Impact of Lifestyle Interventions on Multiple Sclerosis: Focus on Adipose Tissue
Abstract
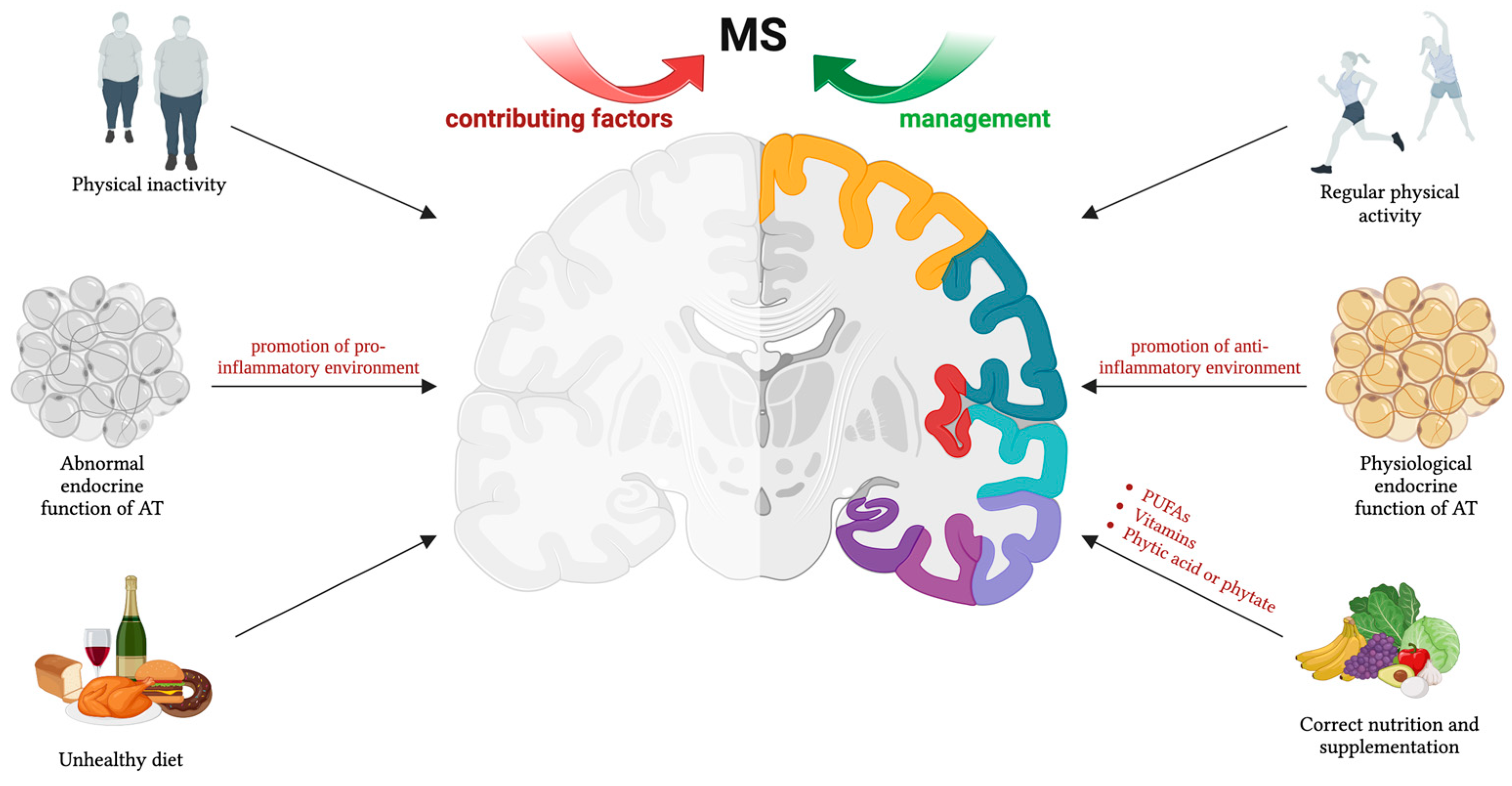
1. Introduction
2. MS and Physical Exercise
3. Involvement of AT in MS Pathophysiology and Adipokine Modulation by Exercise
3.1. Adiponectin
3.2. Leptin
3.3. TNFα
4. Nutrition and Supplements
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Højsgaard Chow, H.; Schreiber, K.; Magyari, M.; Ammitzbøll, C.; Börnsen, L.; Romme Christensen, J.; Ratzer, R.; Soelberg Sørensen, P.; Sellebjerg, F. Progressive multiple sclerosis, cognitive function, and quality of life. Brain Behav. 2018, 8, e00875. [Google Scholar] [CrossRef] [PubMed]
- Klineova, S.; Lublin, F.D. Clinical Course of Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a028928. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, N.G.; Meuth, S.G.; Martinez-Lapiscina, E.H.; Albrecht, P.; Menge, T. Treatment of Patients with Multiple Sclerosis Transitioning Between Relapsing and Progressive Disease. CNS Drugs 2023, 37, 69–92. [Google Scholar] [CrossRef] [PubMed]
- Matthews, P.M. Chronic inflammation in multiple sclerosis—Seeing what was always there. Nat. Rev. Neurol. 2019, 15, 582–593. [Google Scholar] [CrossRef]
- Signoriello, E.; Mallardo, M.; Nigro, E.; Polito, R.; Casertano, S.; Di Pietro, A.; Coletta, M.; Monaco, M.L.; Rossi, F.; Lus, G.; et al. Adiponectin in Cerebrospinal Fluid from Patients Affected by Multiple Sclerosis Is Correlated with the Progression and Severity of Disease. Mol. Neurobiol. 2023, 58, 2663–2670. [Google Scholar] [CrossRef]
- Illiano, M.; Nigro, E.; Sapio, L.; Caiafa, I.; Spina, A.; Scudiero, O.; Bianco, A.; Esposito, S.; Mazzeo, F.; Pedone, P.V.; et al. Adiponectin down-regulates CREB and inhibits proliferation of A549 lung cancer cells. Pulm. Pharmacol. Ther. 2017, 45, 114–120. [Google Scholar] [CrossRef]
- Kalusche, W.J.; Case, C.T.; Taylor, E.B. Leptin antagonism attenuates hypertension and renal injury in an experimental model of autoimmune disease. Clin. Sci. 2023, 137, 1771–1785. [Google Scholar] [CrossRef]
- Guerrero-García, J.J.; Carrera-Quintanar, L.; López-Roa, R.I.; Márquez-Aguirre, A.L.; Rojas-Mayorquín, A.E.; Ortuño-Sahagún, D. Multiple Sclerosis and Obesity: Possible Roles of Adipokines. Mediat. Inflamm. 2016, 2016, 4036232. [Google Scholar] [CrossRef] [PubMed]
- Keyhanian, K.; Saxena, S.; Gombolay, G.; Healy, B.C.; Misra, M.; Chitnis, T. Adipokines are associated with pediatric multiple sclerosis risk and course. Mult. Scler. Relat. Disord. 2019, 36, 101384. [Google Scholar] [CrossRef]
- Correale, J.; Marrodan, M. Multiple sclerosis and obesity: The role of adipokines. Front. Immunol. 2022, 13, 1038393. [Google Scholar] [CrossRef] [PubMed]
- Grazioli, E.; Nigro, E.; Cerulli, C.; Borriello, G.; Mancini, A.; Tranchita, E.; Polito, R.; Parisi, A.; Buono, P.; Daniele, A. Case Report: Concurrent Resistance and Aerobic Training Regulate Adiponectin Expression and Disease Severity in Multiple Sclerosis: A Case Study. Front. Neurosci. 2020, 14, 567302. [Google Scholar] [CrossRef] [PubMed]
- Signoriello, E.; Lus, G.; Polito, R.; Casertano, S.; Scudiero, O.; Coletta, M.; Monaco, M.L.; Rossi, F.; Nigro, E.; Daniele, A. Adiponectin profile at baseline is correlated to progression and severity of multiple sclerosis. Eur. Neurol. 2019, 26, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, F.; Volpe, R.A. From gene doping to athlete biological passport. Sport Sci. 2016, 9, 97–103. [Google Scholar]
- Madsen, L.T.; Dalgas, U.; Hvid, L.G.; Bansi, J. A cross-sectional study on the relationship between cardiorespiratory fitness, disease severity and walking speed in persons with multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 29, 35–40. [Google Scholar] [CrossRef]
- Montesano, P.; Mazzeo, F. Sports activities in obese teenagers improve social inclusion and health. Sport Mont 2019, 17, 55–60. [Google Scholar] [CrossRef]
- Zong, B.; Yu, F.; Zhang, X.; Zhao, W.; Li, S.; Li, L. Mechanisms underlying the beneficial effects of physical exercise on multiple sclerosis: Focus on immune cells. Front. Immunol. 2023, 14, 1260663. [Google Scholar] [CrossRef]
- Khan, F.; Amatya, B. Rehabilitation in Multiple Sclerosis: A Systematic Review of Systematic Reviews. Arch. Phys. Med. Rehabil. 2017, 98, 353–367. [Google Scholar] [CrossRef]
- Halabchi, F.; Alizadeh, Z.; Sahraian, M.A.; Abolhasani, M. Exercise prescription for patients with multiple sclerosis; potential benefits and practical recommendations. BMC Neurol. 2017, 17, 185. [Google Scholar] [CrossRef]
- Faramarzi, M.; Banitalebi, E.; Raisi, Z.; Samieyan, M.; Saberi, Z.; Mardaniyan Ghahfarrokhi, M.; Negaresh, R.; Motl, R.W. Effect of combined exercise training on pentraxins and pro-inflammatory cytokines in people with multiple sclerosis as a function of disability status. Cytokine 2020, 134, 155196. [Google Scholar] [CrossRef]
- Choi, I.Y.; Piccio, L.; Childress, P.; Bollman, B.; Ghosh, A.; Brandhorst, S.; Suarez, J.; Michalsen, A.; Cross, A.H.; Morgan, T.E.; et al. A Diet Mimicking Fasting Promotes Regeneration and Reduces Autoimmunity and Multiple Sclerosis Symptoms. Cell Rep. 2016, 15, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, F.; Jessri, M.; Behrooz, M.; Mirghotbi, M.; Rashidkhani, B. Mediterranean diet adherence and risk of multiple sclerosis: A case-control study. Asia Pac. J. Clin. Nutr. 2016, 25, 377–384. [Google Scholar]
- Tryfonos, C.; Mantzorou, M.; Fotiou, D.; Vrizas, M.; Vadikolias, K.; Pavlidou, E.; Giaginis, C. Dietary Supplements on Controlling Multiple Sclerosis Symptoms and Relapses: Current Clinical Evidence and Future Perspectives. Medicines 2019, 6, 95. [Google Scholar] [CrossRef]
- Miller, E.; Markiewicz, L.; Kabzinski, J.; Odrobina, D.; Majsterek, I. Potential of redox therapies in neurodegenerative disorders. Front. Biosci. 2017, 9, 214–234. [Google Scholar] [CrossRef]
