Maternal Psychological Well-Being as a Protector in Infantile Colic
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Study Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collado, M.C.; Rautava, S.; Isolauri, E.; Salminen, S. Gut Microbiota: A Source of Novel Tools to Reduce the Risk of Human Disease? Pediatr. Res. 2015, 77, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Grönlund, M.M.; Grzeskowiak, Ł.; Isolauri, E.; Salminen, S. Influence of Mother’s Intestinal Microbiota on Gut Colonization in the Infant. Gut Microbes 2011, 2, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Nuriel-Ohayon, M.; Neuman, H.; Koren, O. Microbial Changes during Pregnancy, Birth, and Infancy. Front. Microbiol. 2016, 7, 1031. [Google Scholar] [CrossRef] [PubMed]
- Jost, T.; Lacroix, C.; Braegger, C.P.; Chassard, C. New Insights in Gut Microbiota Establishment in Healthy Breast Fed Neonates. PLoS ONE 2012, 7, e44595. [Google Scholar] [CrossRef] [PubMed]
- Sung, V. Probiotic Interventions in Infantile Colic. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Shamir, R.; St James-Roberts, I.; Lorenzo, C.; Di Burns, A.J.; Thapar, N.; Indrio, F.; Riezzo, G.; Raimondi, F.; Di Mauro, A.; Francavilla, R.; et al. Infant Crying, Colic, and Gastrointestinal Discomfort in Early Childhood: A Review of the Evidence and Most Plausible Mechanisms. J. Pediatr. Gastroenterol. Nutr. 2013, 57 (Suppl. S1), S1–S2. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hang, C.; Hu, J.; Li, C.; Zhan, C.; Pan, J.; Yuan, T. Role of Gut-Brain Axis in Neurodevelopmental Impairment of Necrotizing Enterocolitis. Front. Neurosci. 2023, 17, 1059552. [Google Scholar] [CrossRef] [PubMed]
- Netsi, E.; Pearson, R.M.; Murray, L.; Cooper, P.; Craske, M.G.; Stein, A. Association of Persistent and Severe Postnatal Depression with Child Outcomes. JAMA Psychiatry 2018, 75, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Bolten, M.I.; Fink, N.S.; Stadler, C. Maternal Self-Efficacy Reduces the Impact of Prenatal Stress on Infant’s Crying Behavior. J. Pediatr. 2012, 161, 104–109. [Google Scholar] [CrossRef]
- Despriee, Å.W.; Småstuen, M.C.; Glavin, K.; Lødrup Carlsen, K.C.; Magi, C.A.O.; Söderhäll, C.; Hedlin, G.; Nordhagen, L.; Jonassen, C.M.; Rehbinder, E.M.; et al. Infant Colic and Abdominal Pain; Associations with Infant Multimorbidity and Maternal Perceived Stress up to 3 Months Postpartum—A Cross-Sectional/Cohort Study in the PreventADALL Study. J. Clin. Nurs. 2023, 32, 7605–7617. [Google Scholar] [CrossRef]
- Míguez, M.C.; Vázquez, M.B. Prevalence of Postpartum Major Depression and Depressive Symptoms in Spanish Women: A Longitudinal Study up to 1 Year Postpartum. Midwifery 2023, 126, 103808. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, J.I. Predictive Validity of the Edinburgh Postnatal Depression Scale and Other Tools for Screening Depression in Pregnant and Postpartum Women: A Systematic Review and Meta-Analysis. Arch. Gynecol. Obstet. 2023, 307, 1331–1345. [Google Scholar] [CrossRef]
