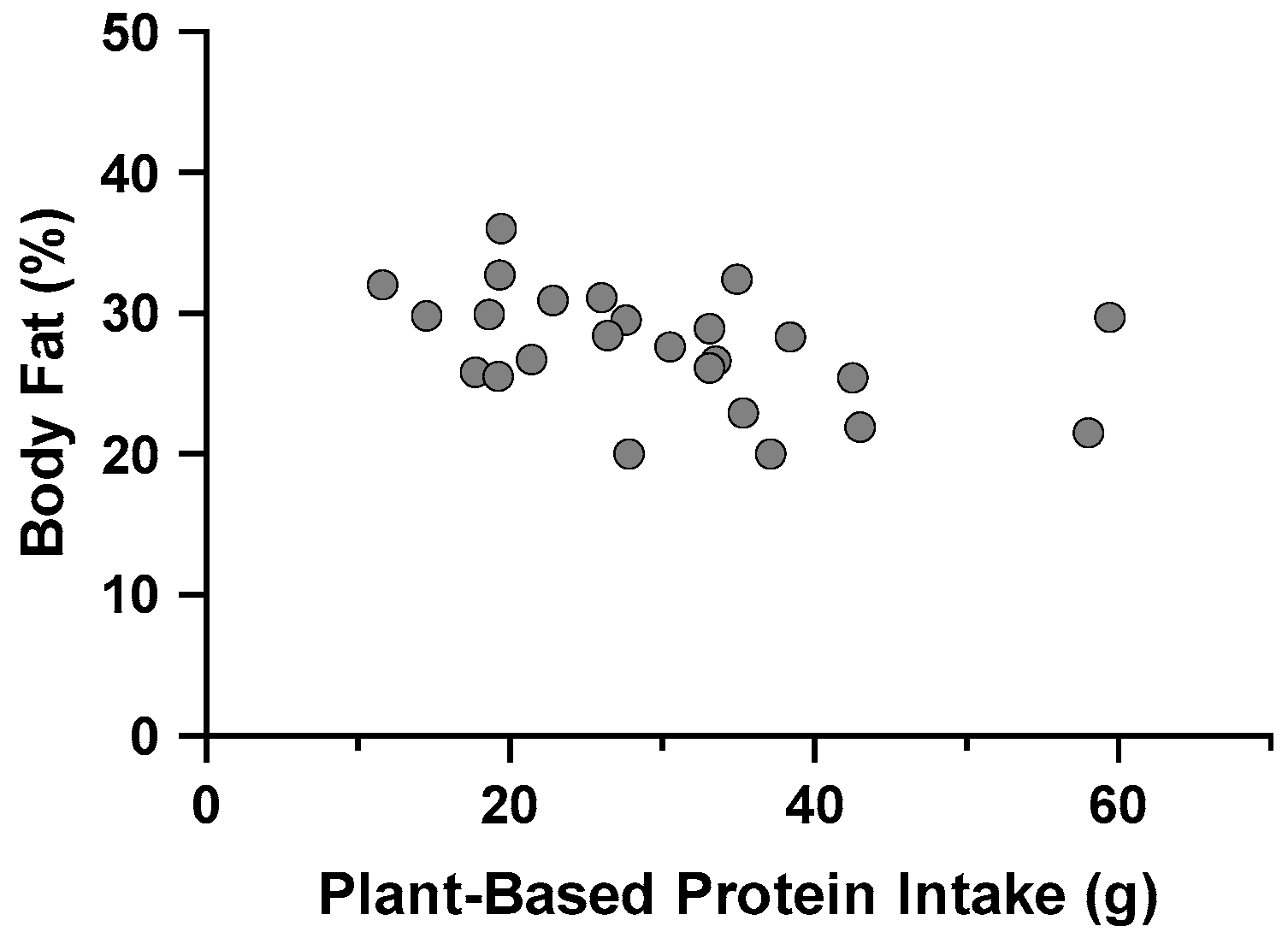
3.6.1. MetS and Dietary Protein Source and Quality
3.6.2. Body Composition, Dietary Protein Source and Quality, and Protein Pacing
4. Discussion
4.1. MetS Risk of Division III Female Athletes
4.2. Dietary Intake of Division III Female Athletes
4.3. Body Composition, Dietary Protein Source and Quality, and Protein Pacing of Division III Female Athletes
4.4. Limitations
4.5. Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malik, S.; Wong, N.D.; Franklin, S.S.; Kamath, T.V.; L’Italien, G.J.; Pio, J.R.; Williams, G.R. Impact of the Metabolic Syndrome on Mortality from Coronary Heart Disease, Cardiovascular Disease, and All Causes in United States Adults. Circulation 2004, 110, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Chee Cheong, K.; Lim, K.H.; Ghazali, S.M.; Teh, C.H.; Cheah, Y.K.; Baharudin, A.; Lim, H.L.; Abdul Hamid, A.M.; Mustapha, F.I.; Omar, M.A. Association of Metabolic Syndrome with Risk of Cardiovascular Disease Mortality and All-Cause Mortality among Malaysian Adults: A Retrospective Cohort Study. BMJ Open 2021, 11, e047849. [Google Scholar] [CrossRef] [PubMed]
- Hirode, G.; Wong, R.J. Trends in the Prevalence of Metabolic Syndrome in the United States, 2011–2016. JAMA 2020, 323, 2526. [Google Scholar] [CrossRef]
- Cornier, M.-A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The Metabolic Syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; And International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Gallardo-Alfaro, L.; Del Mar Bibiloni, M.; Mascaró, C.M.; Montemayor, S.; Ruiz-Canela, M.; Salas-Salvad, J.; Corella, D.; Fitó, M.; Romaguera, D.; Vioque, J.; et al. Leisure-Time Physical Activity, Sedentary Behaviour and Diet Quality Are Associated with Metabolic Syndrome Severity: The PREDIMED-plus Study. Nutrients 2020, 12, 1013. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, B.; Stone, K.A.; Kotarsky, C.J.; Johnson, N.; Bradley, A.; Scheffert, R.A.; Hackney, K.J.; Byun, W.; Stastny, S. Animal-Based Dietary Protein Intake Is Not A Risk Factor For Metabolic Syndrome Among Young Or Middle-Aged Females. Nutr. Metab. Insights 2022, 15, 11786388221107800. [Google Scholar] [CrossRef] [PubMed]
- Chiang, T.L.; Chen, C.; Hsu, C.H.; Lin, Y.C.; Wu, H.J. Is the Goal of 12,000 Steps per Day Sufficient for Improving Body Composition and Metabolic Syndrome? The Necessity of Combining Exercise Intensity: A Randomized Controlled Trial. BMC Public Health 2019, 19, 1215. [Google Scholar] [CrossRef]
- Ying, M.; Hu, X.; Li, Q.; Dong, H.; Zhou, Y.; Chen, Z. Long-Term Trajectories of BMI and Cumulative Incident Metabolic Syndrome: A Cohort Study. Front. Endocrinol. 2022, 13, 915394. [Google Scholar] [CrossRef]
- Poon, V.T.W.; Kuk, J.L.; Ardern, C.I. Trajectories of Metabolic Syndrome Development in Young Adults. PLoS ONE 2014, 9, e111647. [Google Scholar] [CrossRef]
