Synergistic Effect of β-Cryptoxanthin and Epigallocatechin Gallate on Obesity Reduction
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Generation of Chronically Obese Mice and Body Weight Measurement
2.3. Measurement of Green Tea Catechins
2.4. Administration of Green Tea Catechins and β-Cryptoxanthin
2.5. Blood Biochemistry Analysis
2.6. Histological Analysis
2.7. Statistical Analyses
3. Results
3.1. Measurement of Catechin Concentration
3.2. Weight Change Associated with Intake of Each Catechin and β-Cryptoxanthin
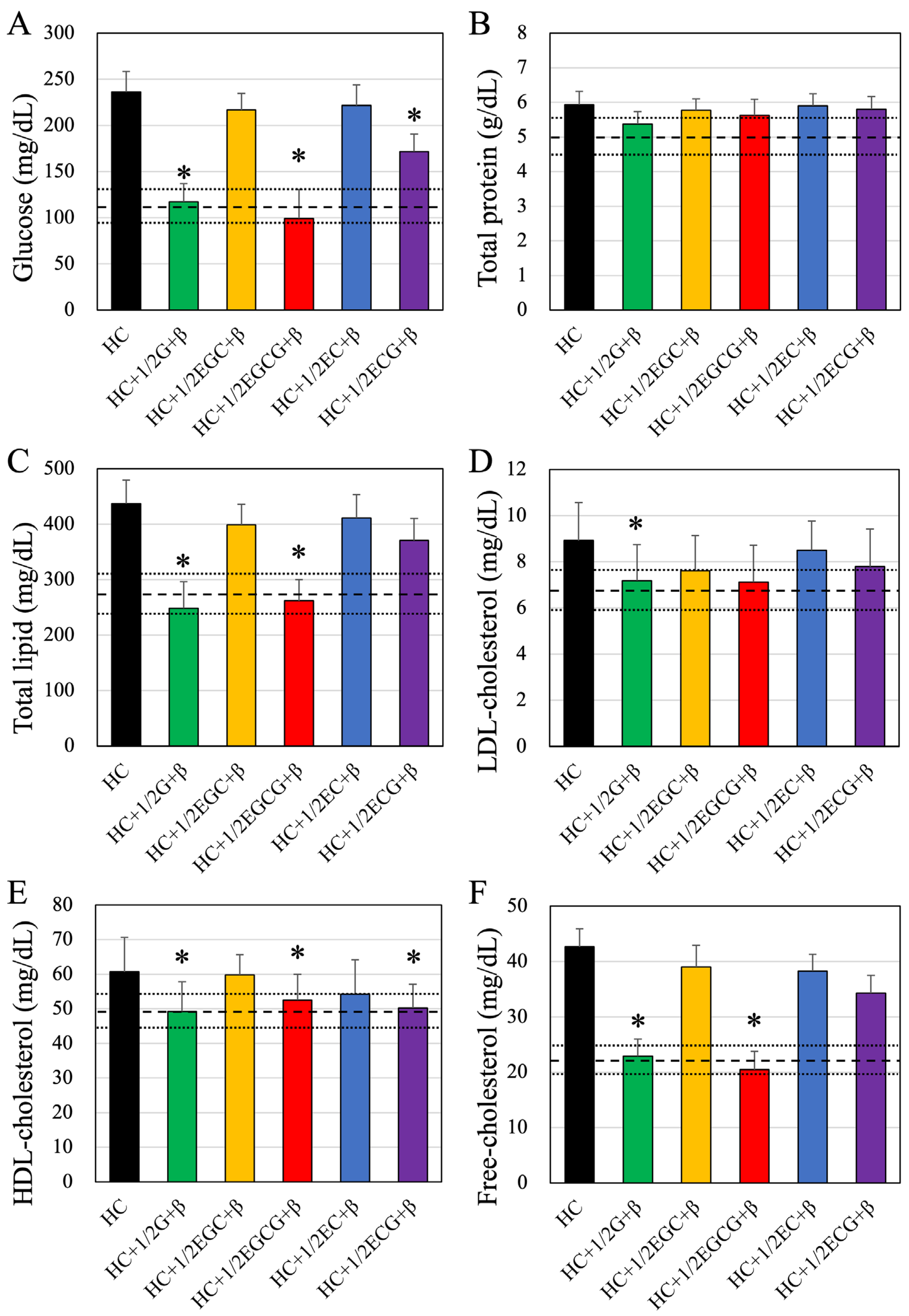
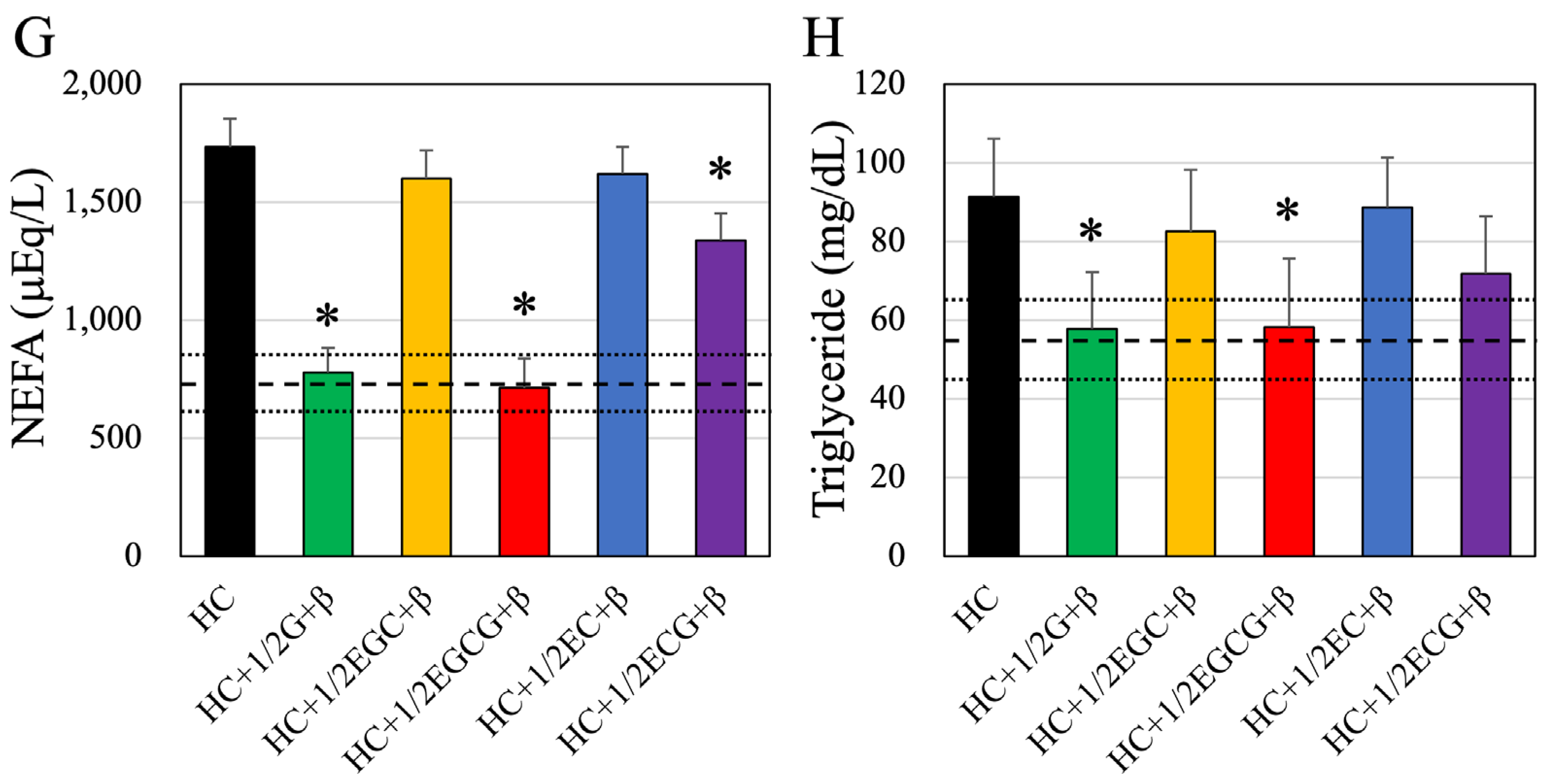
3.3. Blood Biochemistry Tests
3.4. Liver Function Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Zhang, M.; Yu, J.; Pei, Z.; Sun, C.; He, J.; Qian, T.; Luo, F.; Zhang, S.; Xu, Z. Nationwide Trends of Pediatric Obesity and BMI z-Score From 2017-2021 in China: Comparable Findings From Real-World Mobile- and Hospital-Based Data. Front. Endocrinol. 2022, 13, 859245. [Google Scholar] [CrossRef] [PubMed]
- Rozen, G.; Elbaz-Greener, G.; Margolis, G.; Marai, I.; Heist, E.K.; Ruskin, J.N.; Carasso, S.; Roguin, A.; Birati, E.Y.; Amir, O. The Obesity Paradox in Real-World Nation-Wide Cohort of Patients Admitted for a Stroke in the U.S. J. Clin. Med. 2022, 11, 1678. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Lobstein, T. Exploring an algorithm to harmonize International Obesity Task Force and World Health Organization child overweight and obesity prevalence rates. Pediatr. Obes. 2022, 17, e12905. [Google Scholar] [CrossRef] [PubMed]
- Celind, J.; Bygdell, M.; Martikainen, J.; Ohlsson, C.; Kindblom, J.M. Childhood overweight and risk of obesity-related adult cancer in men. Cancer Commun. 