- Gutiérrez-Zotes, A. Factor Structure of the Spanish Version of the Edinburgh Postnatal Depression Scale. Actas Españolas Psiquiatr. 2018, 46, 174–182. [Google Scholar]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of Postnatal Depression: Development of the 10-Item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar] [CrossRef] [PubMed]
- García Marqués, S.; Chillón Martínez, R.; González Zapata, S.; Rebollo Salas, M.; Jiménez Rejano, J.J. Tools Assessment and Diagnosis to Infant Colic: A Systematic Review. Child Care Health Dev. 2017, 43, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Esteve, L.; Ascaso, C.; Ojuel, J.; Navarro, P. Validation of the Edinburgh Postnatal Depression Scale (EPDS) in Spanish Mothers. J. Affect. Disord. 2003, 75, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Flor-Alemany, M.; Migueles, J.H.; Alemany-Arrebola, I.; Aparicio, V.A.; Baena-García, L. Exercise, Mediterranean Diet Adherence or Both during Pregnancy to Prevent Postpartum Depression—GESTAFIT Trial Secondary Analyses. Int. J. Environ. Res. Public Health 2022, 19, 14450. [Google Scholar] [CrossRef] [PubMed]
- Haro, J.M.; Palacín, C.; Vilagut, G.; Martínez, M.; Bernal, M.; Luque, I.; Codony, M.; Dolz, M.; Alonso, J. Prevalence of Mental Disorders and Associated Factors: Results from the ESEMeD-Spain Study. Med. Clin. 2006, 126, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Mateu, F.; Tormo, M.J.; Salmerón, D.; Vilagut, G.; Navarro, C.; Ruíz-Merino, G.; Escámez, T.; Júdez, J.; Martínez, S.; Kessler, R.C.; et al. Prevalence of Mental Disorders in the South-East of Spain, One of the European Regions Most Affected by the Economic Crisis: The Cross-Sectional PEGASUS-Murcia Project. PLoS ONE 2015, 10, e0137293. [Google Scholar] [CrossRef]
- McLaughlin, K.A.; Green, J.G.; Hwang, I.; Sampson, N.A.; Zaslavsky, A.M.; Kessler, R.C. Intermittent Explosive Disorder in the National Comorbidity Survey Replication Adolescent Supplement. Arch. Gen. Psychiatry 2012, 69, 1131–1139. [Google Scholar] [CrossRef]
- Hope, M.L.; Page, A.C.; Hooke, G.R. The Value of Adding the Quality of Life Enjoyment and Satisfaction Questionnaire to Outcome Assessments of Psychiatric Inpatients with Mood and Affective Disorders. Qual. Life Res. 2009, 18, 647–655. [Google Scholar] [CrossRef]
- Waserstein, G.; Partin, C.; Cohen, D.; Schettler, P.; Kinkead, B.; Rapaport, M.H. The Prevalence and Impact of Psychiatric Symptoms in an Undiagnosed Diseases Clinical Program. PLoS ONE 2019, 14, e0216937. [Google Scholar] [CrossRef]
- Lopuszanska-Dawid, M. Life Satisfaction as a Health Determinant among Polish Adult Population. Anthropol. Anz. 2018, 75, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Muraca, G.M.; Joseph, K.S. The Association Between Maternal Age and Depression. J. Obstet. Gynaecol. Can. 2014, 36, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xiang, Q.; Yan, C.; Liao, H.; Wang, J. Relationship between Chronic Diseases and Depression: The Mediating Effect of Pain. BMC Psychiatry 2021, 21, 436. [Google Scholar] [CrossRef]