- Franco, O.H.; Massaro, J.M.; Civil, J.; Cobain, M.R.; O’Malley, B.; D’Agostino, R.B. Trajectories of Entering the Metabolic Syndrome: The Framingham Heart Study. Circulation 2009, 120, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Sygo, J.; Morton, J.P. Fuelling the Female Athlete: Carbohydrate and Protein Recommendations. Eur. J. Sport. Sci. 2022, 22, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Riviere, A.J.; Leach, R.; Mann, H.; Robinson, S.; Burnett, D.O.; Babu, J.R.; Frugé, A.D. Nutrition Knowledge of Collegiate Athletes in the United States and the Impact of Sports Dietitians on Related Outcomes: A Narrative Review. Nutrients 2021, 13, 1772. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, B.; Ackerman, K.E. Recommendations and Nutritional Considerations for Female Athletes: Health and Performance. Sports Med. 2021, 51, 43–57. [Google Scholar] [CrossRef]
- Fiorini, S.; Neri, L.D.C.L.; Guglielmetti, M.; Pedrolini, E.; Tagliabue, A.; Quatromoni, P.A.; Ferraris, C. Nutritional Counseling in Athletes: A Systematic Review. Front. Nutr. 2023, 10, 1250567. [Google Scholar]
- Beermann, B.L.; Lee, D.G.; Almstedt, H.C.; McCormack, W.P. Nutritional Intake and Energy Availability of Collegiate Distance Runners. J. Am. Coll. Nutr. 2020, 39, 747–755. [Google Scholar] [CrossRef]
- Krick, R.L.; Brown, A.F.; Brown, K.N. Increased Female Athlete Triad Knowledge Following a Brief Video Educational Intervention. J. Nutr. Educ. Behav. 2019, 51, 1126–1129. [Google Scholar] [CrossRef]
- Logue, D.M.; Madigan, S.M.; Melin, A.; Delahunt, E.; Heinen, M.; Mc Donnell, S.J.; Corish, C.A. Low Energy Availability in Athletes 2020: An Updated Narrative Review of Prevalence, Risk, within-Day Energy Balance, Knowledge, and Impact on Sports Performance. Nutrients 2020, 12, 835. [Google Scholar] [CrossRef]
- Magee, M.K.; Lockard, B.L.; Zabriskie, H.A.; Schaefer, A.Q.; Luedke, J.A.; Erickson, J.L.; Jones, M.T.; Jagim, A.R. Prevalence of Low Energy Availability in Collegiate Women Soccer Athletes. J. Funct. Morphol. Kinesiol. 2020, 5, 96. [Google Scholar] [CrossRef]
- Castro-Barquero, S.; Ruiz-León, A.M.; Sierra-Pérez, M.; Estruch, R.; Casas, R. Dietary Strategies for Metabolic Syndrome: A Comprehensive Review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef]
- Semnani-Azad, Z.; Khan, T.A.; Blanco Mejia, S.; De Souza, R.J.; Leiter, L.A.; Kendall, C.W.C.; Hanley, A.J.; Sievenpiper, J.L. Association of Major Food Sources of Fructose-Containing Sugars with Incident Metabolic Syndrome: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2020, 3, e209993. [Google Scholar] [CrossRef] [PubMed]
- Balgoon, M.J.; Al-Zahrani, M.H.; Alkhattabi, N.A.; Alzahrani, N.A. The Correlation between Obesity and Metabolic Syndrome in Young Female University Students in the Kingdom of Saudi Arabia. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2399–2402. [Google Scholar] [CrossRef] [PubMed]
- Buell, J.L.; Calland, D.; Hanks, F.; Johnston, B.; Pester, B.; Sweeney, R.; Thorne, R. Presence of Metabolic Syndrome in Football Linemen. J. Athl. Train. 2008, 43, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, X.; Wang, L.; Guo, Y.; Xie, M. Prevalence of Metabolic Syndrome and Its Components among Chinese Professional Athletes of Strength Sports with Different Body Weight Categories. PLoS ONE 2013, 8, e79758. [Google Scholar] [CrossRef] [PubMed]