- Bonanni, R.; Cariati, I.; Tarantino, U.; D’Arcangelo, G.; Tancredi, V. Physical Exercise and Health: A Focus on Its Protective Role in Neurodegenerative Diseases. J. Funct. Morphol. Kinesiol. 2022, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, D.; Lamers, I.; Bertoni, R.; Feys, P.; Jonsdottir, J. Participation Restriction in People With Multiple Sclerosis: Prevalence and Correlations With Cognitive, Walking, Balance, and Upper Limb Impairments. Arch. Phys. Med. Rehabil. 2017, 98, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Dalgas, U.; Stenager, E.; Ingemann-Hansen, T. Multiple sclerosis and physical exercise: Recommendations for the application of resistance-, endurance- and combined training. Mult. Scler. J. 2008, 14, 35–53. [Google Scholar] [CrossRef]
- Petajan, J.H.; White, A.T. Recommendations for physical activity in patients with multiple sclerosis. Sport. Med. 1999, 27, 179–191. [Google Scholar] [CrossRef]
- Aidar, F.J.; Carneiro, A.L.; Costa Moreira, O.; Patrocínio de Oliveira, C.E.; Garrido, N.D.; Machado Reis, V.; Raineh, I.; Vilaça, J.M.; Gama de Matos, D. Effects of resistance training on the physical condition of people with multiple sclerosis. J. Sport. Med. Phys. Fit. 2018, 58, 1127–1134. [Google Scholar] [CrossRef]
- Negaresh, R.; Motl, R.; Mokhtarzade, M.; Ranjbar, R.; Majdinasab, N.; Khodadoost, M.; Zimmer, P.; Baker, J.S.; Patel, D. Effect of Short-Term Interval Exercise Training on Fatigue, Depression, and Fitness in Normal Weight vs. Overweight Person with Multiple Sclerosis. Explore 2019, 15, 134–141. [Google Scholar] [CrossRef]
- Abbaspoor, E.; Zolfaghari, M.; Ahmadi, B.; Khodaei, K. The effect of combined functional training on BDNF, IGF-1, and their association with health-related fitness in the multiple sclerosis women. Growth Horm. IGF Res. 2020, 52, 101320. [Google Scholar] [CrossRef] [PubMed]
- Döring, A.; Pfueller, C.F.; Paul, F.; Dörr, J. Exercise in multiple sclerosis--an integral component of disease management. EPMA J. 2012, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Razazian, N.; Kazeminia, M.; Moayedi, H.; Daneshkhah, A.; Shohaimi, S.; Mohammadi, M.; Jalali, R.; Salari, N. The impact of physical exercise on the fatigue symptoms in patients with multiple sclerosis: A systematic review and meta-analysis. BMC Neurol. 2020, 20, 93. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, F.; Liccardo, A. Respiratory responses to exercise in sport. Sport Sci. 2019, 12, 49–52. [Google Scholar]
- Shariat, A.; Ghayour Najafabadi, M.; Soroush Fard, Z.; Nakhostin-Ansari, A.; Shaw, B.S. A systematic review with meta-analysis on balance, fatigue, and motor function following aquatic therapy in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 68, 104107. [Google Scholar] [CrossRef] [PubMed]
- Giesser, B.S. Exercise in the management of persons with multiple sclerosis. Ther. Adv. Neurol. Disord. 2015, 8, 123–130. [Google Scholar] [CrossRef]
- Rampello, A.; Franceschini, M.; Piepoli, M.; Antenucci, R.; Lenti, G.; Olivieri, D.; Chetta, A. Effect of aerobic training on walking capacity and maximal exercise tolerance in patients with multiple sclerosis: A randomized crossover controlled study. Phys. Ther. 2007, 87, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Zhang, X.; Chen, P. Effects of Different Exercise Therapies on Balance Function and Functional Walking Ability in Multiple Sclerosis Disease Patients-A Network Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2022, 19, 7175. [Google Scholar] [CrossRef]
- Grossman, P.; Kappos, L.; Gensicke, H.; D’Souza, M.; Mohr, D.C.; Penner, I.K.; Steiner, C. MS quality of life, depression, and fatigue improve after mindfulness training: A randomized trial. Neurology 2010, 75, 1141–1149. [Google Scholar] [CrossRef]
- Sá, M.J. Exercise therapy and multiple sclerosis: A systematic review. J. Neurol. 2014, 261, 1651–1661. [Google Scholar] [CrossRef]
- Golzari, Z.; Shabkhiz, F.; Soudi, S.; Kordi, M.R.; Hashemi, S.M. Combined exercise training reduces IFN-γ and IL-17 levels in the plasma and the supernatant of peripheral blood mononuclear cells in women with multiple sclerosis. Int. Immunopharmacol. 2010, 10, 1415–1419. [Google Scholar] [CrossRef] [PubMed]
- Pilutti, L.A.; Lelli, D.A.; Paulseth, J.E.; Crome, M.; Jiang, S.; Rathbone, M.P.; Hicks, A.L. Effects of 12 weeks of supported treadmill training on functional ability and quality of life in progressive multiple sclerosis: A pilot study. Arch. Phys. Med. Rehabil. 2011, 92, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Learmonth, Y.C.; Motl, R.W. Exercise Training for Multiple Sclerosis: A Narrative Review of History, Benefits, Safety, Guidelines, and Promotion. Int. J. Environ. Res. Public Health 2021, 18, 13245. [Google Scholar] [CrossRef] [PubMed]
- Fasczewski, K.S.; Gill, D.L.; Rothberger, S.M. Physical activity motivation and benefits in people with multiple sclerosis. Disabil. Rehabil. 2018, 40, 1517–1523. [Google Scholar] [CrossRef]
- Savšek, L.; Stergar, T.; Strojnik, V.; Ihan, A.; Koren, A.; Špiclin, Ž.; Šega Jazbec, S. Impact of aerobic exercise on clinical and magnetic resonance imaging biomarkers in persons with multiple sclerosis: An exploratory randomized controlled trial. J. Rehabil. Med. 2021, 53, 2772. [Google Scholar] [CrossRef]
- Best, J.R.; Chiu, B.K.; Liang Hsu, C.; Nagamatsu, L.S.; Liu-Ambrose, T. Long-Term Effects of Resistance Exercise Training on Cognition and Brain Volume in Older Women: Results from a Randomized Controlled Trial. J. Int. Neuropsychol. Soc. 2015, 21, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Barry, A.; Cronin, O.; Ryan, A.M.; Sweeney, B.; Yap, S.M.; O’Toole, O.; Allen, A.P.; Clarke, G.; O’Halloran, K.D.; Downer, E.J. Impact of Exercise on Innate Immunity in Multiple Sclerosis Progression and Symptomatology. Front. Physiol. 2016, 7, 194. [Google Scholar] [CrossRef]
- Ozkul, C.; Guclu-Gunduz, A.; Irkec, C.; Fidan, I.; Aydin, Y.; Ozkan, T.; Yazici, G. Effect of combined exercise training on serum brain-derived neurotrophic factor, suppressors of cytokine signaling 1 and 3 in patients with multiple sclerosis. J. Neuroimmunol. 2018, 316, 121–129. [Google Scholar] [CrossRef]