2022, 42, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Turner, L.; Gauthier, M.F.; Lafortune, A.; Tchernof, A.; Santosa, S. Adipocyte size, adipose tissue fibrosis, macrophage infiltration and disease risk are different in younger and older individuals with childhood versus adulthood onset obesity. Int. J. Obes. 2022, 46, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Pineros-Leano, M.; Grafft, N.; Aguayo, L. Childhood obesity risk factors by race and ethnicity. Obesity 2022, 30, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Igusa, T.; Wang, G.; Buckley, J.P.; Hong, X.; Bind, E.; Steffens, A.; Mukherjee, J.; Haltmeier, D.; Ji, Y.; et al. In-utero co-exposure to toxic metals and micronutrients on childhood risk of overweight or obesity: New insight on micronutrients counteracting toxic metals. Int. J. Obes. 2022, 46, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Cassader, M.; Gambino, R. PNPLA3 rs738409 and TM6SF2 rs58542926 gene variants affect renal disease and function in nonalcoholic fatty liver disease. Hepatology 2015, 62, 658–659. [Google Scholar] [CrossRef]
- Li, F.; Yang, Y.; Yang, L.; Wang, K.; Zhang, X.; Zong, Y.; Ding, Y.; Wang, C.; Zhang, L.; Ji, G. Resveratrol alleviates FFA and CCl4 induced apoptosis in HepG2 cells via restoring endoplasmic reticulum stress. Oncotarget 2017, 8, 43799–43809. [Google Scholar] [CrossRef]
- Cao, X.; Thyfault, J.P. Exercise drives metabolic integration between muscle, adipose and liver metabolism and protects against aging-related diseases. Exp. Gerontol. 2023, 176, 112178. [Google Scholar] [CrossRef]
- Nier, A.; Huber, Y.; Labenz, C.; Michel, M.; Bergheim, I.; Schattenberg, J.M. Adipokines and Endotoxemia Correlate with Hepatic Steatosis in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2020, 12, 699. [Google Scholar] [CrossRef] [PubMed]