- Wall, C.C.; Coughlin, M.A.; Jones, M.T. Surveying The Nutritional Habits And Behaviors Of NCAA-Division III Athletes. J. Strength Cond. Res. 2010, 24, 1. [Google Scholar] [CrossRef]
- Zanders, B.R.; Currier, B.S.; Harty, P.S.; Zabriskie, H.A.; Smith, C.R.; Stecker, R.A.; Richmond, S.R.; Jagim, A.R.; Kerksick, C.M. Changes in Energy Expenditure, Dietary Intake, and Energy Availability Across an Entire Collegiate Women’s Basketball Season. J. Strength Cond. Res. 2021, 35, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Kumahara, H.; Ohta, C.; Nabeshima, E.; Nakayama, A.; Mine, S.; Yamato, T. Dietary Intake and Energy Expenditure During Two Different Phases of Athletic Training in Female Collegiate Lacrosse Players. J. Strength Cond. Res. 2020, 34, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Pinckaers, P.J.M.; Trommelen, J.; Snijders, T.; van Loon, L.J.C. The Anabolic Response to Plant-Based Protein Ingestion. Sports Med. 2021, 51, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Fogelholm, M. Dairy Products, Meat and Sports Performance. Sports Med. 2003, 33, 615–631. [Google Scholar] [CrossRef]
- Zhu, J.W.; Reed, J.L.; Van Spall, H.G.C. The Underrepresentation of Female Athletes in Sports Research: Considerations for Cardiovascular Health. Eur. Heart J. 2022, 43, 1609–1611. [Google Scholar] [CrossRef]
- Emmonds, S.; Heyward, O.; Jones, B. The Challenge of Applying and Undertaking Research in Female Sport. Sports Med. Open 2019, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Gay, B.; Caine-Bish, N.; Gordon, K.; Miracle, A. P120 Nutrition Knowledge of Vegetarian Diets in College Students. J. Nutr. Educ. Behav. 2019, 51, S86. [Google Scholar] [CrossRef]
- Arciero, P.J.; Poe, M.; Mohr, A.E.; Ives, S.J.; Arciero, A.; Sweazea, K.L.; Gumpricht, E.; Arciero, K.M. Intermittent Fasting and Protein Pacing Are Superior to Caloric Restriction for Weight and Visceral Fat Loss. Obesity 2023, 31, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Arciero, P.J.; Edmonds, R.; He, F.; Ward, E.; Gumpricht, E.; Mohr, A.; Ormsbee, M.J.; Astrup, A. Protein-Pacing Caloric-Restriction Enhances Body Composition Similarly in Obese Men and Women during Weight Loss and Sustains Efficacy during Long-Term Weight Maintenance. Nutrients 2016, 8, 476. [Google Scholar] [CrossRef] [PubMed]
- Arciero, P.J.; Ormsbee, M.J.; Gentile, C.L.; Nindl, B.C.; Brestoff, J.R.; Ruby, M. Increased Protein Intake and Meal Frequency Reduces Abdominal Fat during Energy Balance and Energy Deficit. Obesity 2013, 21, 1357–1366. [Google Scholar] [CrossRef]
- Arciero, P.J.; Ives, S.J.; Norton, C.; Escudero, D.; Minicucci, O.; O’Brien, G.; Paul, M.; Ormsbee, M.J.; Miller, V.; Sheridan, C.; et al. Protein-Pacing and Multi-Component Exercise Training Improves Physical Performance Outcomes in Exercise-Trained Women: The PRISE 3 Study. Nutrients 2016, 8, 332. [Google Scholar] [CrossRef]
