Effects of β-Alanine Supplementation on Subjects Performing High-Intensity Functional Training
Abstract
1. Introduction
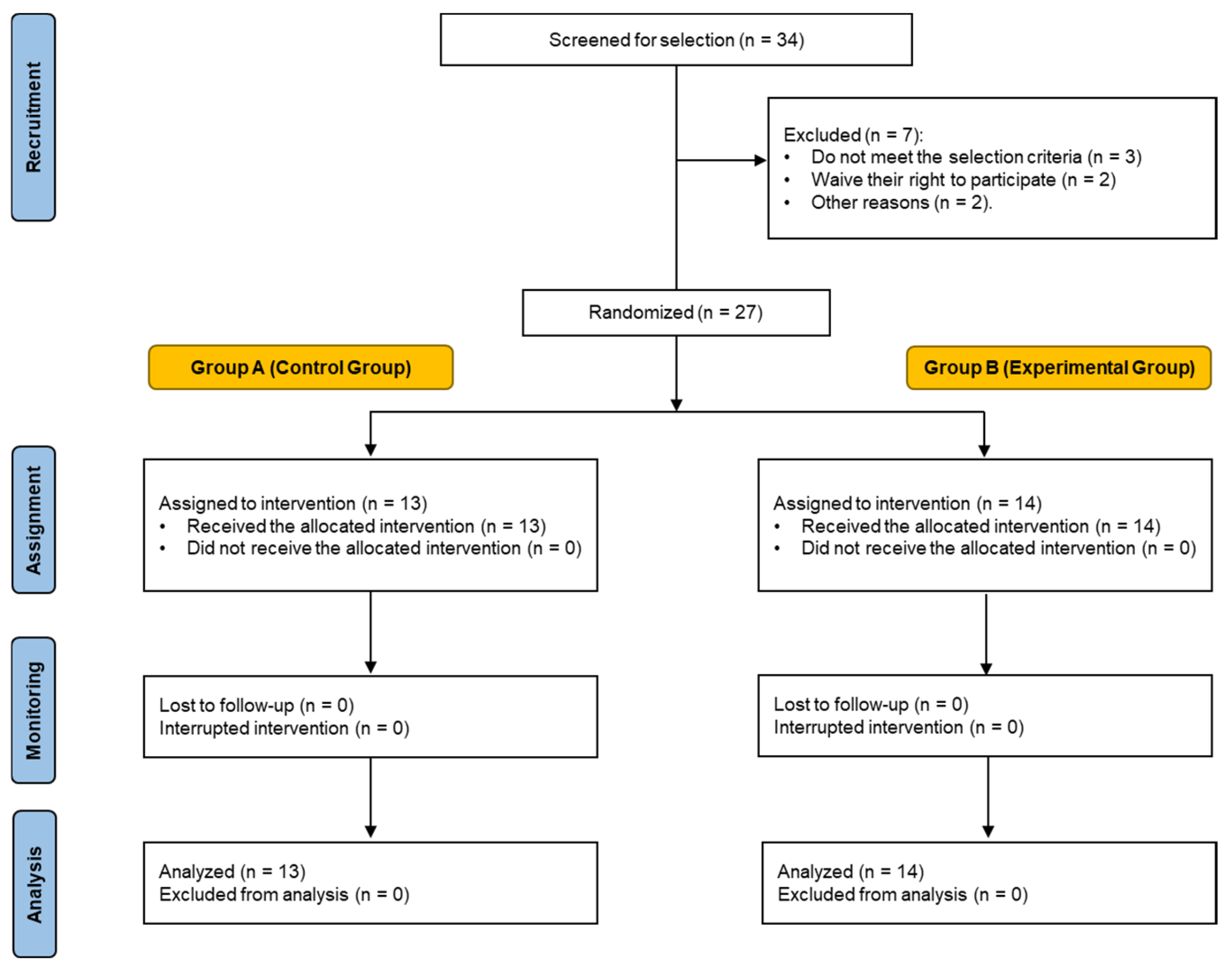
2. Materials and Methods
2.1. Experimental Design
2.2. Subjects
2.3. Supplementation
2.4. High-Intensity Functional Training Workout
- -
- 6 power cleans (50 kg for men, 35 kg for women)
- -
- 10 objects over the shoulder (20 kg for men, 15 kg for women)
- -
- 14 wall ball shots (9 kg for men, 7 kg for women)
- -
- 18 shoulders to overhead dumbbell (DB) movements (20 kg for men, 10 kg for women)
- -
- 200 m run
- -
- 6 pull-ups
- -
- 10 bodyweight squats
- -
- 14 DB power snatches (20 kg for men, 10 kg for women)
- -
- 18 box jumps (60 cm for men, 50 cm for women)
- -
- 100 m run (Table 1).