- Campos, R.M.; de Piano, A.; da Silva, P.L.; Carnier, J.; Sanches, P.L.; Corgosinho, F.C.; Masquio, D.C.; Lazaretti-Castro, M.; Oyama, L.M.; Nascimento, C.M.; et al. The role of pro/anti-inflammatory adipokines on bone metabolism in NAFLD obese adolescents: Effects of long-term interdisciplinary therapy. Endocrine 2012, 42, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Torrezan, R.; Malta, A.; de Souza Rodrigues, W.D.N.; Dos Santos, A.A.A.; Miranda, R.A.; Moura, E.G.; Lisboa, P.C.; de Freitas Mathias, P.C. Monosodium l-glutamate-obesity onset is associated with disruption of central control of the hypothalamic-pituitary-adrenal axis and autonomic nervous system. J. Neuroendocrinol. 2019, 31, e12717. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Bautista, R.J.; Mahmoud, A.M.; Konigsberg, M.; Lopez Diaz Guerrero, N.E. Obesity: Pathophysiology, monosodium glutamate-induced model and anti-obesity medicinal plants. Biomed. Pharmacother. 2019, 111, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Bousova, I.; Kostakova, S.; Matouskova, P.; Bartikova, H.; Szotakova, B.; Skalova, L. Monosodium glutamate-induced obesity changed the expression and activity of glutathione S-transferases in mouse heart and kidney. Pharmazie 2017, 72, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Nakadate, K.; Kawakami, K.; Yamazaki, N. Anti-Obesity and Anti-Inflammatory Synergistic Effects of Green Tea Catechins and Citrus β-Cryptoxanthin Ingestion in Obese Mice. Int. J. Mol. Sci. 2023, 24, 7054. [Google Scholar] [CrossRef]
- Nakadate, K.; Hirakawa, T.; Tanaka-Nakadate, S. Small intestine barrier function failure induces systemic inflammation in monosodium glutamate-induced chronically obese mice. Appl. Physiol. Nutr. Metab. 2019, 44, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Nakadate, K.; Motojima, K.; Hirakawa, T.; Tanaka-Nakadate, S. Progressive Depletion of Rough Endoplasmic Reticulum in Epithelial Cells of the Small Intestine in Monosodium Glutamate Mice Model of Obesity. Biomed. Res. Int. 2016, 2016, 5251738. [Google Scholar] [CrossRef]
- Nakadate, K.; Kawakami, K.; Yamazaki, N. Combined Ingestion of Tea Catechin and Citrus beta-Cryptoxanthin Improves Liver Function via Adipokines in Chronic Obesity. Nutrients 2023, 15, 3345. [Google Scholar] [CrossRef]