- Prentice, R.L.; Mossavar-Rahmani, Y.; Huang, Y.; Van Horn, L.; Beresford, S.A.A.; Caan, B.; Tinker, L.; Schoeller, D.; Bingham, S.; Eaton, C.B.; et al. Evaluation and Comparison of Food Records, Recalls, and Frequencies for Energy and Protein Assessment by Using Recovery Biomarkers. Am. J. Epidemiol. 2011, 174, 591–603. [Google Scholar] [CrossRef]
- Carey, M.; Markham, C.; Gaffney, P.; Boran, G.; Maher, V. Validation of a Point of Care Lipid Analyser Using a Hospital Based Reference Laboratory. Ir. J. Med. Sci. 2006, 175, 30–35. [Google Scholar] [CrossRef]
- Dale, R.A.; Jensen, L.H.; Krantz, M.J. Comparison of Two Point-of-Care Lipid Analyzers for Use in Global Cardiovascular Risk Assessments. Ann. Pharmacother. 2008, 42, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Issa, J.S.; Strunz, C.; Giannini, S.D.; Forti, N.; Diament, J. Precision and Accuracy of Blood Lipid Analyses by a Portable Device (Cholestech-LDX). Arq. Bras. Cardiol. 1996, 66, 339–342. [Google Scholar]
- Soldatovic, I.; Vukovic, R.; Culafic, D.; Gajic, M.; Dimitrijevic-Sreckovic, V. SiMS Score: Simple Method for Quantifying Metabolic Syndrome. PLoS ONE 2016, 11, e0146143. [Google Scholar] [CrossRef] [PubMed]
- Sebekova, K.; Sebek, J. Continuous Metabolic Syndrome Score (SiMS) Enables Quantifi Cation of Severity of Cardiometabolic Affliction in Individuals Not Presenting with Metabolic Syndrome. Bratisl. Med. J. 2018, 119, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jäger, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN Exercise & Sports Nutrition Review Update: Research & Recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar] [PubMed]
- National Academies of Sciences Engineering and Medicine; Health and Medicine Division; Food and Nutrition Board; Committee to Review the Dietary Reference Intakes for Sodium and Potassium. Dietary Reference Intakes for Sodium and Potassium; Stallings, V.A., Harrison, M., Oria, M., Eds.; National Academies Press: Washington, DC, USA, 2019; ISBN 978-0-309-48834-1. [Google Scholar]
- Phillips, J.A. Dietary Guidelines for Americans, 2020–2025. Workplace Health Saf. 2021, 69, 395. [Google Scholar] [CrossRef]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory from the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef]
- Imboden, M.T.; Welch, W.A.; Swartz, A.M.; Montoye, A.H.K.; Finch, H.W.; Harber, M.P.; Kaminsky, L.A. Reference Standards for Body Fat Measures Using GE Dual Energy X-Ray Absorptiometry in Caucasian Adults. PLoS ONE 2017, 12, e0175110. [Google Scholar] [CrossRef]
- Ferrari, L.; Panaite, S.A.; Bertazzo, A.; Visioli, F. Animal- and Plant-Based Protein Sources: A Scoping Review of Human Health Outcomes and Environmental Impact. Nutrients 2022, 14, 5115. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.; Johnston, C.; Wharton, C. Plant-Based Diets: Considerations for Environmental Impact, Protein Quality, and Exercise Performance. Nutrients 2018, 10, 1841. [Google Scholar] [CrossRef] [PubMed]