2.5. Blood Lactate
2.6. Muscular Fatigue
2.7. Rating of Perceived Exertion
2.8. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoffman, J.R.; Varanoske, A.; Stout, J.R. Effects of β-Alanine Supplementation on Carnosine Elevation and Physiological Performance. Adv. Food Nutr. Res. 2018, 84, 183–206. [Google Scholar] [CrossRef]
- Li, G.; Li, Z.; Liu, J. Amino acids regulating skeletal muscle metabolism: Mechanisms of action, physical training dosage recommendations and adverse effects. Nutr. Metab. 2024, 21, 41. [Google Scholar] [CrossRef] [PubMed]
- Berezhnoy, D.S.; Stvolinsky, S.L.; Lopachev, A.V.; Devyatov, A.A.; Lopacheva, O.M.; Kulikova, O.I.; Abaimov, D.A.; Fedorova, T.N. Carnosine as an effective neuroprotector in brain pathology and potential neuromodulator in normal conditions. Amino Acids 2019, 51, 139–150. [Google Scholar] [CrossRef]
- Saunders, B.; de Salles Painelli, V.; de Oliveira, L.F.; da Eira Silva, V.; da Silva, R.P.; Riani, L.; Franchi, M.; de Souza Gonçalves, L.; Harris, R.C.; Roschel, H.; et al. Twenty-four Weeks of β-Alanine Supplementation on Carnosine Content, Related Genes, and Exercise. Med. Sci. Sports Exerc. 2017, 49, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R.; Emerson, N.S.; Stout, J.R. ß-alanine supplementation. Curr. Sports Med. Rep. 2012, 11, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Perim, P.; Marticorena, F.M.; Ribeiro, F.; Barreto, G.; Gobbi, N.; Kerksick, C.; Dolan, E.; Saunders, B. Can the Skeletal Muscle Carnosine Response to Beta-Alanine Supplementation Be Optimized? Front. Nutr. 2019, 6, 135. [Google Scholar] [CrossRef]
- Song, P.; Zhang, X.; Wang, S.; Xu, W.; Wei, F. Advances in the Synthesis of β-alanine. Front. Bioeng. Biotechnol. 2023, 11, 1283129. [Google Scholar] [CrossRef] [PubMed]
- Derave, W.; Everaert, I.; Beeckman, S.; Baguet, A. Muscle Carnosine Metabolism and β-Alanine Supplementation in Relation to Exercise and Training. Sports Med. 2010, 40, 247–263. [Google Scholar] [CrossRef]
- Stellingwerff, T.; Decombaz, J.; Harris, R.C.; Boesch, C. Optimizing human in vivo dosing and delivery of ß-alanine supple- ments for muscle carnosine synthesis. Amino Acids 2012, 43, 57–65. [Google Scholar] [CrossRef]
- Cesak, O.; Vostalova, J.; Vidlar, A.; Bastlova, P.; Student, V., Jr. Carnosine and Beta-Alanine Supplementation in Human Medicine: Narrative Review and Critical Assessment. Nutrients 2023, 15, 1770. [Google Scholar] [CrossRef]
- Schön, M.; Mousa, A.; Berk, M.; Chia, W.L.; Ukropec, J.; Majid, A.; Ukropcová, B.; de Courten, B. The Potential of Carnosine in Brain-Related Disorders: A Comprehensive Review of Current Evidence. Nutrients 2019, 11, 1196. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.J.; Artioli, G.G.; Turner, M.D.; Sale, C. The Physiological Roles of Carnosine and β-Alanine in Exercising Human Skeletal Muscle. Med. Sci. Sports Exerc. 2019, 51, 2098–2108. [Google Scholar] [CrossRef] [PubMed]