- Gentil, L.; Grenier, G.; Meng, X.; Fleury, M.J. Impact of Co-Occurring Mental Disorders and Chronic Physical Illnesses on Frequency of Emergency Department Use and Hospitalization for Mental Health Reasons. Front. Psychiatry 2021, 12, 735005. [Google Scholar] [CrossRef]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and Aging-Related Diseases: From Molecular Mechanisms to Interventions and Treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef]
- Cho, S.J.; Lee, H.J.; Rhee, S.J.; Kim, E.Y.; Kim, K.N.; Yoon, D.H.; Ahn, Y.M. The Relationship between Visceral Adiposity and Depressive Symptoms in the General Korean Population. J. Affect. Disord. 2019, 244, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Daley, A.; Jolly, K.; MacArthur, C. The Effectiveness of Exercise in the Management of Post-Natal Depression: Systematic Review and Meta-Analysis. Fam. Pract. 2009, 26, 154–162. [Google Scholar] [CrossRef]
- Davenport, M.H.; McCurdy, A.P.; Mottola, M.F.; Skow, R.J.; Meah, V.L.; Poitras, V.J.; Jaramillo Garcia, A.; Gray, C.E.; Barrowman, N.; Riske, L.; et al. Impact of Prenatal Exercise on Both Prenatal and Postnatal Anxiety and Depressive Symptoms: A Systematic Review and Meta-Analysis. Br. J. Sports Med. 2018, 52, 1376–1385. [Google Scholar] [CrossRef]
- Marconcin, P.; Peralta, M.; Gouveia, É.R.; Ferrari, G.; Carraça, E.; Ihle, A.; Marques, A. Effects of Exercise during Pregnancy on Postpartum Depression: A Systematic Review of Meta-Analyses. Biology 2021, 10, 1331. [Google Scholar] [CrossRef]
- Sparling, T.M.; Henschke, N.; Nesbitt, R.C.; Gabrysch, S. The Role of Diet and Nutritional Supplementation in Perinatal Depression: A Systematic Review. Matern. Child Nutr. 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Bekem, Ö.; Günay, İ.; Çelik, F.; Apa, H. Interaction of Functional Gastrointestinal Disorders with Postpartum Conditions Related to Mother and Baby. Turk. J. Pediatr. 2021, 63, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Gianni, M.L.; Bettinelli, M.E.; Manfra, P.; Sorrentino, G.; Bezze, E.; Plevani, L.; Cavallaro, G.; Raffaeli, G.; Crippa, B.L.; Colombo, L.; et al. Breastfeeding Difficulties and Risk for Early Breastfeeding Cessation. Nutrients 2019, 11, 2266. [Google Scholar] [CrossRef] [PubMed]
- WHO. Exclusive Breastfeeding for Optimal Growth, Development and Health of Infants; WHO: Geneva, Switzerland; Available online: https://WwwWhoInt/Health-Topics/Breastfeeding#tab=tab_2 (accessed on 20 June 2024).
- Kramer, M.S.; Aboud, F.; Mironova, E.; Vanilovich, I.; Platt, R.W.; Matush, L.; Igumnov, S.; Fombonne, E.; Bogdanovich, N.; Ducruet, T.; et al. Breastfeeding and Child Cognitive Development New Evidence from a Large Randomized Trial. Arch. Gen. Psychiatry 1996, 65, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Hauck, F.R.; Thompson, J.M.D.; Tanabe, K.O.; Moon, R.Y.; Vennemann, M.M. Breastfeeding and Reduced Risk of Sudden Infant Death Syndrome: A Meta-Analysis. Pediatrics 2011, 128, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Horta, B.L.; Loret De Mola, C.; Victora, C.G. Long-Term Consequences of Breastfeeding on Cholesterol, Obesity, Systolic Blood Pressure and Type 2 Diabetes: A Systematic Review and Meta-Analysis. Acta Paediatr. Int. J. Paediatr. 