- Sun, X.; Dey, P.; Bruno, R.S.; Zhu, J. EGCG and catechin relative to green tea extract differentially modulate the gut microbial metabolome and liver metabolome to prevent obesity in mice fed a high-fat diet. J. Nutr. Biochem. 2022, 109, 109094. [Google Scholar] [CrossRef]
- Nirengi, S.; Amagasa, S.; Homma, T.; Yoneshiro, T.; Matsumiya, S.; Kurosawa, Y.; Sakane, N.; Ebi, K.; Saito, M.; Hamaoka, T. Daily ingestion of catechin-rich beverage increases brown adipose tissue density and decreases extramyocellular lipids in healthy young women. Springerplus 2016, 5, 1363. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Osaki, N.; Shimotoyodome, A. Green tea catechins enhance norepinephrine-induced lipolysis via a protein kinase A-dependent pathway in adipocytes. Biochem. Biophys. Res. Commun. 2015, 461, 1–7. [Google Scholar] [CrossRef]
- Nagao, T.; Meguro, S.; Hase, T.; Otsuka, K.; Komikado, M.; Tokimitsu, I.; Yamamoto, T.; Yamamoto, K. A catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity 2009, 17, 310–317. [Google Scholar] [CrossRef]
- Maki, K.C.; Reeves, M.S.; Farmer, M.; Yasunaga, K.; Matsuo, N.; Katsuragi, Y.; Komikado, M.; Tokimitsu, I.; Wilder, D.; Jones, F.; et al. Green tea catechin consumption enhances exercise-induced abdominal fat loss in overweight and obese adults. J. Nutr. 2009, 139, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Abiri, B.; Amini, S.; Hejazi, M.; Hosseinpanah, F.; Zarghi, A.; Abbaspour, F.; Valizadeh, M. Tea’s anti-obesity properties, cardiometabolic health-promoting potentials, bioactive compounds, and adverse effects: A review focusing on white and green teas. Food Sci. Nutr. 2023, 11, 5818–5836. [Google Scholar] [CrossRef]
