Association of Dietary Selenium Intake with Type 2 Diabetes in Middle-Aged and Older Adults in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Evaluation Indicators
2.2.1. Dietary Nutrients
2.2.2. Diagnostic Criteria for Diabetes
2.2.3. Evaluation of Confounding Factors
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Effect of Dietary Selenium Intake on the Risk of T2D
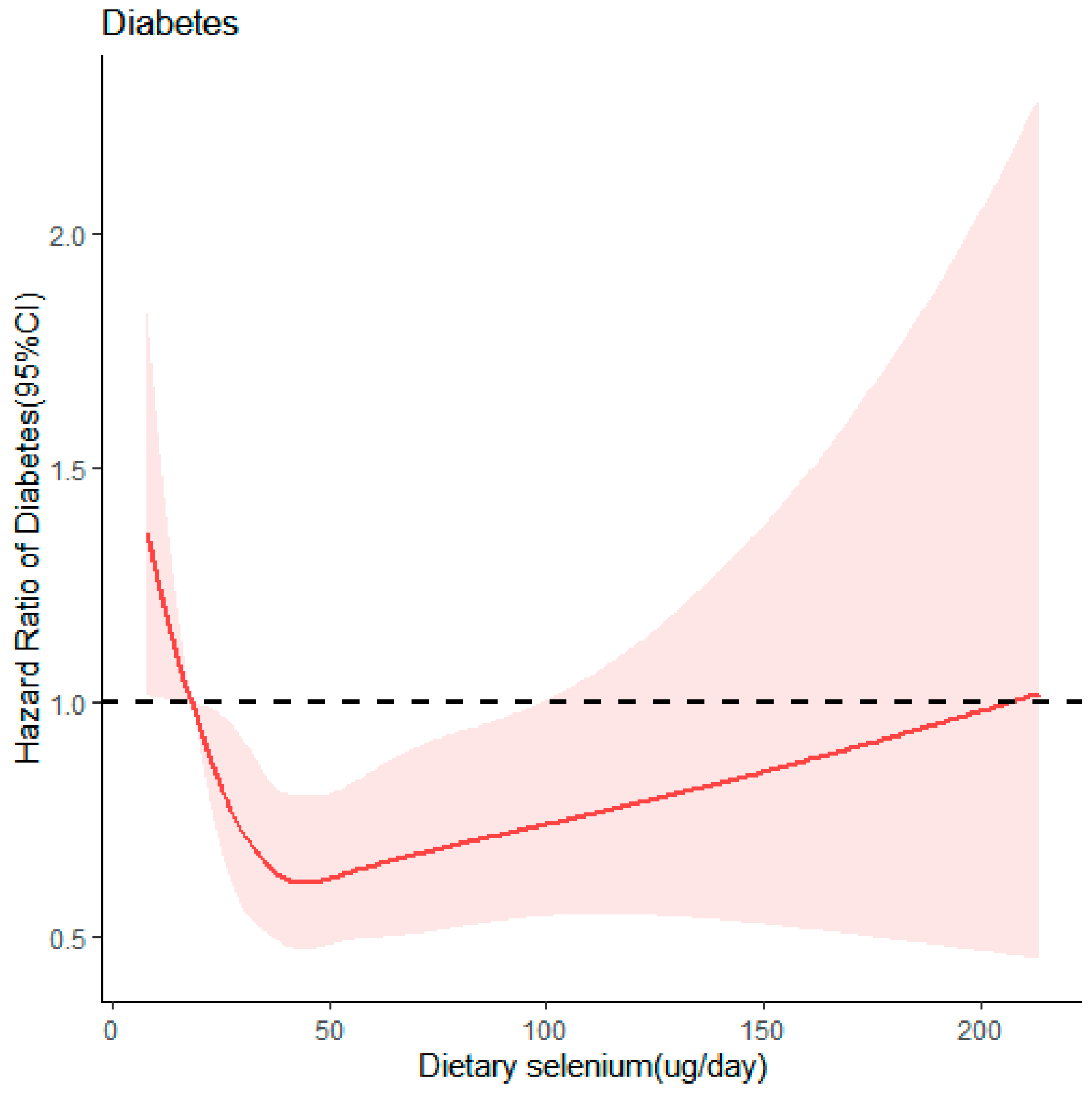
3.3. Dose–Response Relationship between Dietary Selenium Intake and T2D
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amirhossein, M.; Samaneh, A.; Maryam, T.; Sara, K.-A.; Fereidoun, A.; Farzad, H. Change in fasting plasma glucose and incident type 2 diabetes mellitus: Results from a prospective cohort study. BMJ Open 2016, 6, e010889. [Google Scholar] [CrossRef]
- Hong, S.; Pouya, S.; Suvi, K.; Moritz, P.; Katherine, O.; Bruce, B.D.; Caroline, S.; Abdul, B.; Juliana, C.N.C.; Jean Claude, M.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar]
- Limin, W.; Wen, P.; Zhenping, Z.; Mei, Z.; Zumin, S.; Ziwei, S.; Xiao, Z.; Chun, L.; Zhengjing, H.; Xiaomin, S.; et al. Prevalence and Treatment of Diabetes in China, 2013–2018. JAMA 2021, 326, 2498–2506. [Google Scholar] [CrossRef]
- Alan, S.; Pouya, S.; Abha, K.; Suvi, K.; Belma, M.; Rhys, W. Diabetes and global ageing among 65-99-year-old adults: Findings from the International Diabetes Federation Diabetes Atlas, 9(th) Edition. Diabetes Res. Clin. Pract. 2020, 162, 108078. [Google Scholar] [CrossRef]
- Juliana, C.N.C.; Vasanti, M.; Weiping, J.; Takashi, K.; Chittaranjan, S.Y.; Kun-Ho, Y.; Frank, B.H. Diabetes in Asia: Epidemiology, risk factors, and pathophysiology. JAMA 2009, 301, 2129–2140. [Google Scholar]
- Patrice, F. Protective effects of antioxidant micronutrients (vitamin E, zinc and selenium) in type 2 diabetes mellitus. Clin. Chem. Lab. Med. 2003, 41, 995–998. [Google Scholar] [CrossRef]
- Wenli, H.; Chong, Z.; Hongbo, H.; Shutao, Y. Food Sources of Selenium and Its Relationship with Chronic Diseases. Nutrients 2021, 13, 1739. [Google Scholar] [CrossRef] [PubMed]
- Margaret, P.R. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Antonio, C.; Enrico, M. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 816–823. [Google Scholar] [CrossRef]
- Robertson, R.P. Oxidative stress and impaired insulin secretion in type 2 diabetes. Curr. Opin. Pharmacol. 2006, 6, 615–619. [Google Scholar] [CrossRef]
- Kohler, L.N.; Foote, J.; Kelley, C.P.; Florea, A.; Shelly, C.; Chow, H.-H.S.; Hsu, P.; Batai, K.; Ellis, N.; Saboda, K.; et al. Selenium and Type 2 Diabetes: Systematic review. Nutrients 2018, 10, 1924. [Google Scholar] [CrossRef]