2015, 104, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Ystrom, E. Breastfeeding Cessation and Symptoms of Anxiety and Depression: A Longitudinal Cohort Study. BMC Pregnancy Childbirth 2012, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Sinha, B.; Sankar, M.J.; Taneja, S.; Bhandari, N.; Rollins, N.; Bahl, R.; Martines, J. Breastfeeding and Maternal Health Outcomes: A Systematic Review and Meta-Analysis. Acta Paediatr. Int. J. Paediatr. 2015, 104, 96–113. [Google Scholar] [CrossRef]
- Chuong, C.M.; Nickoloff, B.J.; Elias, P.M.; Goldsmith, L.A.; Macher, E.; Maderson, P.A.; Sundberg, J.P.; Tagami, H.; Plonka, P.M.; Thestrup-Pedersen, K.; et al. What Is the “true” Function of Skin? Exp. Dermatol. 2002, 11, 349–367. [Google Scholar]
- Schirmer, A.; Croy, I.; Ackerley, R. What Are C-Tactile Afferents and How Do They Relate to “Affective Touch”? Neurosci. Biobehav. Rev. 2023, 151, 105236. [Google Scholar] [CrossRef]
- Ackerley, R.; Backlund Wasling, H.; Liljencrantz, J.; Olausson, H.; Johnson, R.D.; Wessberg, J. Human C-Tactile Afferents Are Tuned to the Temperature of a Skin-Stroking Caress. J. Neurosci. 2014, 34, 2879–2883. [Google Scholar] [CrossRef]
- Löken, L.S.; Wessberg, J.; Morrison, I.; McGlone, F.; Olausson, H. Coding of Pleasant Touch by Unmyelinated Afferents in Humans. Nat. Neurosci. 2009, 12, 547–548. [Google Scholar] [CrossRef]
- Manzotti, A.; Cerritelli, F.; Esteves, J.E.; Lista, G.; Lombardi, E.; La Rocca, S.; Gallace, A.; McGlone, F.P.; Walker, S.C. Dynamic Touch Reduces Physiological Arousal in Preterm Infants: A Role for c-Tactile Afferents? Dev. Cogn. Neurosci. 2019, 39, 100703. [Google Scholar] [CrossRef]
- Gursul, D.; Goksan, S.; Hartley, C.; Mellado, G.S.; Moultrie, F.; Hoskin, A.; Adams, E.; Hathway, G.; Walker, S.; McGlone, F.; et al. Stroking Modulates Noxious-Evoked Brain Activity in Human Infants. Curr. Biol. 2018, 28, R1380–R1381. [Google Scholar] [CrossRef]
- Ke, S.; Guimond, A.J.; Tworoger, S.S.; Huang, T.; Chan, A.T.; Liu, Y.Y.; Kubzansky, L.D. Gut Feelings: Associations of Emotions and Emotion Regulation with the Gut Microbiome in Women. Psychol. Med. 2023, 53, 7151–7160. [Google Scholar] [CrossRef]
- Galley, J.D.; Mashburn-Warren, L.; Blalock, L.C.; Lauber, C.L.; Carroll, J.E.; Ross, K.M.; Hobel, C.; Coussons-Read, M.; Dunkel Schetter, C.; Gur, T.L. Maternal Anxiety, Depression and Stress Affects Offspring Gut Microbiome Diversity and Bifidobacterial Abundances. Brain Behav. Immun. 2023, 107, 253–264. [Google Scholar] [CrossRef]
- Mepham, J.; Nelles-McGee, T.; Andrews, K.; Gonzalez, A. Exploring the Effect of Prenatal Maternal Stress on the Microbiomes of Mothers and Infants: A Systematic Review. Dev. Psychobiol. 2023, 65, e22424. [Google Scholar] [CrossRef]