- Mey, G.M.; Mahajan, K.R.; DeSilva, T.M. Neurodegeneration in multiple sclerosis. WIREs Mech. Dis. 2023, 15, e1583. [Google Scholar] [CrossRef]
- Grant, R.W.; Dixit, V.D. Adipose tissue as an immunological organ. Obesity 2015, 23, 512–518. [Google Scholar] [CrossRef]
- Harvey, I.; Boudreau, A.; Stephens, J.M. Adipose tissue in health and disease. Open Biol. 2020, 10, 200291. [Google Scholar] [CrossRef] [PubMed]
- Mallardo, M.; Daniele, A.; Musumeci, G.; Nigro, E. A Narrative Review on Adipose Tissue and Overtraining: Shedding Light on the Interplay among Adipokines, Exercise and Overtraining. Int. J. Mol. Sci. 2024, 25, 4089. [Google Scholar] [CrossRef] [PubMed]
- Huber, K.; Szerenos, E.; Lewandowski, D.; Toczylowski, K.; Sulik, A. The Role of Adipokines in the Pathologies of the Central Nervous System. Int. J. Mol. Sci. 2023, 24, 14684. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. The Role of Adipokines in Health and Disease. Biomedicines 2023, 11, 1290. [Google Scholar] [CrossRef] [PubMed]
- Loonstra, F.C.; Falize, K.F.; de Ruiter, L.R.J.; Schoonheim, M.M.; Strijbis, E.M.M.; Killestein, J.; de Vries, H.E.; Uitdehaag, B.M.J.; Rijnsburger, M. Adipokines in multiple sclerosis patients are related to clinical and radiological measures. J. Neurol. 2023, 270, 2018–2030. [Google Scholar] [CrossRef]
- Ray, I.; Meira, L.B.; Michael, A.; Ellis, P.E. Adipocytokines and disease progression in endometrial cancer: A systematic review. Cancer Metastasis Rev. 2022, 41, 211–242. [Google Scholar] [CrossRef] [PubMed]
- Sharif, K.; Watad, A.; Bragazzi, N.L.; Lichtbroun, M.; Amital, H.; Shoenfeld, Y. Physical activity and autoimmune diseases: Get moving and manage the disease. Autoimmun. Rev. 2018, 17, 53–72. [Google Scholar] [CrossRef]
- Angelini, G.; Bani, A.; Constantin, G.; Rossi, B. The interplay between T helper cells and brain barriers in the pathogenesis of multiple sclerosis. Front. Cell. Neurosci. 2023, 17, 1101379. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, D.D.L.; Latini, A. Exercise-induced immune system response: Anti-inflammatory status on peripheral and central organs. Biochim. Biophys. Acta-Mol. Basis Dis. 2020, 1866, 165823. [Google Scholar] [CrossRef]
- Terra, R.; da Silva, S.A.G.; Pinto, V.S.; Dutra, P.M.L. Effect of Exercise on the Immune System: Response, Adaptation and Cell Signaling. Rev. Bras. Med. Esporte 2012, 18, 208–214. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Golbidi, S.; Laher, I. Exercise induced adipokine changes and the metabolic syndrome. J. Diabetes Res. 2014, 2014, 726861. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise—A Systematic Review. Front. Physiol. 2020, 10, 489354. [Google Scholar] [CrossRef] [PubMed]
- Khoramipour, K.; Chamari, K.; Hekmatikar, A.A.; Ziyaiyan, A.; Taherkhani, S.; Elguindy, N.M.; Bragazzi, N.L. Adiponectin: Structure, Physiological Functions, Role in Diseases, and Effects of Nutrition. Nutrients 2021, 13, 1180. [Google Scholar] [CrossRef] [PubMed]
- Clain, J.; Couret, D.; Planesse, C.; Krejbich-Trotot, P.; Meilhac, O.; Lefebvre d’Hellencourt, C.; Viranaicken, W.; Diotel, N. Distribution of Adiponectin Receptors in the Brain of Adult Mouse: Effect of a Single Dose of the Adiponectin Receptor Agonist, AdipoRON, on Ischemic Stroke. Brain Sci. 2022, 12, 680. [Google Scholar] [CrossRef]
- Nguyen, T.M.D. Adiponectin: Role in Physiology and Pathophysiology. Int. J. Prev. Med. 2020, 11, 136. [Google Scholar] [CrossRef]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef] [PubMed]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Salvator, H.; Grassin-Delyle, S.; Brollo, M.; Couderc, L.J.; Abrial, C.; Victoni, T.; Naline, E.; Devillier, P. Adiponectin Inhibits the Production of TNF-α, IL-6 and Chemokines by Human Lung Macrophages. Front. Pharmacol. 2021, 12, 718929. [Google Scholar] [CrossRef]
- Çoban, A.; Düzel, B.; Tüzün, E.; Tamam, Y. Investigation of the prognostic value of adipokines in multiple sclerosis. Mult. Scler. Relat. Disord. 2017, 15, 11–14. [Google Scholar] [CrossRef]
- Düzel, B.; Tamam, Y.; Çoban, A.; Tüzün, E. Adipokines in Multiple Sclerosis Patients with and without Optic Neuritis as the First Clinical Presentation. Immunol. Investig. 2019, 48, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Bartness, T.; Ryu, V. Neural control of white, beige and brown adipocytes. Int. J. Obes. Suppl. 2015, 5, S35–S39. [Google Scholar] [CrossRef]
- Kraszula, L.; Jasińska, A.; Eusebio, M.; Kuna, P.; Głąbiński, A.; Pietruczuk, M. Evaluation of the relationship between leptin, resistin, adiponectin and natural regulatory T cells in relapsing-remitting multiple sclerosis. Neurol. Neurochir. Pol. 2012, 46, 22–28. [Google Scholar] [CrossRef]
- Kvistad, S.S.; Myhr, K.M.; Holmøy, T.; Benth, J.Š.; Wergeland, S.; Beiske, A.G.; Bjerve, K.S.; Hovdal, H.; Midgard, R.; Sagen, J.V.; et al. Serum levels of leptin and adiponectin are not associated with disease activity or treatment response in multiple sclerosis. J. Neuroimmunol. 2018, 323, 73–77. [Google Scholar] [CrossRef]
- Hietaharju, A.; Kuusisto, H.; Nieminen, R.; Vuolteenaho, K.; Elovaara, I.; Moilanen, E. Elevated cerebrospinal fluid adiponectin and adipsin levels in patients with multiple sclerosis: A Finnish co-twin study. Eur. Neurol. 2010, 17, 332–334. [Google Scholar] [CrossRef]
- Piccio, L.; Cantoni, C.; Henderson, J.G.; Hawiger, D.; Ramsbottom, M.; Mikesell, R.; Ryu, J.; Hsieh, C.S.; Cremasco, V.; Haynes, W.; et al. Lack of adiponectin leads to increased lymphocyte activation and increased disease severity in a mouse model of multiple sclerosis. Eur. J. Immunol. 2013, 43, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Guo, Y.; Ge, Z.; Zhang, Z.; Da, Y.; Li, W.; Zhang, Z.; Xue, Z.; Li, Y.; Ren, Y.; et al. Adiponectin Suppresses T Helper 17 Cell Differentiation and Limits Autoimmune CNS Inflammation via the SIRT1/PPARγ/RORγt Pathway. Mol. Neurobiol. 2017, 54, 4908–4920. [Google Scholar] [CrossRef]