- Heller, S. Micronutrient Needs of Athletes Eating Plant-Based Diets. Nutr. Today 2019, 54, 23–30. [Google Scholar] [CrossRef]
- Rogerson, D. Vegan Diets: Practical Advice for Athletes and Exercisers. J. Int. Soc. Sports Nutr. 2017, 14, 36. [Google Scholar] [CrossRef]
- Clifton, P.M.; Keogh, J.B. A Systematic Review of the Effect of Dietary Saturated and Polyunsaturated Fat on Heart Disease. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 1060–1080. [Google Scholar] [CrossRef]
- Melin, A.K.; Areta, J.L.; Heikura, I.A.; Stellingwerff, T.; Torstveit, M.K.; Hackney, A.C. Direct and Indirect Impact of Low Energy Availability on Sports Performance. Scand. J. Med. Sci. Sports 2023, 34, e14327. [Google Scholar] [CrossRef] [PubMed]
- Fuller, S.; Beck, E.; Salman, H.; Tapsell, L. New Horizons for the Study of Dietary Fiber and Health: A Review. Plant Foods Hum. Nutr. 2016, 71, 1–12. [Google Scholar] [PubMed]
- Mountjoy, M.; Ackerman, K.E.; Bailey, D.M.; Burke, L.M.; Constantini, N.; Hackney, A.C.; Heikura, I.A.; Melin, A.; Pensgaard, A.M.; Stellingwerff, T.; et al. 2023 International Olympic Committee’s (IOC) Consensus Statement on Relative Energy Deficiency in Sport (REDs). Br. J. Sports Med. 2023, 57, 1073–1097. [Google Scholar] [CrossRef] [PubMed]
- Stenqvist, T.B.; Torstveit, M.K.; Faber, J.; Melin, A.K. Impact of a 4-Week Intensified Endurance Training Intervention on Markers of Relative Energy Deficiency in Sport (RED-S) and Performance Among Well-Trained Male Cyclists. Front. Endocrinol. 2020, 11, 512365. [Google Scholar] [CrossRef] [PubMed]
- Volpe, S.L. Magnesium and the Athlete. Curr. Sports Med. Rep. 2015, 14, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Lindinger, M.I.; Cairns, S.P. Regulation of Muscle Potassium: Exercise Performance, Fatigue and Health Implications. Eur. J. Appl. Physiol. 2021, 121, 721–748. [Google Scholar]
- Areta, J.L.; Burke, L.M.; Camera, D.M.; West, D.W.D.; Crawshay, S.; Moore, D.R.; Stellingwerff, T.; Phillips, S.M.; Hawley, J.A.; Coffey, V.G.; et al. Reduced Resting Skeletal Muscle Protein Synthesis Is Rescued by Resistance Exercise and Protein Ingestion Following Short-Term Energy Deficit. Am. J. Physiol. Endocrinol. Metab. 2014, 306, 989–997. [Google Scholar] [CrossRef]
- Oxfeldt, M.; Phillips, S.M.; Andersen, O.E.; Johansen, F.T.; Bangshaab, M.; Risikesan, J.; McKendry, J.; Melin, A.K.; Hansen, M. Low Energy Availability Reduces Myofibrillar and Sarcoplasmic Muscle Protein Synthesis in Trained Females. J. Physiol. 2023, 601, 3481–3497. [Google Scholar] [CrossRef]
- Chaston, T.B.; Dixon, J.B.; O’brien, P.E. Changes in Fat-Free Mass during Significant Weight Loss: A Systematic Review. Int. J. Obes. 2007, 31, 743. [Google Scholar]