- Bhattacharyya, C.; Barman, D.; Tripathi, D.; Dutta, S.; Bhattacharya, C.; Alam, M.; Choudhury, P.; Devi, U.; Mahanta, J.; Rasaily, R.; et al. Influence of Maternal Breast Milk and Vaginal Microbiome on Neonatal Gut Microbiome: A Longitudinal Study during the First Year. Microbiol. Spectr. 2023, 11, e04967-22. [Google Scholar] [CrossRef]
- Hassib, L.; de Oliveira, C.L.; Rouvier, G.A.; Kanashiro, A.; Guimarães, F.S.; Ferreira, F.R. Maternal Microbiome Disturbance Induces Deficits in the Offspring’s Behaviors: A Systematic Review and Meta-Analysis. Gut Microbes 2023, 15, 2226282. [Google Scholar] [CrossRef]
- Wei, Q.; Jiang, Z.; Shi, H.; Zou, J.; Lu, W.; Xiao, X.; Zhang, Y. Associations of Maternal Prenatal Emotional Symptoms with Neurodevelopment of Children and the Neonatal Meconium Microbiota: A Prospective Cohort Study. Psychoneuroendocrinology 2022, 142, 105787. [Google Scholar] [CrossRef]
- Van Tilburg, M.A.L.; Hyman, P.E.; Walker, L.; Rouster, A.; Palsson, O.S.; Kim, S.M.; Whitehead, W.E. Prevalence of Functional Gastrointestinal Disorders in Infants and Toddlers. J. Pediatr. 2015, 166, 684–689. [Google Scholar] [CrossRef]
- Bellaiche, M.; Oozeer, R.; Gerardi-Temporel, G.; Faure, C.; Vandenplas, Y. Multiple Functional Gastrointestinal Disorders Are Frequent in Formula-Fed Infants and Decrease Their Quality of Life. Acta Paediatr. Int. J. Paediatr. 2018, 107, 1276–1282. [Google Scholar] [CrossRef]
- Ames, S.R.; Lotoski, L.C.; Azad, M.B. Comparing Early Life Nutritional Sources and Human Milk Feeding Practices: Personalized and Dynamic Nutrition Supports Infant Gut Microbiome Development and Immune System Maturation. Gut Microbes 2023, 15, 2190305. [Google Scholar] [CrossRef]
- Baldassarre, M.E.; Antonucci, L.A.; Castoro, G.; Di Mauro, A.; Fanelli, M.; Grosso, F.M.; Cassibba, R.; Laforgia, N. Maternal Psychological Factors and Onset of Functional Gastrointestinal Disorders in Offspring. J. Pediatr. Gastroenterol. Nutr. 2021, 73, 30–36. [Google Scholar] [CrossRef]
- González-Rodríguez, R.I.; Jiménez-Escobar, I.; Gutiérrez-Castrellón, P. Human Milk Microbiota and Impact on Health. Gac. Medica Mex. 2020, 156, S58–S66. [Google Scholar] [CrossRef]
- Lara-Cinisomo, S.; McKenney, K.; Di Florio, A.; Meltzer-Brody, S. Associations between Postpartum Depression, Breastfeeding, and Oxytocin Levels in Latina Mothers. Breastfeed. Med. 2017, 12, 436–442. [Google Scholar] [CrossRef]
- Vik, T.; Grote, V.; Escribano, J.; Socha, J.; Verduci, E.; Fritsch, M.; Carlier, C.; Kries, R.V.; Koletzko, B. Infantile Colic, Prolonged Crying and Maternal Postnatal Depression. Acta Paediatr. Int. J. Paediatr. 2009, 98, 1344–1348. [Google Scholar] [CrossRef]
- Gostoli, S.; Nicolucci, L.; Malaguti, C.; Patierno, C.; Carrozzino, D.; Balducci, C.; Zaniboni, S.; Lodi, V.; Petio, C.; Rafanelli, C. Mental Illness and Work-Related Limitations in Healthcare Workers: A Preliminary Retrospective Study. Int. J. Environ. Res. Public Health 2022, 19, 9098. [Google Scholar] [CrossRef]