- Mokhtarzade, M.; Ranjbar, R.; Majdinasab, N.; Patel, D.; Molanouri Shamsi, M. Effect of aerobic interval training on serum IL-10, TNFα, and adipokines levels in women with multiple sclerosis: Possible relations with fatigue and quality of life. Endocrine 2017, 57, 262–271. [Google Scholar] [CrossRef]
- Majdinasab, N.; Motl, R.W.; Mokhtarzade, M.; Zimmer, P.; Ranjbar, R.; Keytsman, C.; Cullen, T.; Negaresh, R.; Baker, J.S. Acute responses of cytokines and adipokines to aerobic exercise in relapsing vs. remitting women with multiple sclerosis. Complement. Ther. Clin. Pract. 2018, 31, 295–301. [Google Scholar] [CrossRef]
- Schön, M.; Kovaničová, Z.; Košutzká, Z.; Nemec, M.; Tomková, M.; Jacková, L.; Máderová, D.; Slobodová, L.; Valkovič, P.; Ukropec, J.; et al. Effects of running on adiponectin, insulin and cytokines in cerebrospinal fluid in healthy young individuals. Sci. Rep. 2019, 9, 1959. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.; Bal, N.C. Adipokines from white adipose tissue in regulation of whole body energy homeostasis. Biochimie 2023, 204, 92–107. [Google Scholar] [CrossRef] [PubMed]
- La Cava, A. Leptin in inflammation and autoimmunity. Cytokine 2017, 98, 51–58. [Google Scholar] [CrossRef]
- Tartaglia, L.A.; Dembski, M.; Weng, X.; Deng, N.; Culpepper, J.; Devos, R.; Richards, G.J.; Campfield, L.A.; Clark, F.T.; Deeds, J.; et al. Identification and expression cloning of a leptin receptor, OB-R. Cell 1995, 83, 1263–1271. [Google Scholar] [CrossRef]
- Gupta, S.; Agrawal, S.; Gollapudi, S. Increased activation and cytokine secretion in B cells stimulated with leptin in aged humans. Immun. Ageing 2013, 10, 3. [Google Scholar] [CrossRef]
- Marrodan, M.; Farez, M.F.; Balbuena Aguirre, M.E.; Correale, J. Obesity and the risk of Multiple Sclerosis. The role of Leptin. Ann. Clin. Transl. Neurol. 2021, 8, 406–424. [Google Scholar] [CrossRef]
- Shirshev, S.V.; Orlova, E.G. Molecular mechanisms of regulation of functional activity of mononuclear phagocytes by leptin. Biochemistry 2005, 70, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Yousefian, M.; Nemati, R.; Daryabor, G.; Gholijani, N.; Nikseresht, A.; Borhani-Haghighi, A.; Kamali-Sarvestani, E. Gender-Specific Association of Leptin and Adiponectin Genes With Multiple Sclerosis. Am. J. Med. Sci. 2018, 356, 159–167. [Google Scholar] [CrossRef]
- Natarajan, R.; Hagman, S.; Hämälainen, M.; Leppänen, T.; Dastidar, P.; Moilanen, E.; Elovaara, I. Adipsin is associated with multiple sclerosis: A follow-up study of adipokines. Mult. Scler. Int. 2015, 1, 371734. [Google Scholar] [CrossRef] [PubMed]
- Chatzantoni, K.; Papathanassopoulos, P.; Gourzoulidou, E.; Mouzaki, A. Leptin and its soluble receptor in plasma of patients suffering from remitting-relapsing multiple sclerosis (MS) In vitro effects of leptin on type-1 and type-2 cytokine secretion by peripheral blood mononuclear cells, T-cells and monocytes of MS patients. J. Autoimmun. 2004, 23, 169–177. [Google Scholar] [CrossRef]
- Emamgholipour, S.; Eshaghi, S.M.; Hossein-nezhad, A.; Mirzaei, K.; Maghbooli, Z.; Sahraian, M.A. Adipocytokine Profile, Cytokine Levels and Foxp3 Expression in Multiple Sclerosis: A Possible Link to Susceptibility and Clinical Course of Disease. PLoS ONE 2013, 8, e76555. [Google Scholar] [CrossRef]
- Joseph, D.; Kumar, S. Identifying clues to molecular etiology of multiple sclerosis in South Indian patients. Mult. Scler. Relat. Disord. 2016, 5, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Matarese, G.; Carrieri, P.B.; La Cava, A.; Perna, F.; Sanna, V.; De Rosa, V.; Aufiero, D.; Fontana, S.; Zappacosta, S. Leptin increase in multiple sclerosis associates with reduced number of CD4(+)CD25+ regulatory T cells. Proc. Natl. Acad. Sci. USA 2005, 102, 5150–5155. [Google Scholar] [CrossRef] [PubMed]
- Messina, S.; Vargas-Lowy, D.; Musallam, A.; Healy, B.C.; Kivisakk, P.; Gandhi, R.; Bove, R.; Gholipour, T.; Khoury, S.; Weiner, H.L.; et al. Increased leptin and A-FABP levels in relapsing and progressive forms of MS. BMC Neurol. 2013, 13, 172. [Google Scholar] [CrossRef]
- Penesova, A.; Vlcek, M.; Imrich, R.; Vernerova, L.; Marko, A.; Meskova, M.; Grunnerova, L.; Turcani, P.; Jezova, D.; Kollar, B. Hyperinsulinemia in newly diagnosed patients with multiple sclerosis. Metab. Brain Dis. 2015, 30, 895–901. [Google Scholar] [CrossRef]
- Rotondi, M.; Batocchi, A.P.; Coperchini, F.; Caggiula, M.; Zerbini, F.; Sideri, R.; Leporati, P.; Nociti, V.; Frisullo, G.; Mirabella, M.; et al. Severe disability in patients with relapsing-remitting multiple sclerosis is associated with profound changes in the regulation of leptin secretion. Neuroimmunomodulation 2013, 20, 341–347. [Google Scholar] [CrossRef]
- Dashti, M.; Alroughani, R.; Jacob, S.; Al-Temaimi, R. Leptin rs7799039 polymorphism is associated with multiple sclerosis risk in Kuwait. Mult. Scler. Relat. Disord. 2019, 36, 101409. [Google Scholar] [CrossRef] [PubMed]
- Evangelopoulos, M.E.; Koutsis, G.; Markianos, M. Serum leptin levels in treatment-naive patients with clinically isolated syndrome or relapsing-remitting multiple sclerosis. Autoimmune Dis. 2014, 2014, 486282. [Google Scholar] [CrossRef]
- Frisullo, G.; Mirabella, M.; Angelucci, F.; Caggiula, M.; Morosetti, R.; Sancricca, C.; Patanella, A.K.; Nociti, V.; Iorio, R.; Bianco, A.; et al. The effect of disease activity on leptin, leptin receptor and suppressor of cytokine signalling-3 expression in relapsing-remitting multiple sclerosis. J. Neuroimmunol. 2007, 192, 174–183. [Google Scholar] [CrossRef]
- Batocchi, A.P.; Rotondi, M.; Caggiula, M.; Frisullo, G.; Odoardi, F.; Nociti, V.; Carella, C.; Tonali, P.A.; Mirabella, M. Leptin as a marker of multiple sclerosis activity in patients treated with interferon-beta. J. Neuroimmunol. 2003, 139, 150–154. [Google Scholar] [CrossRef]