- Dolan, E.; Saunders, B.; Harris, R.C.; Bicudo, J.E.P.W.; Bishop, D.J.; Sale, C.; Gualano, B. Comparative physiology investigations support a role for histidine-containing dipeptides in intracellular acid-base regulation of skeletal muscle. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 234, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.J., Jr.; Ring, E. A comparative study of the effect of carnosine on myofibrilar-ATPase activity on vertebrate and invertebrate muscles. Comp. Biochem. Physiol. 1970, 37, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Batrukova, M.A.; Rubtsov, A.M. Histidine-containing dipeptides as endogenous regulators of the activity of sarcoplasmic reticulum Ca-release channels. Biochim. Biophys. Acta 1997, 1324, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Rubtsov, A.M. Molecular mechanisms of regulation of the activity of sarcoplasmic reticulum Ca-release channels (ryanodine receptors), muscle fatigue, and Severin’s phenomenon. Biochemistry 2001, 66, 1132–1143. [Google Scholar] [CrossRef]
- Dutka, T.L.; Lamb, G.D. Effect of carnosine on excitation-contraction coupling in mechanically-skinned rat skeletal muscle. J. Muscle Res. Cell Motil. 2004, 25, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Dutka, T.L.; Lamboley, C.R.; Murphy, R.M.; Lamb, G.D.; Wyckelsma, V.L.; Perry, B.D.; McKenna, M.J. Acute effects of taurine on sarcoplasmic reticulum Ca2+ accumulation and contractility in human type I and type II skeletal muscle fibers. J. Appl. Physiol. 2014, 117, 797–805. [Google Scholar] [CrossRef]
- Kalbe, C.; Metzger, K.; Gariépy, C.; Palin, M.-F. Effect of muscle fibre types and carnosine levels on the expression of carnosine-related genes in pig skeletal muscle. Histochem. Cell Biol. 2023, 160, 63–77. [Google Scholar] [CrossRef]
- Harris, R.C.; Wise, J.A.; Price, K.A.; Kim, H.J.; Kim, C.K.; Sale, C. Determinants of muscle carnosine content. Amino Acids 2012, 43, 5–12. [Google Scholar] [CrossRef]
- Harris, R.C.D.; Greenhaff, P.L. Carnosine and taurine contents in individual fibres of human vastus lateralis muscle. J. Sports Sci. 1998, 16, 639–643. [Google Scholar] [CrossRef]
- Morán, M. Tipos de fibras musculares. In Fisiología del Ejercicio; Chicharro, J.L., Fernández, A., Eds.; Médica Panamericana: Buenos Aires, Argentina, 2008; pp. 91–97. [Google Scholar]
- Hall, E.C.R.; Semenova, E.; Bondareva, E.A.; Borisov, O.; Andryushchenko, O.N.; Andryushchenko, L.; Zmijewski, P.; Generozov, E.; Ahmetov, I. Association of muscle fiber composition with health and exercise-related traits in athletes and untrained subjects. Biol. Sport 2021, 38, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Hopwood, H.J.; Bellinger, P.M.; Compton, H.R.; Bourne, M.N.; Minahan, C. The Relevance of Muscle Fiber Type to Physical Characteristics and Performance in Team-Sport Athletes. Int. J. Sports Physiol. Perform. 2023, 18, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Stellingwerff, T.; Anwander, H.; Egger, A.; Buehler, T.; Kreis, R.; Decombaz, J.; Boesch, C. Effect of two A-alanine dosing protocols on muscle carnosine synthesis and washout. Amino Acids 2011, 42, 2461–2472. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Grgic, J.; Van Every, D.W.; Plotkin, D.L. Loading Recommendations for Muscle Strength, Hypertrophy, and Local Endurance: A Re-Examination of the Repetition Continuum. Sports 2021, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, T.S.; Louzada, C.T.N.; Miyashita, G.K.; da Silva, P.H.J.; Sungaila, H.Y.F.; Lara, P.H.S.; Pochini, A.d.C.; Ejnisman, B.; Cohen, M.; Arliani, G.G. CrossFit®: Injury prevalence and main risk factors. Clinics 2019, 74, e1402. [Google Scholar] [CrossRef] [PubMed]