- Jimenez-Zamora, A.; Delgado-Andrade, C.; Rufian-Henares, J.A. Antioxidant capacity, total phenols and color profile during the storage of selected plants used for infusion. Food Chem. 2016, 199, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, I.; Tsuda, K.; Suzuki, Y.; Kobayashi, M.; Unno, T.; Tomoyori, H.; Goto, H.; Kawata, Y.; Imaizumi, K.; Nozawa, A.; et al. Tea catechins with a galloyl moiety suppress postprandial hypertriacylglycerolemia by delaying lymphatic transport of dietary fat in rats. J. Nutr. 2005, 135, 155–159. [Google Scholar] [CrossRef]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Nagasawa, A.; Suzuki, J.; Hase, T.; Tokimitsu, I. Beneficial effects of tea catechins on diet-induced obesity: Stimulation of lipid catabolism in the liver. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 1459–1464. [Google Scholar] [CrossRef]
- Suchacki, K.J.; Stimson, R.H. Nutritional Regulation of Human Brown Adipose Tissue. Nutrients 2021, 13, 1748. [Google Scholar] [CrossRef]
- Ikeda, I.; Kobayashi, M.; Hamada, T.; Tsuda, K.; Goto, H.; Imaizumi, K.; Nozawa, A.; Sugimoto, A.; Kakuda, T. Heat-epimerized tea catechins rich in gallocatechin gallate and catechin gallate are more effective to inhibit cholesterol absorption than tea catechins rich in epigallocatechin gallate and epicatechin gallate. J. Agric. Food Chem. 2003, 51, 7303–7307. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, I.; Imasato, Y.; Sasaki, E.; Nakayama, M.; Nagao, H.; Takeo, T.; Yayabe, F.; Sugano, M. Tea catechins decrease micellar solubility and intestinal absorption of cholesterol in rats. Biochim. Biophys. Acta 1992, 1127, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Kajimoto, O.; Kajimoto, Y.; Yabune, M.; Nakamura, T.; Kotani, K.; Suzuki, Y.; Nozawa, A.; Nagata, K.; Unno, T.; Sagesaka, Y.; et al. Tea Catechins with a Galloyl Moiety Reduce Body Weight and Fat. J. Health Sci. 2005, 51, 161–171. [Google Scholar] [CrossRef]