- Baranowska-Bik, A.; Uchman, D.; Litwiniuk, A.; Kalisz, M.; Martyńska, L.; Baranowska, B.; Bik, W.; Kochanowski, J. Peripheral levels of selected adipokines in patients with newly diagnosed multiple sclerosis. Endokrynol. Pol. 2020, 71, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Sanna, V.; Di Giacomo, A.; La Cava, A.; Lechler, R.I.; Fontana, S.; Zappacosta, S.; Matarese, G. Leptin surge precedes onset of autoimmune encephalomyelitis and correlates with development of pathogenic T cell responses. J. Clin. Investig. 2003, 111, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Abd Elhafeez, M.A.; Zamzam, D.A.; Fouad, M.M.; Elkhawas, H.M.; Abdel Rahman, H.A. Serum leptin and body mass index in a sample of Egyptian multiple sclerosis patients. Egypt. J. Neurol. Psychiatry Neurosurg. 2020, 56, 107. [Google Scholar] [CrossRef]
- Wens, I.; Dalgas, U.; Deckx, N.; Cools, N.; Eijnde, B.O. Does multiple sclerosis affect glucose tolerance? Mult. Scler. J. 2014, 20, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Daryabor, G.; Amirghofran, Z.; Gholijani, N.; Bemani, P. Obesity and adipose tissue-derived cytokines in the pathogenesis of multiple sclerosis. Endocr. Metab. Immune Disord.-Drug Targets 2022, 22, 1217–1231. [Google Scholar] [CrossRef]
- Neumeier, M.; Weigert, J.; Buettner, R.; Wanninger, J.; Schäffler, A.; Müller, A.M.; Killian, S.; Sauerbruch, S.; Schlachetzki, F.; Steinbrecher, A.; et al. Detection of adiponectin in cerebrospinal fluid in humans. Am. J. Physiol.-Endocrinol. Metab. 2007, 293, E965–E969. [Google Scholar] [CrossRef]
- Matarese, G.; Procaccini, C.; De Rosa, V. The intricate interface between immune and metabolic regulation: A role for leptin in the pathogenesis of multiple sclerosis? J. Leucoc. Biol. 2008, 84, 893–899. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Eftekhari, E.; Etemadifar, M. Effects of whole body vibration on hormonal & functional indices in patients with multiple sclerosis. Indian J. Med. Res. 2015, 142, 450–458. [Google Scholar]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Kalliolias, G.; Ivashkiv, L. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef]
- Probert, L. TNF and its receptors in the CNS: The essential, the desirable and the deleterious effects. Neuroscience 2015, 302, 2–22. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.A.; Alleva, L.M.; Vissel, B. The roles of TNF in brain dysfunction and disease. Pharmacol. Ther. 2010, 128, 519–548. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Tweedie, D.; Scerba, M.T.; Greig, N.H. Neuroinflammation as a Factor of Neurodegenerative Disease: Thalidomide Analogs as Treatments. Front. Cell Dev. Biol. 2019, 7, 313. [Google Scholar] [CrossRef] [PubMed]
- Elenkov, I.J.; Chrousos, G.P. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann. N. Y. Acad. Sci. 2002, 966, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.M.; Xiao, B.G.; Ozenci, V.; Kouwenhoven, M.; Teleshova, N.; Fredrikson, S.; Link, H. Multiple sclerosis is associated with high levels of circulating dendritic cells secreting pro-inflammatory cytokines. J. Neuroimmunol. 1999, 99, 82–90. [Google Scholar] [CrossRef]
- Arnett, H.A.; Mason, J.; Marino, M.; Suzuki, K.; Matsushima, G.K.; Ting, J.P. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat. Neurosci. 2001, 4, 1116–1122. [Google Scholar] [CrossRef]
- Arnett, H.A.; Wang, Y.; Matsushima, G.K.; Suzuki, K.; Ting, J.P. Functional genomic analysis of remyelination reveals importance of inflammation in oligodendrocyte regeneration. J. Neurosci. 2003, 23, 9824–9832. [Google Scholar] [CrossRef]
- Sharief, M.K.; Hentges, R. Association between tumor necrosis factor-alpha and disease progression in patients with multiple sclerosis. N. Engl. J. Med. 1991, 325, 467–472. [Google Scholar] [CrossRef]
- Fresegna, D.; Bullitta, S.; Musella, A.; Rizzo, F.R.; De Vito, F.; Guadalupi, L.; Caioli, S.; Balletta, S.; Sanna, K.; Dolcetti, E.; et al. Re-Examining the Role of TNF in MS Pathogenesis and Therapy. Cells 2020, 9, 2290. [Google Scholar] [CrossRef]
- Maguire, A.D.; Bethea, J.R.; Kerr, B.J. TNFα in MS and Its Animal Models: Implications for Chronic Pain in the Disease. Front. Neurol. 2021, 12, 780876. [Google Scholar] [CrossRef]
- Kassiotis, G.; Pasparakis, M.; Kollias, G.; Probert, L. TNF accelerates the onset but does not alter the incidence and severity of myelin basic protein-induced experimental autoimmune encephalomyelitis. Eur. J. Immunol. 1999, 29, 774–780. [Google Scholar] [CrossRef]
- Wajant, H.; Siegmund, D. TNFR1 and TNFR2 in the Control of the Life and Death Balance of Macrophages. Front. Cell Dev. Biol. 2019, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga-Filho, H.; Sacramento, P.M.; Ferreira, T.B.; Hygino, J.; Abreu, J.E.C.; Carvalho, S.R.; Wing, A.C.; Alvarenga, R.M.P.; Bento, C.A.M. Combined exercise training reduces fatigue and modulates the cytokine profile of T-cells from multiple sclerosis patients in response to neuromediators. J. Neuroimmunol. 2016, 293, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Bansi, J.; Bloch, W.; Gamper, U.; Kesselring, J. Training in MS: Influence of two different endurance training protocols (aquatic versus overland) on cytokine and neurotrophin concentrations during three week randomized controlled trial. Mult. Scler. J. 2013, 19, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, S.; Achiron, A.; Gurevich, M.; Sonis, P.; Kalron, A. Acute effects of aerobic intensities on the cytokine response in women with mild multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 31, 82–86. [Google Scholar] [CrossRef]
- Deckx, N.; Wens, I.; Nuyts, A.H.; Hens, N.; De Winter, B.Y.; Koppen, G.; Goossens, H.; Van Damme, P.; Berneman, Z.N.; Eijnde, B.O.; et al. 12 Weeks of Combined Endurance and Resistance Training Reduces Innate Markers of Inflammation in a Randomized Controlled Clinical Trial in Patients with Multiple Sclerosis. Mediat. Inflamm. 2016, 2016, 6789276. [Google Scholar] [CrossRef]