- Mehrab, M.; de Vos, R.J.; Kraan, G.A.; Mathijssen, N.M.C. Injury Incidence and Patterns Among Dutch CrossFit Athletes. Orthop. J. Sports Med. 2017, 5, 2325967117745263, Corrected in Orthop. J. Sports Med. 2021, 9, 23259671211028303. [Google Scholar] [CrossRef]
- Maté-Muñoz, J.L.; Lougedo, J.H.; Barba, M.; Cañuelo-Márquez, A.M.; Guodemar-Pérez, J.; García-Fernández, P.; Lozano-Estevan, M.D.C.; Alonso-Melero, R.; Sánchez-Calabuig, M.A.; Ruíz-López, M.; et al. Cardiometabolic and Muscular Fatigue Responses to Different CrossFit® Workouts. J. Sports Sci. Med. 2018, 17, 668–679. [Google Scholar]
- Zarrouk, N.; Mtibaa, K.; Hammouda, O.; Chtourou, H.; Chaabouni, K.; Ayadi-Makni, F.; Rebai, H. Assessment of acute neuromuscular fatigue manifestations and functional performances after heavy resistance exercise. J. Sports Med. Phys. Fit. 2021, 61, 1596–1604. [Google Scholar] [CrossRef]
- Meeusen, R.; Watson, P.; Dvorak, J. The brain and fatigue: New opportunities for nutritional interventions? J. Sports Sci. 2006, 24, 773–782. [Google Scholar] [CrossRef]
- Tornero-Aguilera, J.F.; Jimenez-Morcillo, J.; Rubio-Zarapuz, A.; Clemente-Suárez, V.J. Central and Peripheral Fatigue in Physical Exercise Explained: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 3909. [Google Scholar] [CrossRef] [PubMed]
- Jayalath, J.L.R.; de Noronha, M.; Weerakkody, N.; Bini, R. Effects of fatigue on ankle biomechanics during jumps: A systematic review. J. Electromyogr. Kinesiol. 2018, 42, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Maté-Muñoz, J.L.; Budurin, M.; González-Lozano, S.; Heredia-Elvar, J.R.; Cañuelo-Márquez, A.M.; Barba-Ruiz, M.; Muriarte, D.; Garnacho-Castaño, M.V.; Hernández-Lougedo, J.; García-Fernández, P. Physiological Responses at 15 Minutes of Recovery after a Session of Functional Fitness Training in Well-Trained Athletes. Int. J. Environ. Res. Public Health 2022, 19, 8864. [Google Scholar] [CrossRef] [PubMed]
- Smilios, I.; Hakkinen, K.; Tokmakidis, S.P. Power Output and Electromyographic Activity during and after a Moderate Load Muscular Endurance Session. J. Strength Cond. Res. 2010, 24, 2122–2131. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Lougedo, J.; Heredia-Elvar, J.R.; Maicas-Pérez, L.; Cañuelo-Márquez, A.M.; Rozalén-Bustín, M.; Franco, F.d.J.; Garnacho-Castaño, M.V.; García-Fernández, P.; Maté-Muñoz, J.L. Neuromuscular Fatigue and Metabolic Stress during the 15 Minutes of Rest after Carrying Out a Bench Press Exercise Protocol. Biology 2022, 11, 1435. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.D.; Bobbert, M.F.; van Soest, A.J.; Gribble, P.L.; Kistemaker, D.A. Optimizing the Distribution of Leg Muscles for Vertical Jumping. PLoS ONE 2016, 11, e0150019. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.; Longobardi, I.; Perim, P.; Duarte, B.; Ferreira, P.; Gualano, B.; Roschel, H.; Saunders, B. Timing of Creatine Supplementation around Exercise: A Real Concern? Nutrients 2021, 13, 2844. [Google Scholar] [CrossRef] [PubMed]
- Coates, A.M.; Joyner, M.J.; Little, J.P.; Jones, A.M.; Gibala, M.J. A Perspective on High-Intensity Interval Training for Performance and Health. Sports Med. 2023, 53 (Suppl. S1), 85–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Lin, S.C.; Hsu, S.C.; Yang, M.T.; Chan, K.H. Effects of Creatine Supplementation on Muscle Strength and Optimal Individual Post-Activation Potentiation Time of the Upper Body in Canoeists. Nutrients 2017, 9, 1169. [Google Scholar] [CrossRef]