- Marco, V.; Tommaso, F.; Rothman, K.J. Selenium exposure and the risk of type 2 diabetes: A systematic review and meta-analysis. Eur. J. Epidemiol. 2018, 33, 789–810. [Google Scholar] [CrossRef]
- Marco, V.; Tommaso, F.; Lauren, A.W.; Kenneth, J.R. A systematic review and dose-response meta-analysis of exposure to environmental selenium and the risk of type 2 diabetes in nonexperimental studies. Environ. Res. 2021, 197, 111210. [Google Scholar] [CrossRef]
- Saverio, S.; Sabina, S.; Marco, V.; Sara, G.; Eliseo, G.; Martin, L.; Paola, M.; Franco, B.; Vittorio, K. A prospective study of dietary selenium intake and risk of type 2 diabetes. BMC Public Health 2010, 10, 564. [Google Scholar] [CrossRef]
- Song, M.; Aihua, Z.; Songming, H. Selenium supplementation and the risk of type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Endocrine 2014, 47, 758–763. [Google Scholar] [CrossRef]
- Mahdi, V.; Shirin, H.; Zeinab, G.; Mohammad, B. Selenium supplementation effect on glycemic control: A GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Pharmacol. Res. 2023, 195, 106888. [Google Scholar] [CrossRef]
- Wei, J.; Zeng, C.; Gong, Q.Y.; Yang, H.B.; Li, X.X.; Lei, G.H.; Yang, T.B. The association between dietary selenium intake and diabetes: A cross-sectional study among middle-aged and older adults. Nutr. J. 2015, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhai, F.Y.; Du, S.F.; Popkin, B.M. The China Health and Nutrition Survey, 1989–2011. Obes. Rev. 2013, 15, 2–7. [Google Scholar] [CrossRef]
- Popkin, B.M.; Shufa, D.; Fengying, Z.; Bing, Z. Cohort Profile: The China Health and Nutrition Survey-monitoring and understanding socio-economic and health change in China, 1989–2011. Int. J. Epidemiol. 2009, 39, 1435–1440. [Google Scholar] [CrossRef]
- Li, W.; Jiao, Y.; Wang, L.; Wang, S.; Hao, L.; Wang, Z.; Wang, H.; Zhang, B.; Ding, G.; Jiang, H. Association of Serum Magnesium with Insulin Resistance and Type 2 Diabetes among Adults in China. Nutrients 2022, 14, 1799. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, W.; Wang, L.; Jiang, H.; Wang, S.; Jia, X.; Wang, Z.; Wang, H.; Zhang, B.; Ding, G. Relationship between Dietary Magnesium Intake and Metabolic Syndrome. Nutrients 2022, 14, 2013. [Google Scholar] [CrossRef]
- Sallis, J.F.; Haskell, W.L.; Wood, P.D.; Fortmann, S.P.; Rogers, T.; Blair, S.N.; Paffenbarger, R.S., Jr. Physical activity assessment methodology in the Five-City Project. Am. J. Epidemiol. 1985, 121, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, H.; Wang, Z.; Zhang, J.; Zhang, B. Association between Toenail Magnesium and Type 2 Diabetes in Chinese Adults. Nutrients 2017, 9, 811. [Google Scholar] [CrossRef]