- Yoshitomi, R.; Yamamoto, M.; Kumazoe, M.; Fujimura, Y.; Yonekura, M.; Shimamoto, Y.; Nakasone, A.; Kondo, S.; Hattori, H.; Haseda, A.; et al. The combined effect of green tea and alpha-glucosyl hesperidin in preventing obesity: A randomized placebo-controlled clinical trial. Sci. Rep. 2021, 11, 19067. [Google Scholar] [CrossRef] [PubMed]
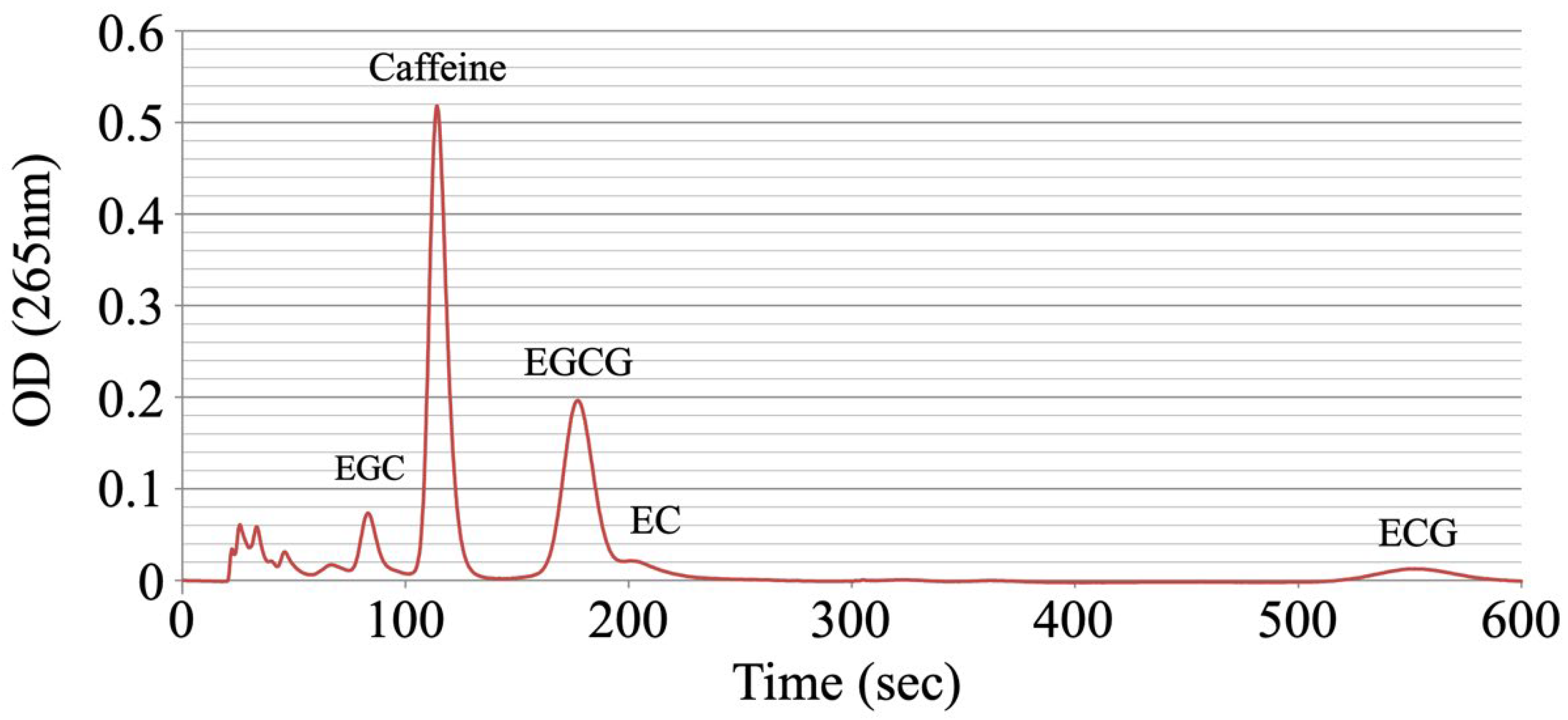

| EGC | Caffeine | EGCG | EC | ECG | |
|---|---|---|---|---|---|
| No.1 Average | 0.900 | 0.619 | 1.942 | 0.231 | 0.196 |
| No.2 Average | 1.050 | 0.583 | 1.833 | 0.192 | 0.174 |
| No.3 Average | 0.788 | 0.583 | 2.035 | 0.240 | 0.239 |
| No.4 Average | 0.775 | 0.631 | 1.987 | 0.183 | 0.250 |
| No.5 Average | 0.963 | 0.607 | 2.067 | 0.212 | 0.185 |
| Total Average | 0.895 | 0.605 | 1.973 | 0.212 | 0.209 |
| S.D. | 0.117 | 0.021 | 0.091 | 0.025 | 0.034 |
| 11 Weeks (g) | 15 Weeks (g) | |
|---|---|---|
| Control | 22.76 ± 1.69 * | 28.84 ± 1.94 * |
| HC | 31.26 ± 0.70 # | 39.86 ± 1.86 # |
| HC + Green Tea | 32.32 ± 1.35 # | 36.02 ± 1.91 # |
| HC + 1/2 Green Tea | 31.73 ± 1.60 # | 39.03 ± 1.33 # |
| HC + β-cryptoxanthin | 31.95 ± 1.36 # | 39.98 ± 1.97 # |
| HC + 1/2 Green Tea + β-cryptoxanthin | 31.66 ± 0.99 # | 30.64 ± 2.13 * |
| HC + 1/2 EGC + β-cryptoxanthin | 32.44 ± 0.84 # | 39.98 ± 2.10 # |
| HC + 1/2 EGCG + β-cryptoxanthin | 31.86 ± 0.84 # | 29.58 ± 1.76 * |
| HC + 1/2 EC + β-cryptoxanthin | 31.66 ± 0.99 # | 39.70 ± 2.03 # |
| HC + 1/2 ECG + β-cryptoxanthin | 31.86 ± 1.14 # | 37.74 ± 1.17 # |
| HC + 1/2 EGC | 32.19 ± 1.57 # | 40.01 ± 1.47 # |
| HC + 1/2 EGCG | 30.91 ± 1.23 # | 37.92 ± 2.01 # |
| HC + 1/2 EC | 32.14 ± 1.51 # | 39.25 ± 1.35 # |
| HC + 1/2 ECG | 31.35 ± 0.94 # | 38.46 ± 2.14 # |
| Weight (g) | |
|---|---|
| Control | 0.79 ± 0.31 * |
| HC | 3.14 ± 0.41 # |
| HC + Green Tea | 2.98 ± 0.40 # |
| HC + 1/2 Green Tea | 3.21 ± 0.35 # |
| HC + β-cryptoxanthin | 3.06 ± 0.42 # |
| HC + 1/2 Green Tea + β-cryptoxanthin | 0.74 ± 0.42 * |
| HC + 1/2EGC + β-cryptoxanthin | 2.88 ± 0.51 # |