- Wang, C.C.; Fang, C.C.; Lee, Y.H.; Yang, M.T.; Chan, K.H. Effects of 4-Week Creatine Supplementation Combined with Complex Training on Muscle Damage and Sport Performance. Nutrients 2018, 10, 1640. [Google Scholar] [CrossRef]
- Ducker, K.J.; Dawson, B.; Wallman, K.E. Effect of Beta-Alanine Supplementation on 2000-m Rowing-Ergometer Performance. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Grgic, J.; Grgic, I.; Del Coso, J.; Schoenfeld, B.J.; Pedisic, Z. Effects of sodium bicarbonate supplementation on exercise performance: An umbrella review. J. Int. Soc. Sports Nutr. 2021, 18, 71. [Google Scholar] [CrossRef] [PubMed]
- Association, W.M. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar]
- Penafiel, R.; Ruzafa, C.; Monserrat, F.; Cremades, A. Gender related differences in carnosine, anserine and lysine content of murine skeletal muscle. Amino Acids 2004, 26, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; Fiandor, E.M.; García, J.F.; Busto, N.; Santamaría-Peláez, M.; Gutiérrez-Abejón, E.; Roche, E.; Mielgo-Ayuso, J. β-Alanine Supplementation in Combat Sports: Evaluation of Sports Performance, Perception, and Anthropometric Parameters and Biochemical Markers-A Systematic Review of Clinical Trials. Nutrients 2023, 15, 3755. [Google Scholar] [CrossRef] [PubMed]
- Tiedje, K.E.; Stevens, K.; Barnes, S.; Weaver, D.F. Beta-alanine as a small molecule neurotransmitter. Neurochem. Int. 2010, 57, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R.; Ratamess, N.A.; Faigenbaum, A.D.; Ross, R.; Kang, J.; Stout, J.R.; Wise, J.A. Short-duration ß -alanine supplementation increases training volume and reduces subjective feelings of fatigue in college football players. Nutr. Res. 2008, 28, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Décombaz, J.; Beaumont, M.; Vuichoud, J.; Bouisset, F.; Stellingwerff, T. The effect of slow-release Aalanine on absorption kinetics and paresthesia. Amino Acids 2012, 43, 67–76. [Google Scholar] [CrossRef]
- García-Fernández, P.; Cimadevilla, E.; Guodemar-Pérez, J.; Cañuelo-Márquez, A.M.; Heredia-Elvar, J.R.; Fernández-Rodríguez, T.; Lozano-Estevan, M.d.C.; Hervás-Pérez, J.P.; Sánchez-Calabuig, M.A.; Garnacho-Castaño, M.V.; et al. Muscle Recovery after a Single Bout of Functional Fitness Training. Int. J. Environ. Res. Public Health 2021, 18, 6634. [Google Scholar] [CrossRef]
- McNaughton, L.R.; Thompson, D.; Philips, G.; Backx, K.; Crickmore, L. A comparison of the lactate Pro, Accusport, Analox GM7 and Kodak Ektachem lactate analysers in normal, hot and humid conditions. Int. J. Sports Med. 2002, 23, 130–135. [Google Scholar] [CrossRef]
- McLean, S.R.; Norris, S.R.; Smith, D.J. Comparison of the Lactate Pro and the YSI 1500 Sport Blood Lactate Analyzers. Int. J. Appl. Sports Sci. 2004, 16, 22–30. [Google Scholar]
- Gorostiaga, E.M.; Asiain, X.; Izquierdo, M.; Postigo, A.; Aguado, R.; Alonso, J.M.; Ibánez, J. Vertical jump performance and blood ammonia and lactate levels during typical training sessions in elite 400-m runners. J. Strength Cond. Res. 2010, 24, 1138–1149. [Google Scholar] [CrossRef]
- Bachero-Mena, B.; González-Badillo, J.J. Mechanical and Metabolic Responses during High-intensity Training in Elite 800-m Runners. Int. J. Sports Med. 2020, 42, 350–356. [Google Scholar] [CrossRef] [PubMed]