- Bushra, H.; Zumin, S. Association between selenium intake, diabetes and mortality in adults: Findings from National Health and Nutrition Examination Survey (NHANES) 2003–2014. Br. J. Nutr. 2021, 127, 1098–1105. [Google Scholar] [CrossRef]
- João Pedro Viana, D.; Paulo de Souza, C.S.; Adriano Marçal, P.; Helen Hermana Miranda, H.; Josefina, B.; Luciana Neri, N. Dietary Selenium Intake and Type-2 Diabetes: A Cross-Sectional Population-Based Study on CUME Project. Front. Nutr. 2021, 8, 678648. [Google Scholar] [CrossRef]
- Lv, Y.; Xie, L.; Dong, C.; Yang, R.; Long, T.; Yang, H.; Chen, L.; Zhang, L.; Chen, X.; Luo, X.; et al. Co-exposure of serum calcium, selenium and vanadium is nonlinearly associated with increased risk of type 2 diabetes mellitus in a Chinese population. Chemosphere 2020, 263, 128021. [Google Scholar] [CrossRef]
- Chinese Nutrition Society. Dietary Reference Intakes for China (2023); People’s Medical Publishing House: Beijing, China, 2023. [Google Scholar]
- Diana, C.-A.; Rodica Mihaela, F.; Luiza, C.; Florin, O. Selenium Analysis and Speciation in Dietary Supplements Based on Next-Generation Selenium Ingredients. Nutrients 2018, 10, 1466. [Google Scholar] [CrossRef]
- Harmon, J.; Bogdani, M.; Parazzoli, S.; Mak, S.; Oseid, E.; Berghmans, M.; Leboeuf, R.; Robertson, R. beta-Cell-specific overexpression of glutathione peroxidase preserves intranuclear MafA and reverses diabetes in db/db mice. Endocrinology 2009, 150, 4855–4862. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dai, C.; Guo, M.; Taylor, B.; Harmon, J.; Sander, M.; Robertson, R.; Powers, A.; Stein, R. Inactivation of specific β cell transcription factors in type 2 diabetes. J. Clin. Investig. 2013, 123, 3305–3316. [Google Scholar] [CrossRef]
- Wang, X.; Vatamaniuk, M.; Wang, S.; Roneker, C.; Simmons, R.; Lei, X. Molecular mechanisms for hyperinsulinaemia induced by overproduction of selenium-dependent glutathione peroxidase-1 in mice. Diabetologia 2008, 51, 1515–1524. [Google Scholar] [CrossRef]
- Hirofumi, M.; Toshinari, T.; Hiroaki, T.; Hiroto, H.; Naoto, M.-N.; Seiichiro, K.; Kazuhide, I.; Hitoshi, A.; Yumie, T.; Tsuguhito, O.; et al. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab. 2010, 12, 483–495. [Google Scholar] [CrossRef]
- Hirofumi, M.; Hiroaki, T.; Yoshiro, S.; Yuichiro, M.; Akihiro, K.; Kiyo-Aki, I.; Keita, C.; Takehiro, K.; Natsumi, T.; Fei, L.; et al. Deficiency of the hepatokine selenoprotein P increases responsiveness to exercise in mice through upregulation of reactive oxygen species and AMP-activated protein kinase in muscle. Nat. Med. 2017, 23, 508–516. [Google Scholar] [CrossRef]