- Stone, K.A.; Barry, A.M.; Kotarsky, C.J.; Dicks, N.D.; Stastny, S.N.; Byun, W.; Mitchell, S.; McGrath, R.; Hackney, K.J. Moderate to Vigorous Physical Activity, Leucine, and Protein Intake Contributions to Muscle Health in Middle Age. J. Frailty Sarcopenia Falls 2022, 07, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Dicks, N.D.; Kotarsky, C.J.; Trautman, K.A.; Barry, A.M.; Keith, J.F.; Mitchell, S.; Byun, W.; Stastny, S.N.; Hackney, K.J. Contribution of Protein Intake and Concurrent Exercise to Skeletal Muscle Quality with Aging. J. Frailty Aging 2020, 9, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.R.; Kotarsky, C.J.; Mahoney, S.J.; Sawyer, B.C.; Stone, K.A.; Byun, W.; Hackney, K.J.; Mitchell, S.; Stastny, S.N. Evenness of Dietary Protein Intake Is Positively Associated with Lean Mass and Strength in Healthy Women. Nutr. Metab. Insights 2022, 15, 11786388221101829. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.R.; Kotarsky, C.J.; Hackney, K.J.; Trautman, K.A.; Dicks, N.D.; Byun, W.; Keith, J.F.; David, S.L.; Stastny, S.N. Measures Derived from Panoramic Ultrasonography and Animal-Based Protein Intake Are Related to Muscular Performance in Middle-Aged Adults. J. Clin. Med. 2021, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.L.; Reguant-Closa, A.; Nemecek, T. Sustainable Diets for Athletes. Curr. Nutr. Rep. 2020, 9, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, S.I.; Baranowski, T.; Subar, A.F.; Tooze, J.A.; Frongillo, E.A. Best Practices for Conducting and Interpreting Studies to Validate Self-Report Dietary Assessment Methods. J. Acad. Nutr. Diet. 2019, 119, 1801–1816. [Google Scholar] [CrossRef] [PubMed]
- Subar, A.F.; Freedman, L.S.; Tooze, J.A.; Kirkpatrick, S.I.; Boushey, C.; Neuhouser, M.L.; Thompson, F.E.; Potischman, N.; Guenther, P.M.; Tarasuk, V.; et al. Addressing Current Criticism Regarding the Value of Self-Report Dietary Data. J. Nutr. 2015, 145, 2639–2645. [Google Scholar] [CrossRef]
- Freedman, L.S.; Commins, J.M.; Moler, J.E.; Willett, W.; Tinker, L.F.; Subar, A.F.; Spiegelman, D.; Rhodes, D.; Potischman, N.; Neuhouser, M.L.; et al. Pooled Results from 5 Validation Studies of Dietary Self-Report Instruments Using Recovery Biomarkers for Potassium and Sodium Intake. Am. J. Epidemiol. 2015, 181, 473–487. [Google Scholar] [CrossRef]
- Freedman, L.S.; Commins, J.M.; Moler, J.E.; Arab, L.; Baer, D.J.; Kipnis, V.; Midthune, D.; Moshfegh, A.J.; Neuhouser, M.L.; Prentice, R.L.; et al. Pooled Results from 5 Validation Studies of Dietary Self-Report Instruments Using Recovery Biomarkers for Energy and Protein Intake. Am. J. Epidemiol. 2014, 180, 172–188. [Google Scholar] [CrossRef]
- Kirkpatrick, S.I.; Subar, A.F.; Douglass, D.; Zimmerman, T.P.; Thompson, F.E.; Kahle, L.L.; George, S.M.; Dodd, K.W.; Potischman, N. Performance of the Automated Self-Administered 24-Hour Recall Relative to a Measure of True Intakes and to an Interviewer-Administered 24-h Recall. Am. J. Clin. Nutr. 2014, 100, 233–240. [Google Scholar] [CrossRef]


| Variables | Total (n = 25) | Off-Season (n = 13) | In-Season (n = 12) | p Value | ES d |
|---|---|---|---|---|---|
| Age (years) | 19.6 ± 1.3 | 19.7 ± 1.4 | 19.4 ± 1.2 | 0.605 | 0.210 |
| Height (cm) | 167.5 ± 6.9 | 169.3 ± 5.5 | 165.5 ± 7.9 | 0.165 | 0.575 |
| Body mass (kg) | 65.9 ± 7.0 | 66.4 ± 8.4 | 65.3 ± 5.3 | 0.714 | 0.149 |
| BMI (kg/m2) | 23.5 ± 2.0 | 23.1 ± 2.1 | 23.9 ± 1.8 | 0.309 | −0.416 |