- Castellano, V.; Patel, D.I.; White, L.J. Cytokine responses to acute and chronic exercise in multiple sclerosis. J. Appl. Physiol. 2008, 104, 1697–1702. [Google Scholar] [CrossRef]
- White, L.J.; Castellano, V.; Mc Coy, S.C. Cytokine responses to resistance training in people with multiple sclerosis. J. Sport. Sci. 2006, 24, 911–914. [Google Scholar] [CrossRef]
- Kjølhede, T.; Dalgas, U.; Gade, A.B.; Bjerre, M.; Stenager, E.; Petersen, T.; Vissing, K. Acute and chronic cytokine responses to resistance exercise and training in people with multiple sclerosis. Scand. J. Med. Sci. Sport. 2016, 26, 824–834. [Google Scholar] [CrossRef]
- Heesen, C.; Gold, S.M.; Hartmann, S.; Mladek, M.; Reer, R.; Braumann, K.M.; Wiedemann, K.; Schulz, K.H. Endocrine and cytokine responses to standardized physical stress in multiple sclerosis. Brain Behav. Immun. 2003, 17, 473–481. [Google Scholar] [CrossRef]
- Rezaee, S.; Kahrizi, S.; Nabavi, S.M.; Hedayati, M. VEGF and TNF-α Responses to Acute and Chronic Aerobic Exercise in the Patients with Multiple Sclerosis. Asian J. Sport. Med. 2020, 11, e98312. [Google Scholar] [CrossRef]
- Olatona, F.A.; Onabanjo, O.O.; Ugbaja, R.N.; Nnoaham, K.E.; Adelekan, D.A. Dietary habits and metabolic risk factors for non-communicable diseases in a university undergraduate population. J. Health Popul. Nutr. 2018, 37, 21. [Google Scholar] [CrossRef] [PubMed]
- Alfredsson, L.; Olsson, T. Lifestyle and Environmental Factors in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2019, 9, a028944. [Google Scholar] [CrossRef]
- Bagur, M.J.; Murcia, M.A.; Jiménez-Monreal, A.M.; Tur, J.A.; Bibiloni, M.M.; Alonso, G.L.; Martínez-Tomé, M. Influence of Diet in Multiple Sclerosis: A Systematic Review. Adv. Nutr. 2017, 8, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Yin, X.; Wang, M.; Chen, T.; Wang, Y.; Gao, Z.; Wang, Z. Effects of Ketogenic Diet on Neuroinflammation in Neurodegenerative Diseases. Aging Dis. 2022, 13, 1146–1165. [Google Scholar] [CrossRef] [PubMed]
- Brenton, J.N.; Banwell, B.; Bergqvist, A.G.C.; Lehner-Gulotta, D.; Gampper, L.; Leytham, E.; Coleman, R.; Goldman, M.D. Pilot study of a ketogenic diet in relapsing-remitting MS. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e565. [Google Scholar] [CrossRef]
- Meulmeester, F.L.; Luo, J.; Martens, L.G.; Mills, K.; van Heemst, D.; Noordam, R. Antioxidant Supplementation in Oxidative Stress-Related Diseases: What Have We Learned from Studies on Alpha-Tocopherol? Antioxidants 2022, 11, 2322. [Google Scholar] [CrossRef]
- Ortí, J.E.R.; Cuerda-Ballester, M.; Sanchis-Sanchis, C.E.; Lajara Romance, J.M.; Navarro-Illana, E.; García Pardo, M.P. Exploring the impact of ketogenic diet on multiple sclerosis: Obesity, anxiety, depression, and the glutamate system. Front. Nutr. 2023, 10, 1227431. [Google Scholar] [CrossRef]
- Evans, E.; Levasseur, V.; Cross, A.H.; Piccio, L. An overview of the current state of evidence for the role of specific diets in multiple sclerosis. Mult. Scler. Relat. Disorders. 2019, 36, 101393. [Google Scholar] [CrossRef]
- Yu, M.; Jelinek, G.; Simpson-Yap, S.; Neate, S.; Nag, N. Self-reported ongoing adherence to diet is associated with lower depression, fatigue, and disability, in people with multiple sclerosis. Front. Nutr. 2023, 10, 979380. [Google Scholar] [CrossRef]
- Hadgkiss, E.J.; Jelinek, G.A.; Taylor, K.L.; Marck, C.H.; van der Meer, D.M.; Pereira, N.G.; Weiland, T.J. Engagement in a program promoting lifestyle modification is associated with better patient-reported outcomes for people with MS. Ital. J. Neurol. Sci. 2015, 36, 845–852. [Google Scholar] [CrossRef][Green Version]
- AlAmmar, W.A.; Albeesh, F.H.; Ibrahim, L.M.; Algindan, Y.Y.; Yamani, L.Z.; Khattab, R.Y. Effect of omega-3 fatty acids and fish oil supplementation on multiple sclerosis: A systematic review. Nutr. Neurosci. 2021, 24, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Stoiloudis, P.; Kesidou, E.; Bakirtzis, C.; Sintila, S.A.; Konstantinidou, N.; Boziki, M.; Grigoriadis, N. The Role of Diet and Interventions on Multiple Sclerosis: A Review. Nutrients 2022, 14, 1150. [Google Scholar] [CrossRef] [PubMed]
- Bjørnevik, K.; Chitnis, T.; Ascherio, A.; Munger, K.L. Polyunsaturated fatty acids and the risk of multiple sclerosis. Mult. Scler. J. 2017, 23, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Harbige, L.S.; Sharief, M.K. Polyunsaturated fatty acids in the pathogenesis and treatment of multiple sclerosis. Br. J. Nutr. 2007, 98 (Suppl. S1), S46–S53. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Ramirez, V.; Macias-Islas, M.A.; Ortiz, G.G.; Pacheco-Moises, F.; Torres-Sanchez, E.D.; Sorto-Gomez, T.E.; Cruz-Ramos, J.A.; Orozco-Aviña, G.; Celis de la Rosa, A.J. Efficacy of fish oil on serum of TNF α, IL-1 β, and IL-6 oxidative stress markers in multiple sclerosis treated with interferon beta-1b. Oxidative Med. Cell. Longev. 2013, 1, 709493. [Google Scholar]
- Kousparou, C.; Fyrilla, M.; Stephanou, A.; Patrikios, I. DHA/EPA (Omega-3) and LA/GLA (Omega-6) as Bioactive Molecules in Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 10717. [Google Scholar] [CrossRef]
- Shinto, L.; Marracci, G.; Baldauf-Wagner, S.; Strehlow, A.; Yadav, V.; Stuber, L.; Bourdette, D. Omega-3 fatty acid supplementation decreases matrix metalloproteinase-9 production in relapsing-remitting multiple sclerosis. Prostaglandins Leukot. Essent. Fat. Acids 2009, 80, 131–136. [Google Scholar] [CrossRef]
- Al-Naqeb, G.; Kalmpourtzidou, A.; De Giuseppe, R.; Cena, H. Beneficial Effects of Plant Oils Supplementation on Multiple Sclerosis: A Comprehensive Review of Clinical and Experimental Studies. Nutrients 2023, 15, 4827. [Google Scholar] [CrossRef]
- Mazaherioun, M.; Saedisomeolia, A.; Javanbakht, M.H.; Koohdani, F.; Eshraghian, M.R.; Djalali, M. Beneficial effects of n-3 polyunsaturated fatty acids on adiponectin levels and AdipoR gene expression in patients with type 2 diabetes mellitus: A randomized, placebo-controlled, double-blind clinical trial. Arch. Med. Sci. 2017, 13, 716–724. [Google Scholar] [CrossRef]