| HC + 1/2 EGCG + β-cryptoxanthin | 0.72 ± 0.49 * |
| HC + 1/2 EC + β-cryptoxanthin | 2.79 ± 0.46 # |
| HC + 1/2 ECG + β-cryptoxanthin | 1.74 ± 0.52 #* |
| HC + 1/2EGC | 3.02 ± 0.43 # |
| HC + 1/2 EGCG | 3.01 ± 0.38 # |
| HC + 1/2 EC | 3.19 ± 0.36 # |
| HC + 1/2 ECG | 3.00 ± 0.44 # |
| Area (mm2) | |
|---|---|
| Control | 728.75 ± 433.67 * |
| HC | 1999.0 ± 1038.9 # |
| HC + Green Tea | 1798.4 ± 582.41 # |
| HC + 1/2 Green Tea | 1933.2 ± 701.39 # |
| HC + β-cryptoxanthin | 2013.2 ± 821.92 # |
| HC + 1/2 Green Tea + β-cryptoxanthin | 804.38 ± 448.30 * |
| HC + 1/2 EGC + β-cryptoxanthin | 1721.5 ± 835.20 # |
| HC + 1/2 EGCG + β-cryptoxanthin | 847.33 ± 398.32 * |
| HC + 1/2 EC + β-cryptoxanthin | 1902.3 ± 964.12 # |
| HC + 1/2 ECG + β-cryptoxanthin | 1418.4 ± 624.11 # |
| HC + 1/2 EGC | 1897.3 ± 705.28 # |
| HC + 1/2 EGCG | 2083.6 ± 783.88 # |
| HC + 1/2 EC | 2103.8 ± 647.97 # |
| HC + 1/2 ECG | 2001.1 ± 592.17 # |
| Glucose (mg/dL) | Total Protein (g/dL) | Total Lipid (mg/dL) | LDL-Cholesterol (mg/dL) | |
| HC + Green Tea | 208.4 ± 21.40 # | 5.28 ± 0.31 # | 410.3 ± 39.88 # | 8.74 ± 1.63 # |
| HC + 1/2Green Tea | 210.4 ± 21.85 # | 5.44 ± 0.42 # | 436.3 ± 45.82 # | 8.87 ± 1.52 # |
| HC + β-cryptoxanthin | 223.4 ± 19.46 # | 5.79 ± 0.38 # | 449.2 ± 46.38 # | 9.01 ± 1.87 # |
| HC + 1/2 EGC | 231.5 ± 24.87 # | 5.92 ± 0.34 # | 439.0 ± 38.29 # | 8.46 ± 1.93 # |
| HC + 1/2 EGCG | 232.5 ± 22.71 # | 5.71 ± 0.42 # | 421.7 ± 37.87 # | 8.43 ± 1.38 # |
| HC + 1/2 EC | 229.8 ± 20.01 # | 5.94 ± 0.42 # | 428.9 ± 43.44 # | 8.72 ± 1.68 # |
| HC + 1/2 ECG | 238.9 ± 19.78 # | 5.96 ± 0.35 # | 436.6 ± 39.80 # | 8.59 ± 1.55 # |
| HDL-Cholesterol (mg/dL) | Free-Cholesterol (mg/dL) | NEFA (mEq/L) | Triglyceride (mg/dL) | |
| HC + Green Tea | 59.9 ± 8.64 # | 39.7 ± 3.88 # | 1658.4 ± 120.37 # | 90.01 ± 13.77 # |
| HC + 1/2Green Tea | 59.9 ± 6.92 # | 40.4 ± 3.02 # | 1763.4 ± 105.55 # | 91.98 ± 14.89 # |