| Hip C (cm) | 95.8 ± 9.1 | 94.5 ± 12.0 | 97.3 ± 4.2 | 0.427 a | −0.322 |
| Waist C (cm) | 76.8 ± 6.6 | 78.3 ± 8.5 | 75.1 ± 3.4 | 0.222 a | 0.501 |
| Waist-to-hip ratio | 0.8 ± 0.2 | 0.9 ± 0.2 | 0.8 ± 0.0 | 0.218 a | 0.509 |
| SBP (mmHg) | 112.8 ± 9.6 | 112.8 ± 8.9 | 112.9 ± 10.8 | 0.970 | −0.015 |
| DBP (mmHg) | 69.1 ± 6.8 | 67.3 ± 6.2 | 71.0 ± 7.2 | 0.182 | −0.551 |
| Variables | Total (n = 25) | Off-Season (n = 13) | In-Season (n = 12) | p Value | ES d | |
|---|---|---|---|---|---|---|
| Energy (kcal) | A (/d) | 2280.1 ± 568.3 | 2249.9 ± 597.8 | 2312.8 ± 559.0 | 0.789 | −0.109 |
| R (/kg/d) | 35.0 ± 9.4 | 34.6 ± 11.1 | 35.4 ± 7.7 | 0.838 | −0.083 | |
| Carbs (g) | A (/d) | 267.1 ± 84.1 | 271.3 ± 89.9 | 262.5 ± 81.1 | 0.799 | 0.103 |
| R (/kg/d) | 4.1 ± 1.4 | 4.2 ± 1.6 | 4.0 ± 1.1 | 0.778 | 0.114 | |
| Dietary fiber (g) | A (/d) | 23.9 ± 8.9 | 23.5 ± 6.6 | 24.3 ± 11.2 | 0.842 | −0.081 |
| R (/kg/d) | - | - | - | - | - | |
| Lipids (g) | A (/d) | 91.9 ± 27.1 | 85.7 ± 28.0 | 98.6 ± 25.6 | 0.242 | −0.481 |
| R (/kg/d) | 1.4 ± 0.4 | 1.3 ± 0.5 | 1.5 ± 0.4 | 0.284 | −0.439 | |
| Saturated fat (g) | A (/d) | 28.7 ± 10.4 | 25.9 ± 10.0 | 31.7 ± 10.4 | 0.174 | −0.561 |
| R (/kg/d) | - | - | - | - | - | |
| Proteins (g) | A (/d) | 104.5 ± 30.3 | 107.7 ± 28.2 | 101.0 ± 33.3 | 0.590 | 0.219 |
| R (/kg/d) | 1.6 ± 0.5 | 1.7 ± 0.5 | 1.6 ± 0.5 | 0.577 | 0.226 | |
| ABP (g) | A (/d) | 71.7 ± 28.2 | 75.9 ± 28.2 | 67.3 ± 28.7 | 0.456 | 0.304 |
| R (/kg/d) | 1.5 ± 0.9 | 1.2 ± 0.4 | 1.9 ± 1.2 | 0.097 a | −0.726 | |
| PBP (g) | A (/d) | 30.0 ± 12.2 | 29.2 ± 12.2 | 30.9 ± 12.6 | 0.741 | −0.134 |
| R (/kg/d) | 0.6 ± 0.3 | 0.4 ± 0.2 | 0.8 ± 0.3 | 0.004 ^ | −1.267 | |
| ABP:PBP | - | 2.8 ± 1.6 | 3.1 ± 1.7 | 2.5 ± 1.5 | 0.379 | 0.359 |
| ABP QR | - | 1.9 ± 1.8 | 2.4 ± 2.0 | 1.4 ± 1.4 | 0.194 | 0.414 |
| ABP HQ (%) | - | 55.5 ± 19.9 | 61.7 ± 17.0 | 48.8 ± 21.4 | 0.107 | −0.671 |
| Variables | DRI | Total (n = 25) | Off-Season (n = 13) | In-Season (n = 12) | p Value | ES d |
|---|---|---|---|---|---|---|
| Calcium (mg) | 1000 # | 905.1 ± 365.2 | 873.6 ± 357.0 | 939.2 ± 386.7 | 0.663 | −0.177 |
| Iron (mg) | 18 # | 17.0 ± 6.6 | 16.9 ± 7.5 | 17.0 ± 5.7 | 0.989 | −0.006 |
| Magnesium (mg) | 310 # | 280.1 ± 73.7 | 280.0 ± 66.4 | 280.2 ± 83.8 | 0.996 | −0.002 |
| Potassium (mg) | 2600 * | 2551.9 ± 790.1 | 2527.9 ± 720.2 | 2577.8 ± 891.5 | 0.879 | −0.062 |
| Sodium (mg) | 1500 * | 3622.7 ± 1352.1 | 3522.4 ± 1595.4 | 3731.2 ± 1089.6 | 0.708 | −0.152 |
| Vitamin A (mcg) | 700 # | 946.3 ± 643.6 | 847.4 ± 451.7 | 1053.4 ± 810.6 | 0.436 | −0.317 |
| Vitamin B12 (mcg) | 2.4 # | 3.7 ± 2.6 | 3.1 ± 2.5 | 4.4 ± 2.6 | 0.226 | −0.499 |
| Vitamin C (mg) | 75 # | 100.0 ± 61.5 | 90.3 ± 48.3 | 110.6 ± 74.0 | 0.430 a | −0.326 |
| Vitamin D (mcg) | 15 # | 5.0 ± 10.6 | 2.7 ± 3.3 | 7.5 ± 14.8 | 0.270 | −0.452 |
| Vitamin E (mg) | 15 # | 5.1 ± 3.2 | 3.2 ± 1.6 | 7.2 ± 3.3 | <0.001 ^ | −1.571 |
| Vitamin K (mcg) | 90 * | 207.3 ± 170.1 | 202.4 ± 149.8 | 212.5 ± 196.5 | 0.886 | −0.058 |
| Variables | Total (n = 25) | Off-Season (n = 13) | In-Season (n = 12) | p Value | ES d |