- Coombs, N.C.; Meriwether, W.E.; Caringi, J.; Newcomer, S.R. Barriers to Healthcare Access among U.S. Adults with Mental Health Challenges: A Population-Based Study. SSM-Popul. Health 2021, 15, 100847. [Google Scholar] [CrossRef]
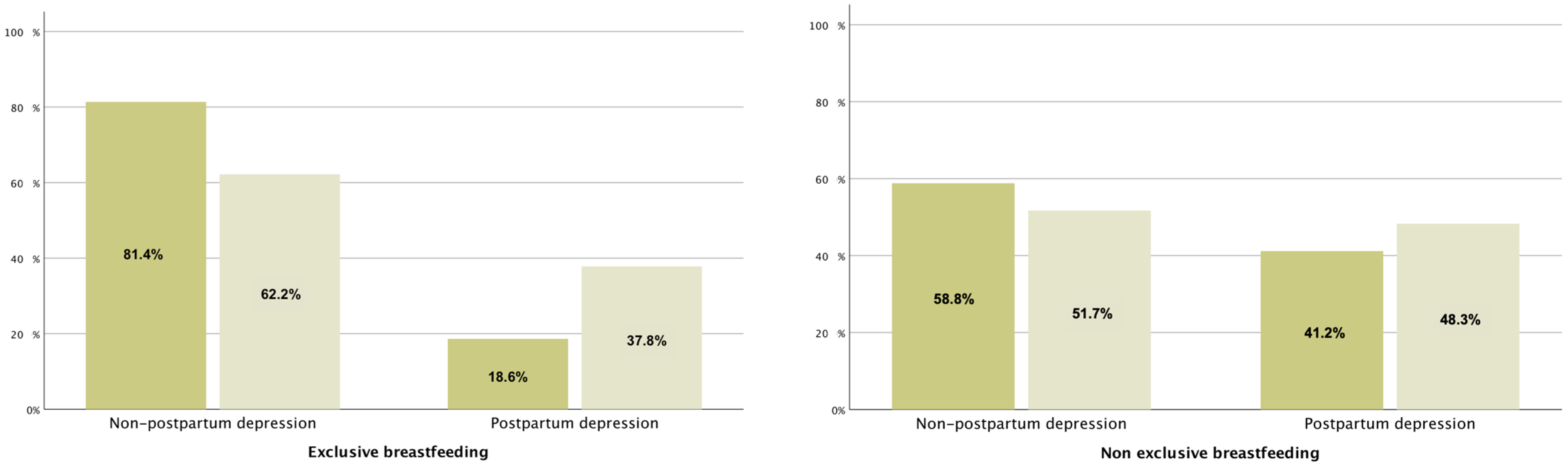
| Total Group (n = 528) | Postpartum Depression | Infantile Colic | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Present (n = 170) | Absent (n = 358) | p-Value | Present (n = 206) | Absent (n = 322) | p-Value | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Age (years) | 35.36 | 3.98 | 35.21 | 3.98 | 35.43 | 3.98 | 0.566 a | 35.29 | 3.68 | 35.40 | 4.16 | 0.752 a |
| BMI (kg/m2) | 23.74 | 4.68 | 23.76 | 4.37 | 23.74 | 4.83 | 0.956 a | 23.91 | 5.02 | 23.64 | 4.46 | 0.515 a |
| Maternal education level | ||||||||||||
| Illiterate/literate (n. %) | 1 | 0.2% | 0 | 0 | 1 | 100% | 0.024 b | 1 | 100% | 0 | 0 | 0.461 b |
| Elementary school (n. %) | 13 | 3.2% | 6 | 46.2% | 7 | 53.8% | 4 | 30.8% | 9 | 69.2% | ||
| Secondary school (n. %) | 75 | 28.5% | 34 | 45.3% | 41 | 54.6% | 7 | 35.0% | 13 | 65% | ||
| University (n. %) | 343 | 79.4% | 101 | 29.4% | 242 | 70.6% | 159 | 40.0% | 239 | 60% | ||
| Lifestyle | ||||||||||||
| Smoke (Yes (n. %)) | 26 | 4.9% | 11 | 42.3% | 15 | 57.7% | 0.529 b | 11 | 42.3% | 15 | 57.7% | 0.392 b |
| Alcohol (Yes (n. %)) | 2 | 0.4% | 1 | 50% | 1 | 50% | 1.000 b | 0 | 0% | 2 | 100% | 0.082 b |
| Walking | ||||||||||||
| Hours/day | 2.31 | 0.70 | 2.31 | 0.78 | 2.31 | 0.66 | 0.942 a | 2.40 | 0.77 | 2.25 | 0.64 | 0.017 a |
| Days/week | 5.28 | 1.78 | 5.15 | 1.84 | 5.34 | 1.74 | 0.263 a | 5.29 | 1.74 | 5.28 | 1.80 | 0.921 a |
| Sports practice | ||||||||||||
| Hours/day | 1.69 | 0.76 | 1.66 | 0.86 | 1.71 | 0.71 | 0.551 a | 1.69 | 0.75 | 1.69 | 0.77 | 0.914 a |
| Days/week | 2.50 | 1.61 | 2.28 | 1.61 | 2.60 | 1.60 | 0.057 a | 2.57 | 1.73 | 2.47 | 1.53 | 0.523 a |
| Obstetrics data | ||||||||||||
| Postpartum days | 149.20 | 96.22 | 165.81 | 102.46 | 141.30 | 92.22 | 0.006 a | 142.78 | 95.36 | 153.30 | 96.70 | 0.221 a |
| Parity | ||||||||||||
| Primiparous (n. %) | 341 | 64.6% | 229 | 67.2% | 112 | 32.8% | 0.698 b | 146 | 42.8% | 195 | 57.2% | 0.020 b |
| Multiparous (n. %) | 187 | 35.4% | 58 | 31% | 129 | 69% | 60 | 32.1% | 127 | 67.9% | ||
| Number of children | 1.4 | 0.59 | 1.36 | 0.53 | 1.42 | 0.628 | 0.356 a | 1.33 | 0.53 | 1.45 | 0.63 | 0.022 a |
| ICSQ score | 47.32 | 8.16 | 49.90 | 9.19 | 46.09 | 7.32 | <0.001 a | 55.37 | 4.95 | 42.17 | 5.04 | <0.001 a |
| EPDS score | 9.54 | 5.36 | 15.76 | 3.54 | 6.59 | 3.06 | <0.001 a | 11.24 | 5.69 | 8.45 | 4.85 | <0.001 a |
| Total Group (n = 528) | Postpartum Depression | Infantile Colic | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Present (n = 170) | Absent (n = 358) | p-Value a | Present (n = 206) | Absent (n = 322) | p-Value a | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Age groups | ||||||||||||
| Less than 1 month | 48 | 9.1% | 12 | 25% | 36 | 75% | 0.089 | 15 | 31.3% | 33 | 68.8% | 0.045 |