- De Pedro, M.B.; de Pedro, A.I.B.; Rubio, Á.A.; Muñoz, J.L.M.; Lougedo, J.H. Changes in the Activity of the Erector Spinae and Gluteus Medius Muscles with the Presence of Simulated Lower Limb Dysmetria. Sensors 2024, 24, 1223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sánchez-Medina, L.; González-Badillo, J.J. Velocity Loss as an indicator of neuromuscular fatigue during resistance training. Med. Sci. Sports Exerc. 2011, 43, 1725–1734. [Google Scholar] [CrossRef]
- Linthorne, N.P. Analysis of standing vertical jumps using a force platform. Am. J. Phys. 2001, 69, 1198–1204. [Google Scholar] [CrossRef]
- Street, G.; McMillan, S.; Board, W.; Rasmussen, M.; Heneghan, J.M. Sources of error in determining countermovement jump height with the impulse method. J. Appl. Biomech. 2001, 17, 43–54. [Google Scholar] [CrossRef]
- Flora, P.; Gómez-Landero, L.A.; Suárez-Arrones, L.; Harrison, A.J. Kinetic and kinematic analysis for assessing the differences in countermovement jump performance in rugby players. J. Strength Cond. Res. 2016, 30, 2533–2539. [Google Scholar] [CrossRef]
- Owen, N.J.; Watkins, J.; Kilduff, L.P.; Bevan, H.R.; Bennett, M.A. Development of a criterion method to determine peak mechanical power output in a countermovement jump. J. Strength Cond. Res. 2014, 28, 1552–1558. [Google Scholar] [CrossRef]
- Borg, G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Garnacho-Castaño, M.V.; Cuenca, E.; García-Fernández, P.; Muñoz-González, A.; De Jesús, F.; Lozano-Estevan, M.C.; Fernandes da Silva, S.; Veiga-Herreros, P.; Maté-Muñoz, J.L. Effects of Beetroot Juice Supplementation on a 30-s High-Intensity Inertial Cycle Ergometer Test. Nutrients 2017, 9, 1360. [Google Scholar] [CrossRef] [PubMed]
- Huerta Ojeda, Á.; Contreras-Montilla, O.; Galdames Maliqueo, S.; Jorquera-Aguilera, C.; Fuentes-Kloss, R.; Guisado-Barrilao, R. Efectos de la suplementación aguda con beta-alanina sobre una prueba de tiempo límite a velocidad aeróbica máxima en atletas de resistencia. Nutr. Hosp. 2019, 36, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.C.; Edwards, R.H.; Hultman, E.; Nordesjö, L.O.; Nylind, B.; Sahlin, K. The time course of phosphorylcreatine resynthesis during recovery of the quadriceps muscle in man. Pflugers Arch. 1976, 367, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, B.; Danforth, W.H. Effect of pH on the kinetics of frog muscle phosphofructokinase. J. Biol. Chem. 1966, 241, 4110–4112. [Google Scholar] [CrossRef] [PubMed]
- Swank, A.M.; Robertson, R.J. Effect of induced alkalosis on perception of exertion during exercise recovery. J. Strength Cond. Res. 2002, 16, 491–499. [Google Scholar] [PubMed]
- Hermansen, L.; Osnes, J.B. Blood and muscle pH after maximal exercise in man. J. Appl. Physiol. 1972, 32, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Spriet, L.L.; Lindinger, M.I.; McKelvie, R.S.; Heigenhauser, G.J.; Jones, N.L. Muscle glycogenolysis and H+ concentration during maximal intermittent cycling. J. Appl. Physiol. 1989, 66, 8–13. [Google Scholar] [CrossRef]
- Precio, M.; Moss, P. Los efectos del trabajo: La duración del descanso en las respuestas fisiológicas y de percepción durante el ejercicio y el rendimiento intermitentes. J. Sports Sci. 2007, 25, 1613–1621. [Google Scholar] [CrossRef]