|---|---|---|---|---|---|
| Tissue mass (kg) | 63.3 ± 6.8 | 63.7 ± 8.1 | 62.8 ± 5.3 | 0.735 | 0.137 |
| Fat mass (kg) | 19.5 ± 10.4 | 21.0 ± 14.4 | 17.8 ± 2.0 | 0.434 a | 0.318 |
| Lean mass (kg) | 45.7 ± 4.5 | 46.3 ± 4.3 | 45.0 ± 4.8 | 0.499 | 0.275 |
| Visceral fat mass (kg) | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.860 | −0.072 |
| BMD (g∙cm2) | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 0.847 | 0.078 |
| Body fat tissue (%) | 27.6 ± 4.1 | 26.9 ± 5.0 | 28.4 ± 3.0 | 0.378 | −0.360 |
| Variables | Total (n = 24) | Off-Season (n = 13) | In-Season (n = 11) | p Value | ES d |
|---|---|---|---|---|---|
| Glucose (mg/dL) | 85.3 ± 6.4 | 86.7 ± 6.4 | 83.6 ± 6.2 | 0.249 | 0.485 |
| Triglycerides (mg/dL) | 78.0 ± 33.1 | 59.2 ± 15.3 | 100.1 ± 35.2 | 0.003 ^,a | −1.505 |
| T cholesterol (mg/dL) | 152.5 ± 28.9 | 147.8 ± 30.2 | 158.1 ± 27.7 | 0.399 | −0.352 |
| LDL (mg/dL) | 79.6 ± 21.2 | 77.5 ± 20.6 | 82.1 ± 22.6 | 0.605 | −0.215 |
| HDL (mg/dL) | 57.4 ± 13.1 | 58.6 ± 17.3 | 55.9 ± 5.9 a | 0.604 a | 0.210 |
| Non-HDL (mg/dL) | 95.2 ± 23.0 | 89.2 ± 20.7 | 102.2 ± 24.6 | 0.175 | −0.574 |
| Variables | Total (n = 24) | Off-Season (n = 13) | In-Season (n = 11) | p Value | ES d |
|---|---|---|---|---|---|
| siMS score | 1.99 ± 0.33 | 1.86 ± 0.29 | 2.14 ± 0.30 | 0.036 ^ | −0.917 |
| siMS risk score | 0.78 ± 0.14 | 0.73 ± 0.12 | 0.84 ± 0.13 | 0.050 | −0.850 |
| β ± SE | p-Value | R2 | Adjusted R2 | |
|---|---|---|---|---|
| siMS score | 0.200 | 0.164 | ||
| Constant | 2.339 ± 0.161 | <0.001 | ||
| PBP (g) | −0.012 ± 0.005 | 0.028 | ||
| siMS risk score | 0.257 | 0.223 | ||
| Constant | 0.947 ± 0.064 | <0.001 | ||
| PBP (g) | −0.006 ± 0.002 | 0.011 | ||
| Body fat percentage | 0.191 | 0.156 | ||
| Constant | 32.031 ± 2.053 | <0.001 | ||
| PBP (g) | −0.148 ± 0.064 | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotarsky, C.J.; Frenett, M.L.; Hoerle, W.F.; Kim, J.; Lockwood, J.; Cryer, L.; Ives, S.J. Plant-Based Dietary Protein Is Associated with Lower Metabolic Syndrome Risk in Division III Female Athletes: A Pilot Study. Nutrients 2024, 16, 3486. https://doi.org/10.3390/nu16203486
Kotarsky CJ, Frenett ML, Hoerle WF, Kim J, Lockwood J, Cryer L, Ives SJ. Plant-Based Dietary Protein Is Associated with Lower Metabolic Syndrome Risk in Division III Female Athletes: A Pilot Study. Nutrients. 2024; 16(20):3486. https://doi.org/10.3390/nu16203486
Chicago/Turabian StyleKotarsky, Christopher J., Marissa L. Frenett, William F. Hoerle, Jiseung Kim, Jillian Lockwood, Liala Cryer, and Stephen J. Ives. 2024. "Plant-Based Dietary Protein Is Associated with Lower Metabolic Syndrome Risk in Division III Female Athletes: A Pilot Study" Nutrients 16, no. 20: 3486. https://doi.org/10.3390/nu16203486
APA StyleKotarsky, C. J., Frenett, M. L., Hoerle, W. F., Kim, J., Lockwood, J., Cryer, L., & Ives, S. J. (2024). Plant-Based Dietary Protein Is Associated with Lower Metabolic Syndrome Risk in Division III Female Athletes: A Pilot Study. Nutrients, 16(20), 3486. https://doi.org/10.3390/nu16203486