- Farimani, A.R.; Hariri, M.; Azimi-Nezhad, M.; Borji, A.; Zarei, S.; Hooshmand, E. The effect of n-3 PUFAs on circulating adiponectin and leptin in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. 2018, 55, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Mostowik, M.; Gajos, G.; Zalewski, J.; Nessler, J.; Undas, A. Omega-3 polyunsaturated fatty acids increase plasma adiponectin to leptin ratio in stable coronary artery disease. Cardiovasc. Drugs Ther. 2013, 27, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Ascherio, A.; Munger, K.L. Epidemiology of Multiple Sclerosis: From Risk Factors to Prevention-An Update. Semin. Neurol. 2016, 36, 103–114. [Google Scholar] [CrossRef]
- Munger, K.L.; Zhang, S.M.; O’Reilly, E.; Hernán, M.A.; Olek, M.J.; Willett, W.C.; Ascherio, A. Vitamin D intake and incidence of multiple sclerosis. Neurology 2004, 62, 60–65. [Google Scholar] [CrossRef]
- Feige, J.; Moser, T.; Bieler, L.; Schwenker, K.; Hauer, L.; Sellner, J. Vitamin D Supplementation in Multiple Sclerosis: A Critical Analysis of Potentials and Threats. Nutrients 2020, 12, 783. [Google Scholar] [CrossRef]
- Cassard, S.D.; Fitzgerald, K.C.; Qian, P.; Emrich, S.A.; Azevedo, C.J.; Goodman, A.D.; Sugar, E.A.; Pelletier, D.; Waubant, E.; Mowry, E.M. High-dose vitamin D3 supplementation in relapsing-remitting multiple sclerosis: A randomised clinical trial. EClinicalMedicine 2023, 59, 101957. [Google Scholar] [CrossRef]
- Royal, W., III; Gartner, S.; Gajewski, C.D. Retinol measurements and retinoid receptor gene expression in pa-tients with multiple sclerosis. Mult. Scler. J. 2002, 8, 452–458. [Google Scholar] [CrossRef]
- Runia, T.F.; Hop, W.C.; de Rijke, Y.B.; Hintzen, R.Q. Vitamin A is not associated with exacerbations in multiple sclerosis. Mult. Scler. Relat. Disord. 2014, 3, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Bitarafan, S.; Saboor-Yaraghi, A.; Sahraian, M.A.; Soltani, D.; Nafissi, S.; Togha, M.; Moghadam, N.B.; Roostaei, T.; Honarvar, N.M.; Harirchian, M.H. Effect of Vitamin A Supplementation on fatigue and depression in Multiple Sclerosis patients: A Double-Blind Placebo-Controlled Clinical Trial. Iran. J. Allergy Asthma Immunol. 2016, 15, 13–19. [Google Scholar]
- Perez-Gregorio, R.; Simal-Gandara, J. A Critical Review of Bioactive Food Components, and of their Functional Mechanisms, Biological Effects and Health Outcomes. Curr. Pharm. Des. 2017, 23, 2731–2741. [Google Scholar] [CrossRef] [PubMed]
- Pujol, A.; Sanchis, P.; Grases, F.; Masmiquel, L. Phytate Intake, Health and Disease: “Let Thy Food Be Thy Medicine and Medicine Be Thy Food”. Antioxidants 2023, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Pires, S.M.G.; Reis, R.S.; Cardoso, S.M.; Pezzani, R.; Paredes-Osses, E.; Seilkhan, A.; Ydyrys, A.; Martorell, M.; Sönmez Gürer, E.; Setzer, W.N.; et al. Phytates as a natural source for health promotion: A critical evaluation of clinical trials. Front. Chem. 2023, 11, 1174109. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Hou, L.; Wang, Y.; Zuo, J.; Xue, M.; Li, X.; Liu, Y.; Song, J.; Pan, F.; et al. Phytic acid attenuates upregulation of GSK-3β and disturbance of synaptic vesicle recycling in MPTP-induced Parkinson’s disease models. Neurochem. Int. 2019, 129, 104507. [Google Scholar] [CrossRef]
- Seidl, S.E.; Potashkin, J.A. The promise of neuroprotective agents in Parkinson’s disease. Front. Neurol. 2011, 2, 68. [Google Scholar] [CrossRef]
- Jahromi, S.R.; Toghae, M.; Jahromi, M.J.; Aloosh, M. Dietary pattern and risk of multiple sclerosis. Iran. J. Neurol. 2012, 11, 47–53. [Google Scholar]
- Sanchis, P.; Calvo, P.; Pujol, A.; Rivera, R.; Berga, F.; Fortuny, R.; Costa-Bauza, A.; Grases, F.; Masmiquel, L. Daily phytate intake increases adiponectin levels among patients with diabetes type 2: A randomized crossover trial. Nutr. Diabetes 2023, 13, 2. [Google Scholar] [CrossRef]
- Mirmiran, P.; Hosseini, S.; Hosseinpour-Niazi, S.; Azizi, F. Legume consumption increase adiponectin concentrations among type 2 diabetic patients: A randomized crossover clinical trial. Endocrinol. Diabetes Nutr. 2019, 66, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef]
- Jangi, S.; Gandhi, R.; Cox, L.M.; Li, N.; von Glehn, F.; Yan, R.; Patel, B.; Mazzola, M.A.; Liu, S.; Glanz, B.L.; et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 2016, 7, 12015. [Google Scholar] [CrossRef]
- Asghari, K.M.; Dolatkhah, N.; Ayromlou, H.; Mirnasiri, F.; Dadfar, T.; Hashemian, M. The effect of probiotic supplementation on the clinical and para-clinical findings of multiple sclerosis: A randomized clinical trial. Sci. Rep. 2023, 13, 18577. [Google Scholar] [CrossRef] [PubMed]
- Kouchaki, E.; Tamtaji, O.R.; Salami, M.; Bahmani, F.; Daneshvar Kakhaki, R.; Akbari, E.; Tajabadi-Ebrahimi, M.; Jafari, P.; Asemi, Z. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2017, 36, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
| Adipocytokine | Study Population | Main Findings | Reference |
|---|---|---|---|
| Adiponectin | Case report: a 39-year-old RRMS patient | Total serum adiponectin and HMW oligomers were reduced after 4 months of training at moderate intensity (65% heart rate reserve); in addition, a reduction in BMI (−0.9%) and FAT (−2.6%) and an improvement in the disability level were also demonstrated | [12] |
| 40 MS women randomized divided into either a non-exercising control or training group | Blood adiponectin levels considerably increased in the training group (8 weeks of aerobic interval training). In addition, the aerobic interval training was associated with improvements in fatigue, quality of life, and maximal oxygen consumption | [79] | |
| 30 MS women and 15 healthy controls | Adiponectin showed no significant difference between non-exercising and training group (a single bout of aerobic exercise at 60–70% maximal heart rate) | [80] | |