| 1–2 months | 128 | 24.2% | 37 | 28.9% | 91 | 71.1% | 63 | 49.2% | 65 | 50.8% | ||
| 3–5 months | 177 | 33.5 | 51 | 28.8% | 126 | 71.2% | 68 | 38.4% | 109 | 61.6% | ||
| 6–8 months | 95 | 18% | 36 | 37.9% | 59 | 62.1% | 29 | 30.5% | 66 | 69.5% | ||
| 9–12 months | 79 | 15% | 33 | 41.8% | 46 | 58.2% | 30 | 38% | 49 | 62% | ||
| Birth data | ||||||||||||
| Mode of delivery | ||||||||||||
| Vaginally without complications | 330 | 62.5% | 97 | 29.4% | 233 | 70.6% | 0.274 | 124 | 37.6% | 206 | 62.4% | 0.851 |
| Vaginally with complications | 64 | 12.1% | 21 | 32.8% | 43 | 67.2% | 26 | 40.6% | 38 | 59.4% | ||
| Planned cesarean | 38 | 7.2% | 15 | 39.5% | 23 | 60.5% | 16 | 42.1% | 22 | 57.9% | ||
| Emergency cesarean | 96 | 18.2% | 37 | 38.5% | 59 | 61.5% | 40 | 41.7% | 56 | 58.3% | ||
| Gestation weeks | ||||||||||||
| 28 to 37 weeks | 25 | 4.7% | 9 | 36.0% | 16 | 64.0% | 0.535 | 10 | 40.0% | 15 | 61.2% | 0.957 |
| 38 to 41 weeks | 479 | 90.7% | 151 | 31.5% | 328 | 68.5% | 186 | 38.8% | 293 | 58.3% | ||
| 42 weeks or more | 24 | 4.5% | 10 | 41.7% | 14 | 58.3% | 10 | 41.7% | 14 | 60.0% | ||
| Birth weight | ||||||||||||
| More than 3 kg | 383 | 72.5% | 122 | 31.9% | 261 | 68.1% | 0.945 | 148 | 38.6% | 235 | 61.4% | 0.491 |
| 2–3 kg | 139 | 26.3% | 46 | 33.1% | 93 | 66.9% | 57 | 41.0% | 82 | 59.0% | ||
| 1.5–2 kg | 6 | 1.1% | 2 | 33.3% | 4 | 66.7% | 1 | 16.7% | 5 | 83.3% | ||
| Feeding | ||||||||||||
| Feeding type | ||||||||||||
| Exclusive breastfeeding | 339 | 64.2% | 86 | 25.4% | 253 | 74.6% | <0.001 | 119 | 35.1% | 220 | 64.9% | 0.045 |
| Non-breastfeeding | 52 | 9.8% | 25 | 48.1% | 27 | 51.9% | 25 | 48.1% | 27 | 51.9% | ||
| Mixed | 137 | 25.9% | 59 | 43.1% | 78 | 56.9% | 62 | 45.3% | 75 | 54.7% | ||
| Feeding frequency | ||||||||||||
| Request | 339 | 64.2% | 86 | 25.4% | 253 | 74.6% | <0.001 | 119 | 35.1% | 220 | 64.9% | 0.009 |
| Fixed hours | 189 | 35.8% | 84 | 44.4% | 105 | 55.6% | 87 | 46.0% | 102 | 54.0% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garnacho-Garnacho, V.E.; Rodríguez-López, E.S.; Oliva-Pascual-Vaca, A.; Goenaga-Echave, L.; Otero-Campos, Á. Maternal Psychological Well-Being as a Protector in Infantile Colic. Nutrients 2024, 16, 2342. https://doi.org/10.3390/nu16142342
Garnacho-Garnacho VE, Rodríguez-López ES, Oliva-Pascual-Vaca A, Goenaga-Echave L, Otero-Campos Á. Maternal Psychological Well-Being as a Protector in Infantile Colic. Nutrients. 2024; 16(14):2342. https://doi.org/10.3390/nu16142342
Chicago/Turabian StyleGarnacho-Garnacho, Victoria Eugenia, Elena Sonsoles Rodríguez-López, Ángel Oliva-Pascual-Vaca, Leire Goenaga-Echave, and Álvaro Otero-Campos. 2024. "Maternal Psychological Well-Being as a Protector in Infantile Colic" Nutrients 16, no. 14: 2342. https://doi.org/10.3390/nu16142342
APA StyleGarnacho-Garnacho, V. E., Rodríguez-López, E. S., Oliva-Pascual-Vaca, A., Goenaga-Echave, L., & Otero-Campos, Á. (2024). Maternal Psychological Well-Being as a Protector in Infantile Colic. Nutrients, 16(14), 2342. https://doi.org/10.3390/nu16142342