| HC + β-cryptoxanthin | 61.4 ± 5.74 # | 42.0 ± 3.21 # | 1759.5 ± 123.97 # | 92.02 ± 15.88 # |
| HC + 1/2 EGC | 58.8 ± 6.37 # | 42.0 ± 3.09 # | 1698.9 ± 128.34 # | 90.85 ± 13.89 # |
| HC + 1/2 EGCG | 59.4 ± 8.83 # | 39.5 ± 3.24 # | 1735.8 ± 104.76 # | 91.74 ± 13.85 # |
| HC + 1/2 EC | 61.3 ± 9.35 # | 42.3 ± 3.89 # | 1719.7 ± 119.33 # | 90.93 ± 15.03 # |
| HC + 1/2 ECG | 62.1 ± 8.00 # | 42.9 ± 3.16 # | 1692.5 ± 113.79 # | 91.65 ± 14.58 # |
| Alkaline Phosphatase (IU/L) | AST (IU/L) | ALT (IU/L) | |
|---|---|---|---|
| HC + Green Tea | 553.8 ± 59.43 # | 1438.3 ± 139.21 # | 793.32 ± 138.25 # |
| HC + 1/2Green Tea | 569.2 ± 46.39 # | 1592.9 ± 148.89 # | 804.85 ± 148.12 # |
| HC + β-cryptoxanthin | 583.9 ± 49.21 # | 1629.4 ± 148.81 # | 822.96 ± 131.09 # |
| HC + 1/2 EGC | 592.5 ± 38.39 # | 1673.7 ± 134.27 # | 808.29 ± 118.92 # |
| HC + 1/2 EGCG | 583.2 ± 38.44 # | 1539.3 ± 129.99 # | 797.74 ± 142.26 # |
| HC + 1/2 EC | 591.2 ± 40.39 # | 1577.5 ± 151.49 # | 806.17 ± 123.47 # |
| HC + 1/2 ECG | 584.7 ± 55.24 # | 1502.1 ± 137.74 # | 818.08 ± 139.01 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakadate, K.; Kawakami, K.; Yamazaki, N. Synergistic Effect of β-Cryptoxanthin and Epigallocatechin Gallate on Obesity Reduction. Nutrients 2024, 16, 2344. https://doi.org/10.3390/nu16142344
Nakadate K, Kawakami K, Yamazaki N. Synergistic Effect of β-Cryptoxanthin and Epigallocatechin Gallate on Obesity Reduction. Nutrients. 2024; 16(14):2344. https://doi.org/10.3390/nu16142344
Chicago/Turabian StyleNakadate, Kazuhiko, Kiyoharu Kawakami, and Noriko Yamazaki. 2024. "Synergistic Effect of β-Cryptoxanthin and Epigallocatechin Gallate on Obesity Reduction" Nutrients 16, no. 14: 2344. https://doi.org/10.3390/nu16142344
APA StyleNakadate, K., Kawakami, K., & Yamazaki, N. (2024). Synergistic Effect of β-Cryptoxanthin and Epigallocatechin Gallate on Obesity Reduction. Nutrients, 16(14), 2344. https://doi.org/10.3390/nu16142344