- Lu, C.; Chang, H.; Yang, K.; Kuo, C.; Lee, L.; Huang, K. High serum selenium levels are associated with increased risk for diabetes mellitus independent of central obesity and insulin resistance. BMJ Open Diabetes Res. Care 2016, 4, e000253. [Google Scholar] [CrossRef]
- Stapleton, S. Selenium: An insulin-mimetic. Cell. Mol. Life Sci. CMLS 2000, 57, 1874–1879. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.; Pallauf, J. Compendium of the antidiabetic effects of supranutritional selenate doses. In vivo and in vitro investigations with type II diabetic db/db mice. J. Nutr. Biochem. 2006, 17, 548–560. [Google Scholar] [CrossRef]
- Campbell, S.; Aldibbiat, A.; Marriott, C.; Landy, C.; Ali, T.; Ferris, W.; Butler, C.; Shaw, J.; Macfarlane, W. Selenium stimulates pancreatic beta-cell gene expression and enhances islet function. FEBS Lett. 2008, 582, 2333–2337. [Google Scholar] [CrossRef]
- Frączek, A.; Pasternak, K. Selenium in medicine and treatment. J. Elemntol. 2012, 18, 145–163. [Google Scholar] [CrossRef]
- Richard, M. Effect of genotype on micronutrient absorption and metabolism: A review of iron, copper, iodine and selenium, and folates. Int. J. Vitam. Nutr. Res. 2008, 77, 205–216. [Google Scholar] [CrossRef]
- Ha, H.Y.; Naghum, A.; Berry, M.J.; Seale, L.A. From Selenium Absorption to Selenoprotein Degradation. Biol. Trace Elem. Res. 2019, 192, 26–37. [Google Scholar] [CrossRef]
- Patterson, B.H.; Combs, G.F., Jr.; Taylor, P.R.; Patterson, K.Y.; Moler, J.E.; Wastney, M.E. Selenium Kinetics in Humans Change Following 2 Years of Supplementation with Selenomethionine. Front. Endocrinol. 2021, 12, 624687. [Google Scholar] [CrossRef] [PubMed]

| Baseline Characteristics | Quintile of Dietary Selenium Intake (µg/day) | |||||
|---|---|---|---|---|---|---|
| Q1 22.05 (18.05~25.14) | Q2 32.27 (30.03~34.53) | Q3 41.04 (38.66~43.40) | Q4 51.57 (48.55~55.39) | Q5 74.38 (65.96~89.58) | p-Value | |
| Age, % | <0.0001 | |||||
| 50~64 | 64.49 | 73.2 | 77.81 | 77.22 | 80.82 | |
| 65~79 | 31.74 | 24.12 | 21.02 | 21.27 | 18.26 | |
| 80~ | 3.77 | 2.68 | 1.17 | 1.51 | 0.92 | |
| Male, % | 32.66 | 42.21 | 45.98 | 51.93 | 60.64 | <0.0001 |
| Household income per capital, % | <0.0001 | |||||
| Low (<6128.13 yuan) | 42.46 | 37.94 | 31.66 | 30.57 | 24.04 | |
| Median (6128.13–17,611.37 yuan) | 31.16 | 32.24 | 34.76 | 35.51 | 33.00 | |
| High (>17,611.37 yuan) | 26.38 | 29.82 | 33.58 | 33.92 | 42.96 | |
| Education, % | <0.0001 | |||||