- Stavrinou, P.S.; Bogdanis, G.C.; Giannaki, C.D.; Terzis, G.; Hadjicharalambous, M. High-intensity Interval Training Frequency: Cardiometabolic Effects and Quality of Life. Int. J. Sports Med. 2018, 39, 210–217. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Ratamess, N.A.; Kang, J.; Mangine, G.; Faigenbaum, A.; Stout, J. Effect of creatine and A-alanine supplementation on performance and endocrine responses in strength/power athletes. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 430–446. [Google Scholar] [CrossRef]
- Van Thienen, R.; Van Proeyen, K.; Van Eynde, B.; Lefere, T.; Hespel, P. Beta-alanine improves sprint performance in endurance cycling. Med. Sci. Sports Exerc. 2009, 41, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Bogdanis, G.C.; Nevill, M.E.; Lakomy, H.K.; Boobis, L.H. Power output and muscle metabolism during and following recovery from 10 and 20 s of maximal sprint exercise in humans. Acta Physiol. Scand. 1998, 163, 261–272. [Google Scholar] [CrossRef] [PubMed]
| 2 rounds (r) × (6 power clean) | 2 r × (6 pull-up) | ||
|---|---|---|---|
 | A power clean is a weightlifting exercise in which the athlete must catch the barbell in the front rack position without reaching the bottom of a squat. |  | To complete the pull-up, the subject must grip a suspended horizontal bar with both hands and fully extend both arms at the repetition bottom. At the top of the repetition, the chin must break the uppermost horizontal plane of the bar. A kip of any style may be used. The repetition is counted when the athlete’s chin breaks the top-most horizontal plane of the bar. |
| Men: 50 kg | Women: 35 kg | ||
| 2 r × (10 objects over shoulder) | 2 r × (10 bodyweight squat) | ||
 | To perform the object-over-shoulder exercise, the athlete must first select the designated object and then lift it up and over their shoulder while simultaneously extending their hips fully. The athlete may employ any technique to lift the object. |  | The bodyweight squat is a bodyweight movement in which the athlete begins in a standing position with an open hip angle, descends to a full squat with the creases of both hips below the plane of the top of the knees, and returns to the standing position with the hips returning to an open angle. |
| Men: 20 kg | Women: 15 kg | ||
| 2 r × (14 wall ball shot) | |||
 | The wallball shot is executed with a medicine ball and an elevated target. The athlete must descend to a bottom-of-squat position with the medicine ball in the frontal plane, after which they must ascend while throwing the ball so that it makes contact at or above a designated height. While this repetition is permitted, it is not obligatory to jump during the execution. |  | The power snatch is a weightlifting exercise in which the athlete must catch the dumbbell overhead with fully extended elbows without achieving the bottom of a squat during the task. |
| Men: 9 kg | Women: 7 kg | Men: 20 kg | Women: 10 kg |
| 2 r × (18 shoulder to overhead) | 2 r × (18 box jump) | ||
 | Shoulder-to-overhead movements entail the elevation of dumbbells from a static position at the shoulder to a static position overhead. The athlete may utilize a single, simultaneous flexion of the hips and/or knees to facilitate the elevation of the dumbbells to the top of the repetition. |  | The box jump is a test of the athlete’s ability to initiate a jump from the ground with both feet simultaneously, land on a designated object (e.g., a box) with both feet, and demonstrate static control. |