| Leptin | 30 MS women and 15 healthy controls | Participants performed a single bout of aerobic exercise at 60–70% maximal heart rate. Immediately following exercise, leptin levels significantly decreased in MS subjects | [80] |
| 34 MS patients with mild to moderate disability randomly divided into a training group (n = 17) and a control group (n = 17) | Non-significantly changed the serum levels of leptin, ghrelin, ghrelin/leptin ratio, testosterone, and testosterone/leptin ratio between no exercise and training subjects (low-intensity exercise three times a week for 10 weeks) | [108] | |
| TNFα | 40 MS women randomized into either a non-exercising control or training group. | TNFα levels significantly decreased subsequent to the aerobic interval training (8 weeks of aerobic interval training) | [79] |
| 30 MS women and 15 healthy controls | TNFα levels were significantly decreased immediately after exercise (a single bout of aerobic exercise at 60–70% maximal heart rate) | [80] | |
| 8 MS patients with low disability | Decrease in fatigue at the end of physical activity intervention (12-week series of combining Pilates and aerobic exercises) accompanied by a significant reduction in TNFα | [123] | |
| A randomized controlled clinical trial in 60 MS patients | In response to cardiopulmonary exercise test (30 min training at 60% of VO2max), TNFα levels stayed unchanged. | [124] | |
| 15 MS women and 10 healthy women. | Blood samples were taken at baseline. TNFα remained unchanged immediately after exercise and two hours after exercise [15 min treadmill (~50% VO2 peak)] | [125] | |
| 67 MS patients | Decrease in the production of TNFα at the end of the exercise program (12-week combined exercise) | [126] | |
| 11 MS and 11 non-MS control subjects (8 women and 3 men in both groups) | TNFα increased in MS compared with controls after exercise (30 min of cycle ergometry at 60% of peak O(2) uptake, 3 day/wk for 8 wk at weeks) | [127] | |
| 10 MS female patients | Participants completed 8-week program of twice-weekly progressive resistance training. After training, TNFα showed non-significant reductions | [128] | |
| 35 MS people treated with interferon (IFN)-β | No changes were observed in TNFα levels after a 24-week progressive resistance training respect to a control group | [129] | |
| 15 MS patients and 13 in control group. Twenty healthy controls | TNFα levels were slightly inducible in MS patients completing an eight-week aerobic training program | [130] | |
| 20 subjects (n = 10 MS patients and n = 10 controls) | Serum concentration of the TNFα decreased significantly after a single bout and 6 weeks of aerobic exercise training in the intervention group | [131] |
| Dietary Approach and/or Supplement | Study Population | Main Findings | Reference |
|---|---|---|---|
| Adherence to the ketogenic diet | 99 MS subjects | Amelioration of fatigue and depression accompanied by weight loss and reduction in pro-inflammatory cytokines | [136] |
| Adherence to the OMS diet | Data from an international population of MS followed over 7.5 years | Lower depression rate | [140] |
| High intake of grain or meat, fat, and milk from animals (elevate content of phytic acid) | 75 MS women and 75 healthy controls | Positive correlation with the prevalence of MS | [166] |
| Omega-3 fatty acid and fish oils supplementation | Systematic review of 5554 studies | Beneficial effects on reducing relapsing rate, inflammatory markers, and improving quality of life | [142] |
| Diets enriched in PUFAs | 80,920 women from Nurses’ Health Study and 94,511 women from Nurses’ Health Study II | Lower incidence of MS. Among the specific types of PUFA, only α-linolenic acid was inversely associated with MS risk | [144] |
| Omega-3 PUFAs supplementation | 10 MS patients | Improvement in quality of life by decreasing relapse rates | [148] |
| Vitamin D deficiency | 92,253 women from the Nurses’ Health Study (NHS) and 95,310 women from the Nurses’ Health Study II (NHS II) | Higher risk of MS incidence | [155] |
| Low vitamin D intake | Review of literature data | Increased incidence of MS, but the risk–benefit profile of dosage and duration or supplementation needs to be clarified | [156] |
| Vitamin D supplementation | 172 MS patients were randomized: low-dose vitamin D3–high-dose vitamin D3 | Lack of significant effects | [157] |
| Low levels of vitamin A | 31 MS patients and 29 matched controls | Lack of correlation with the incidence of MS | [159] |
| Vitamin A supplementation | 101 MS patients in a placebo randomized clinical trial | Significant improvement in fatigue and depression. Improvement also in psychiatric outcomes during interferon therapy | [160] |
| Probiotics supplementation | 40 MS patients | Significant improvement in inflammatory markers, oxidative stress indicators, pain, fatigue, and quality of life | [171] |
| Probiotics supplementation | 60 MS patients | Significant improvements in disability scores and mental health parameters, such as reduced depressive symptoms, anxiety, and stress | [172] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallardo, M.; Mazzeo, F.; Lus, G.; Signoriello, E.; Daniele, A.; Nigro, E. Impact of Lifestyle Interventions on Multiple Sclerosis: Focus on Adipose Tissue. Nutrients 2024, 16, 3100. https://doi.org/10.3390/nu16183100
Mallardo M, Mazzeo F, Lus G, Signoriello E, Daniele A, Nigro E. Impact of Lifestyle Interventions on Multiple Sclerosis: Focus on Adipose Tissue. Nutrients. 2024; 16(18):3100. https://doi.org/10.3390/nu16183100
Chicago/Turabian StyleMallardo, Marta, Filomena Mazzeo, Giacomo Lus, Elisabetta Signoriello, Aurora Daniele, and Ersilia Nigro. 2024. "Impact of Lifestyle Interventions on Multiple Sclerosis: Focus on Adipose Tissue" Nutrients 16, no. 18: 3100. https://doi.org/10.3390/nu16183100
APA StyleMallardo, M., Mazzeo, F., Lus, G., Signoriello, E., Daniele, A., & Nigro, E. (2024). Impact of Lifestyle Interventions on Multiple Sclerosis: Focus on Adipose Tissue. Nutrients, 16(18), 3100. https://doi.org/10.3390/nu16183100