| Primary and below | 63.4 | 53.85 | 44.56 | 42.63 | 35.85 | |
| Middle and high | 31.07 | 35.26 | 44.3 | 46.06 | 48.66 | |
| College and above | 5.53 | 10.89 | 11.14 | 11.31 | 15.49 | |
| Urban, % | 28.73 | 35.68 | 37.86 | 40.54 | 44.89 | <0.0001 |
| Never smoked, % | 77.72 | 70.77 | 71.02 | 67.84 | 62.56 | <0.0001 |
| Non-drinker in the past year, % | 81.07 | 72.11 | 69.68 | 66.5 | 61.81 | <0.0001 |
| Physical activity, % | 0.60 | |||||
| Low | 33.84 | 31.24 | 33.84 | 34.25 | 33.42 | |
| Median | 31.83 | 33.75 | 32.91 | 33.42 | 34.84 | |
| High | 34.34 | 35.01 | 33.25 | 32.33 | 31.74 | |
| BMI (kg/m2), % | <0.0001 | |||||
| <18.5 | 6.45 | 5.36 | 4.1 | 3.18 | 2.26 | |
| 18.5~23.9 | 51.51 | 52.76 | 49.25 | 47.57 | 45.23 | |
| ≥24.0 | 42.04 | 41.88 | 46.65 | 49.25 | 52.51 | |
| Energy (kcal/day) | 1564.65 ± 492.48 | 1931.00 ± 589.56 | 2134.19 ± 602.86 | 2341.46 ± 654.01 | 2624.06 ± 764.53 | <0.0001 |
| Model | Quintile of Dietary Selenium Intake (µg/day) | p Trend | ||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Model 1 | 1.00 | 0.87 (0.69, 1.10) | 0.68 (0.53, 0.87) * | 0.79 (0.63, 1.01) | 0.84 (0.66, 1.08) | 0.39 |
| Model 2 | 1.00 | 0.87 (0.69, 1.10) | 0.68 (0.53, 0.87) * | 0.80 (0.63, 1.01) | 0.86 (0.67, 1.09) | 0.20 |
| Model 3 | 1.00 | 0.91 (0.71, 1.16) | 0.74 (0.57, 0.95) * | 0.84 (0.65, 1.08) | 0.90 (0.69, 1.17) | 0.43 |
| Model 4 | 1.00 | 0.91 (0.72, 1.16) | 0.73 (0.56, 0.94) * | 0.79 (0.61, 1.03) | 0.85 (0.65, 1.11) | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Hong, X.; Wang, H.; Li, W.; Chen, L.; Wang, L.; Zhao, B.; Wang, S.; Jiang, H.; Wang, Z. Association of Dietary Selenium Intake with Type 2 Diabetes in Middle-Aged and Older Adults in China. Nutrients 2024, 16, 2367. https://doi.org/10.3390/nu16142367
Li F, Hong X, Wang H, Li W, Chen L, Wang L, Zhao B, Wang S, Jiang H, Wang Z. Association of Dietary Selenium Intake with Type 2 Diabetes in Middle-Aged and Older Adults in China. Nutrients. 2024; 16(14):2367. https://doi.org/10.3390/nu16142367
Chicago/Turabian StyleLi, Fangyuan, Xi Hong, Huijun Wang, Weiyi Li, Lili Chen, Liusen Wang, Boya Zhao, Shaoshunzi Wang, Hongru Jiang, and Zhihong Wang. 2024. "Association of Dietary Selenium Intake with Type 2 Diabetes in Middle-Aged and Older Adults in China" Nutrients 16, no. 14: 2367. https://doi.org/10.3390/nu16142367
APA StyleLi, F., Hong, X., Wang, H., Li, W., Chen, L., Wang, L., Zhao, B., Wang, S., Jiang, H., & Wang, Z. (2024). Association of Dietary Selenium Intake with Type 2 Diabetes in Middle-Aged and Older Adults in China. Nutrients, 16(14), 2367. https://doi.org/10.3390/nu16142367