| Men: 20 kg | Women: 10 kg | Men: 60 cm (23.6 inch) | Women: 50 cm (19.7 inch) |
| 2 r × (200 m run) | 2 r × (100 m run) | ||
| Placebo | β-Alanine | Levene | p-Valor | |||
|---|---|---|---|---|---|---|
| M ± SD | Shapiro–Wilk | M ± SD | Shapiro–Wilk | |||
| Pre-intervention Jump 1 | 29.37 ± 5.68 | 0.610 | 30.73 ± 6.51 | 0.210 | 0.882 | 0.551 |
| Pre-intervention Power 1 | 937.19 ± 91.46 | 0.586 | 963.75 ± 117.16 | 0.459 | 0.701 | 0.500 |
| Pre-intervencion Lactic acid | 15.62 ± 5.34 | 0.395 | 17.58 ± 4.05 | 0.769 | 0.342 | 0.279 |
| Pre-intervention Jump 2 | 28.78 ± 5.62 | 0.124 | 30.33 ± 6.37 | 0.201 | 0.983 | 0.490 |
| Pre-intervention Power 2 | 926.95 ± 88.82 | 0.164 | 955.91 ± 110.87 | 0.151 | 0.755 | 0.443 |
| Weight | 72.40 ± 11.90 | 0.104 | 74,21 ± 10.61 | 0.151 | 0.256 | 0.669 |
| Baseline Pre-WOD 1 | Baseline Post-WOD 2 | Intervention Pre-WOD1 | Intervention Post-WOD2 | |||
|---|---|---|---|---|---|---|
| Mean ± DS | Mean ± DS | Mean ± DS | Mean ± DS | p Group × Time | ||
| Jump (cm) | Placebo | 29.37 ± 5.68 | 28.78 ± 5.62 | 28.67 ± 5.14 | 28.60 ± 5.51 | 0.084 |
| β-alanine | 30.73 ± 6.51 | 30.33 ± 6.37 | 31.09 ± 6.24 | 31.87 ± 5.46 | ||
| Power (Newtons) | Placebo | 937.19 ± 91.46 | 926.95 ± 88.82 | 927.27 ± 83.19 | 925.21 ± 88.09 | 0.097 |
| β-alanine | 963.75 ± 117.16 | 955.91 ± 110.87 | 963.51 ± 102.49 | 985.64 ± 92.40 † | ||
| Intra-Group Analysis | Inter-Group Analysis | |||||
|---|---|---|---|---|---|---|
| Pre-Intervention | Post-Intervention | Differences Pre-Post | ||||
| Mean ± DS | Mean ± DS | Mean ± DS | p-Value | Student’s t-Test | ||
| EEP | Placebo | 16.80 ± 1.57 | 17.40 ± 1.35 | −0.6 ± 4.38 | 0.223 | 0.262 |
| β-alanine | 17.36 ± 1.50 | 17.36 ± 1.39 | 0.00 ± 1.41 | 1.000 | ||
| Lactic acid (mmol·L−1) | Placebo | 15.62 ± 5.34 | 17.06 ± 3.75 | −1.44 ± 1.40 | 0.120 | 0.199 |
| β-alanine | 17.58 ± 4.05 | 16.53 ± 5.09 | 1.05 ± 5.77 | 0.508 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cimadevilla-Fernández-Pola, E.; Martínez-Roldán, C.; Maté-Muñoz, J.L.; Guodemar-Pérez, J.; Sánchez-Calabuig, M.A.; García-Fernández, P.; Hervás-Pérez, J.P.; Hernández-Lougedo, J. Effects of β-Alanine Supplementation on Subjects Performing High-Intensity Functional Training. Nutrients 2024, 16, 2340. https://doi.org/10.3390/nu16142340
Cimadevilla-Fernández-Pola E, Martínez-Roldán C, Maté-Muñoz JL, Guodemar-Pérez J, Sánchez-Calabuig MA, García-Fernández P, Hervás-Pérez JP, Hernández-Lougedo J. Effects of β-Alanine Supplementation on Subjects Performing High-Intensity Functional Training. Nutrients. 2024; 16(14):2340. https://doi.org/10.3390/nu16142340
Chicago/Turabian StyleCimadevilla-Fernández-Pola, Eduardo, Cristina Martínez-Roldán, Jose Luis Maté-Muñoz, Jesús Guodemar-Pérez, Maria Aránzazu Sánchez-Calabuig, Pablo García-Fernández, Juan Pablo Hervás-Pérez, and Juan Hernández-Lougedo. 2024. "Effects of β-Alanine Supplementation on Subjects Performing High-Intensity Functional Training" Nutrients 16, no. 14: 2340. https://doi.org/10.3390/nu16142340
APA StyleCimadevilla-Fernández-Pola, E., Martínez-Roldán, C., Maté-Muñoz, J. L., Guodemar-Pérez, J., Sánchez-Calabuig, M. A., García-Fernández, P., Hervás-Pérez, J. P., & Hernández-Lougedo, J. (2024). Effects of β-Alanine Supplementation on Subjects Performing High-Intensity Functional Training. Nutrients, 16(14), 2340. https://doi.org/10.3390/nu16142340










